Optimization of Feed Components to Improve Hermetia illucens Growth and Development of Oil Extractor to Produce Biodiesel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. H. illucens Larvae
2.2. Preparation of Organic Waste
2.3. HIL Breeding
2.4. Extraction of Crude Oil From HIL
2.5. Analysis of HIL Nutritional and Fat Compositions
2.6. Performance Evaluation of Oil Extraction
2.7. Statistical Analysis
3. Results
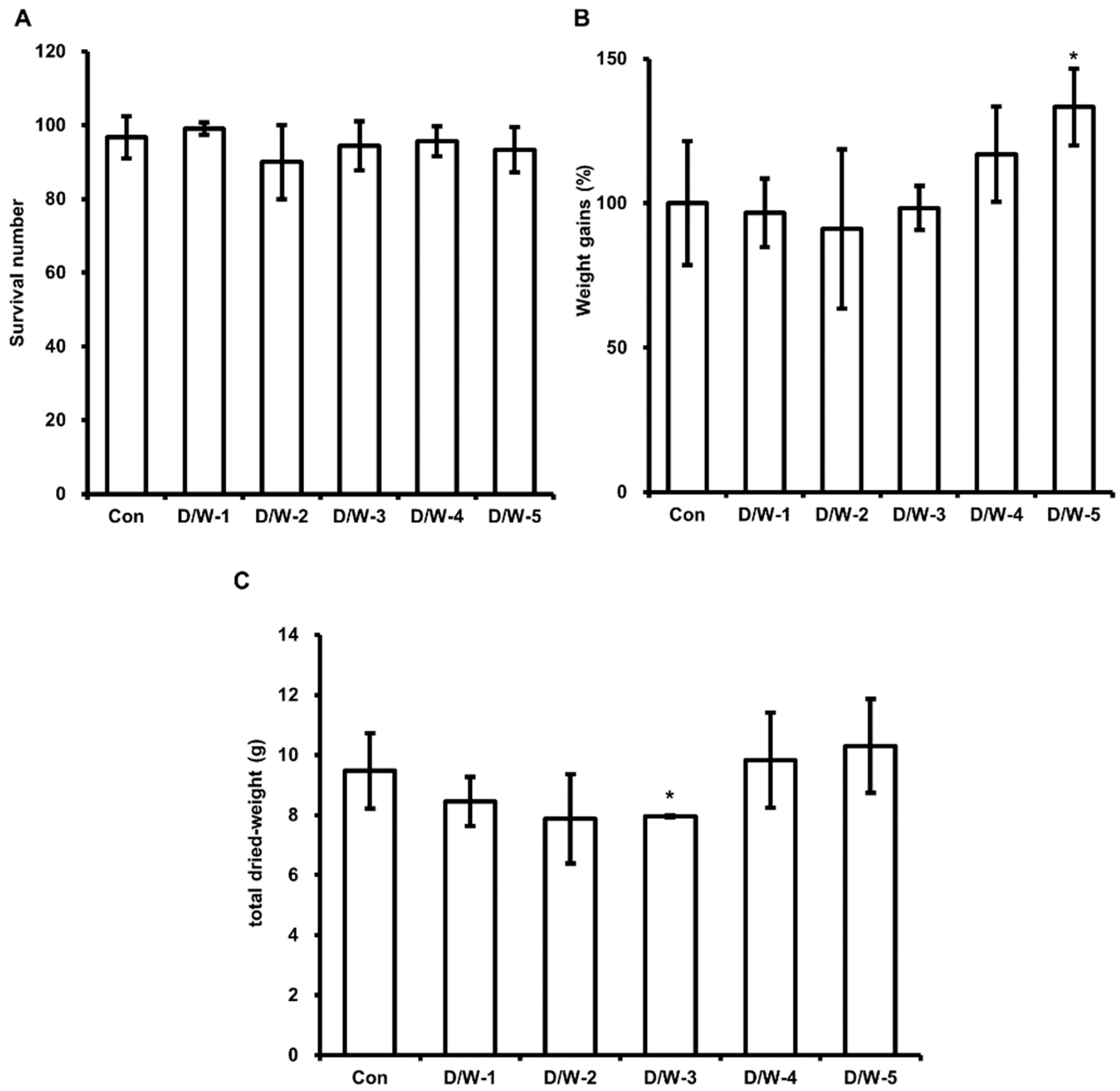
3.1. Effect of DFW/CM on HIL Growth
3.2. Effect of DFW/ WCO on HIL Growth
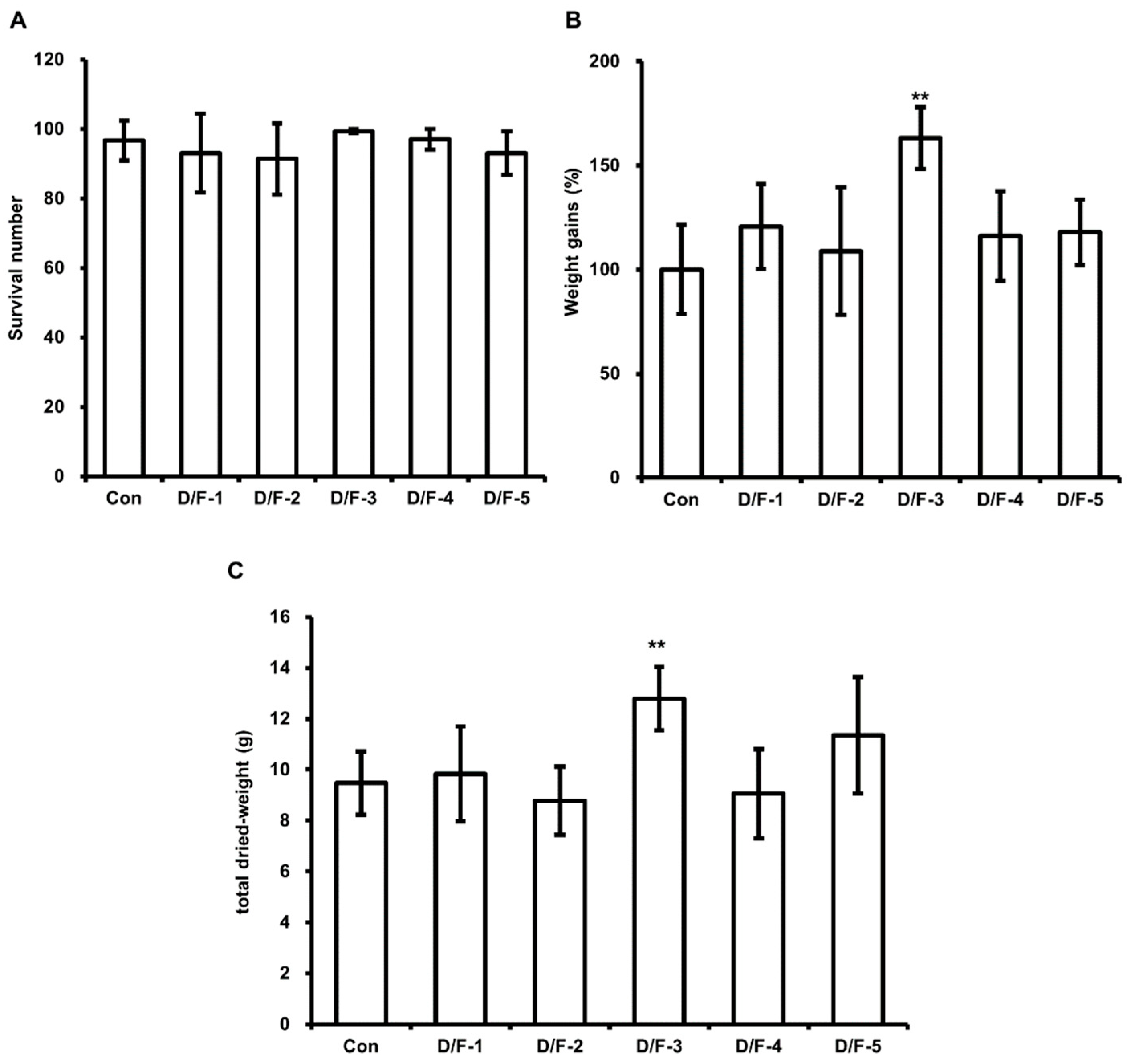
3.3. Effect of DFW/F-EM on HIL Growth
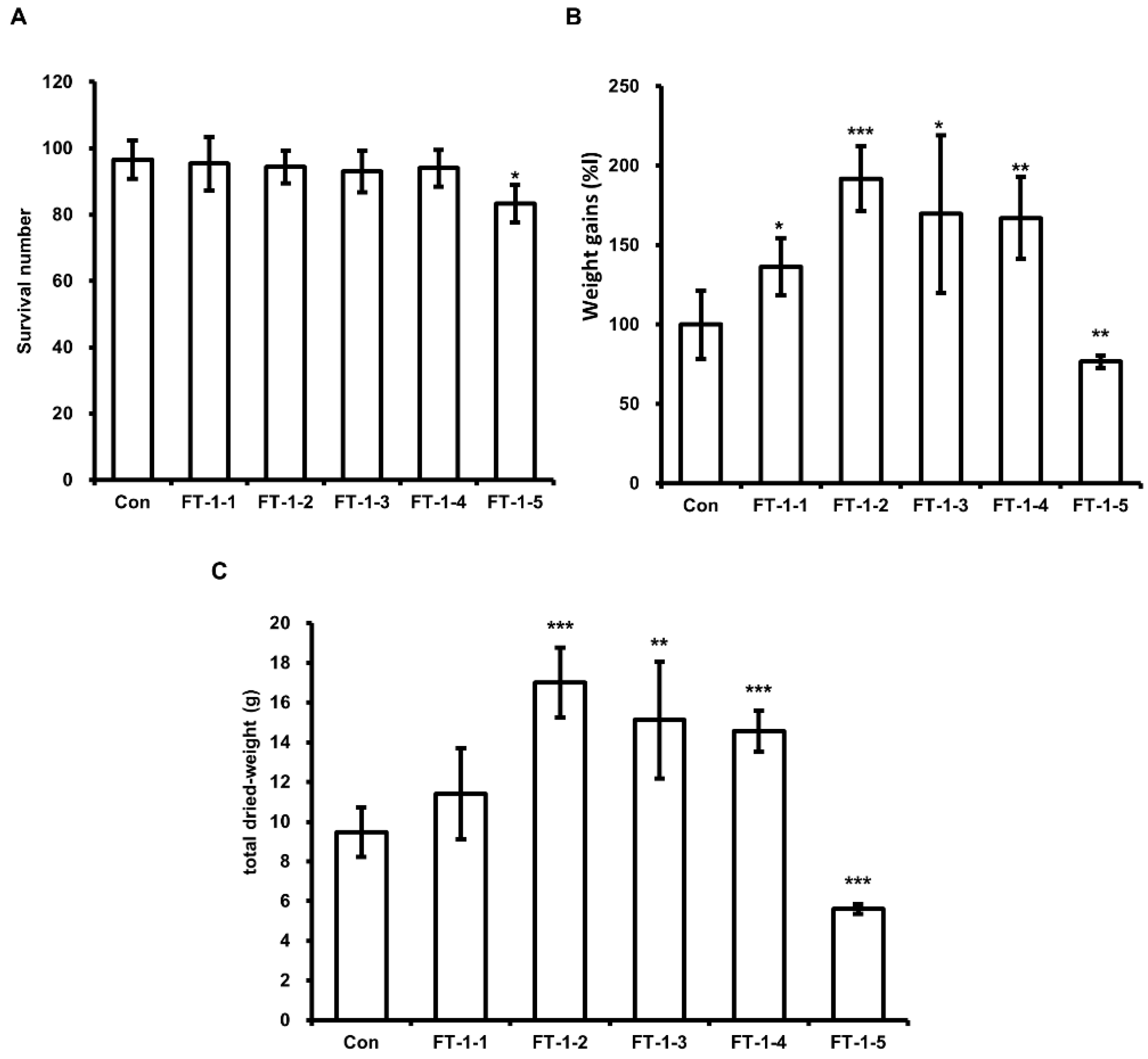
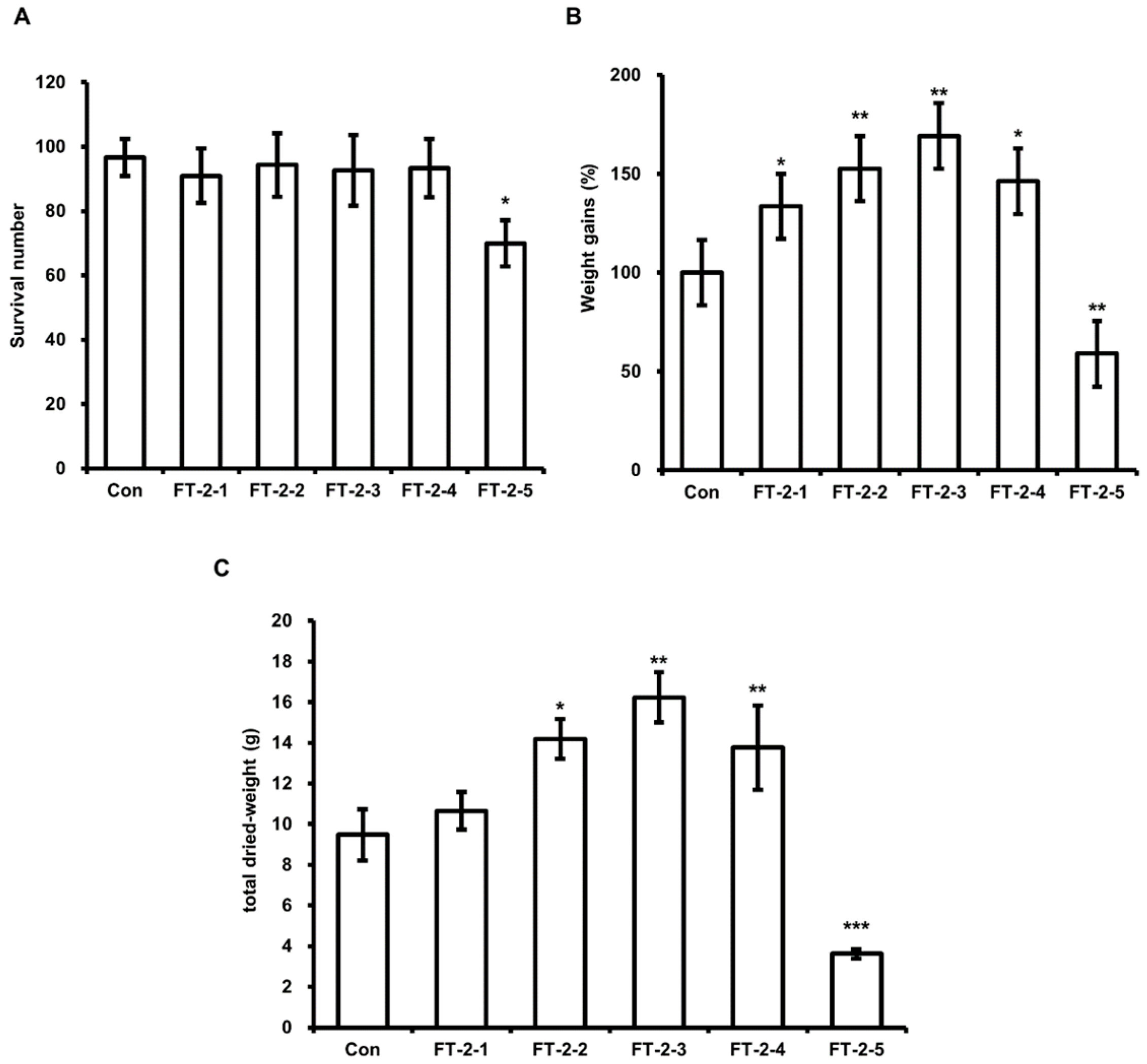
3.4. Effect of DFW/CM/WCO/F-EM on HIL Growth
3.5. Analysis of Nutritional and Fatty Acid Compositions of HIL
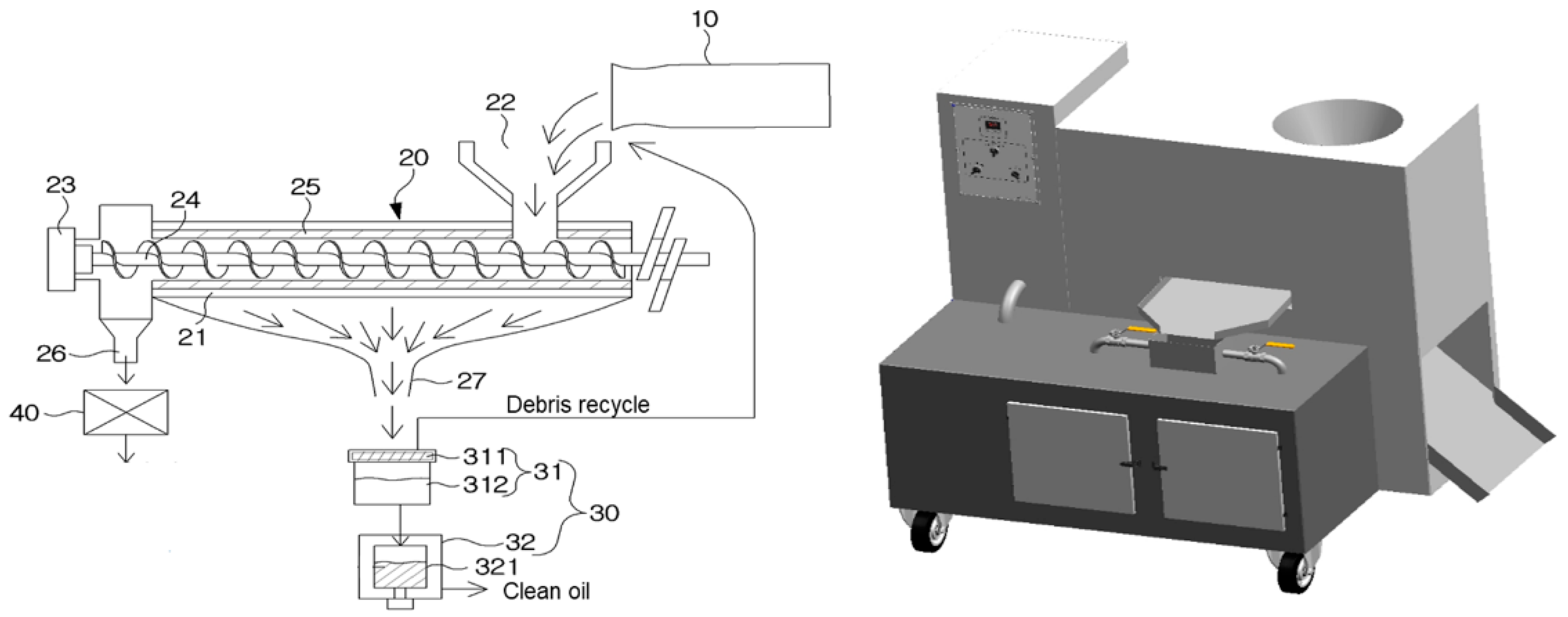
3.6. Development of the Crude Oil Extractor Used to Extract Crude Fat from HIL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-P.; Park, S.-C.; Kim, Y.-J.; Lee, J.-S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Synthesis of biodiesel through catalytic transesterification of various feedstocks using fast solvothermal technology: A critical review. Catal. Rev. 2015, 57, 407–435. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.-y.; Park, K.Y.; Chung, H. Use of Black Soldier Fly Larvae for Food Waste Treatment and Energy Production in Asian Countries: A Review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of life cycle assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Choi, Y.-C.; Choi, J.-Y.; Kim, J.-G.; Kim, M.-S.; Kim, W.-T.; Park, K.-H.; Bae, S.-W.; Jeong, G.-S. Potential usage of food waste as a natural fertilizer after digestion by Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2009, 19, 171–174. [Google Scholar]
- Zahn, N.H.; Quilliam, R. The Effects of Insect Frass Created by Hermetia illucens on Spring Onion Growth and Soil Fertility. Bachelor’s Thesis, University of Stirling, Stirling, UK, 2017. [Google Scholar]
- Lalander, C.; Senecal, J.; Calvo, M.G.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinnerås, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.C.; Nguyen, N.T.; Su, C.-H.; Wang, F.-M.; Tran, T.N.; Liao, Y.-T.; Liang, S.-H. Biodiesel production from insects: From organic waste to renewable energy. Curr. Org. Chem. 2019, 23, 1499–1508. [Google Scholar] [CrossRef]
- Manzano-Agugliaro, F.; Sanchez-Muros, M.; Barroso, F.; Martínez-Sánchez, A.; Rojo, S.; Pérez-Bañón, C. Insects for biodiesel production. Renew. Sustain. Energy Rev. 2012, 16, 3744–3753. [Google Scholar] [CrossRef]
- Pleissner, D.; Rumpold, B.A. Utilization of organic residues using heterotrophic microalgae and insects. Waste Manag. 2018, 72, 227–239. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Bowling, J.J.; Anderson, J.B.; Armbrust, K.L.; Hamann, M.T. Evaluation of potential biodiesel feedstock production from oleaginous insect Solenopsis sp. Fuel 2014, 117, 5–7. [Google Scholar] [CrossRef]
- Yang, S.; Li, Q.; Gao, Y.; Zheng, L.; Liu, Z. Biodiesel production from swine manure via housefly larvae (Musca domestica L.). Renew. Energy 2014, 66, 222–227. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Exploring the potential of grease from yellow mealworm beetle (Tenebrio molitor) as a novel biodiesel feedstock. Appl. Energy 2013, 101, 618–621. [Google Scholar] [CrossRef]
- Yang, S.; Li, Q.; Zeng, Q.; Zhang, J.; Yu, Z.; Liu, Z. Conversion of solid organic wastes into oil via Boettcherisca peregrine (Diptera: Sarcophagidae) larvae and optimization of parameters for biodiesel production. PLoS ONE 2012, 7, e45940. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Zheng, L.; Wang, Y.; Zhang, J.; Yu, Z.; Zhang, Y. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour. Technol. 2015, 194, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Antimicrobial activity of an extract of Hermetia illucens larvae immunized with Lactobacillus casei against Salmonella species. Insects 2020, 11, 704. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Evaluation of the Antimicrobial Activity of an Extract of Lactobacillus casei-Infected Hermetia illucens Larvae Produced Using an Automatic Injection System. Animals 2020, 10, 2121. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, H.J.; Goo, T.W.; Quan, F.S. Novel antibacterial peptides induced by probiotics in Hermetia illucens (Diptera: Stratiomyidae) larvae. Entomol. Res. 2018, 48, 237–247. [Google Scholar] [CrossRef]
- Dörper, A.; Veldkamp, T.; Dicke, M. Use of black soldier fly and house fly in feed to promote sustainable poultry production. J. Insects Food Feed 2020, 1–20. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Diener, S.; Solano, N.M.S.; Gutiérrez, F.R.; Zurbrügg, C.; Tockner, K. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valorization 2011, 2, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Surendra, K.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef] [Green Version]


| No. | DFW/CM | DFW/WCO | DFW/F-EM | |||
|---|---|---|---|---|---|---|
| DFW (g) | CM (g) | DFW (g) | WCO (mL) | DFW (g) | F-EM (%) | |
| Control | 100 | 0 | 100 | 0 | 100 | 0 |
| 1 | 80 | 20 | 100 | 1 | 100 | 0.4 |
| 2 | 60 | 40 | 100 | 2 | 100 | 0.8 |
| 3 | 40 | 60 | 100 | 3 | 100 | 1.2 |
| 4 | 20 | 80 | 100 | 4 | 100 | 1.6 |
| 5 | 0 | 100 | 100 | 5 | 100 | 2.0 |
| Feed Type | Sample Number | Components | |||
|---|---|---|---|---|---|
| DFW (g) | CM (g) | WCO (mL) | F-EM (%) | ||
| FT *-1 | Control | 100 | 0 | 0 | 0 |
| 1 | 80 | 20 | 1 | 0.4 | |
| 2 | 60 | 40 | 2 | 0.8 | |
| 3 | 40 | 60 | 3 | 1.2 | |
| 4 | 20 | 80 | 4 | 1.6 | |
| 5 | 0 | 100 | 5 | 2.0 | |
| FT-2 | Control | 100 | 0 | 0 | 0 |
| 1 | 80 | 20 | 5 | 0.4 | |
| 2 | 60 | 40 | 4 | 0.8 | |
| 3 | 40 | 60 | 3 | 1.2 | |
| 4 | 20 | 80 | 2 | 1.6 | |
| 5 | 0 | 100 | 1 | 2.0 | |
| FT-3 | Control | 100 | 0 | 0 | 0 |
| 1 | 80 | 20 | 5 | 2.0 | |
| 2 | 60 | 40 | 4 | 1.6 | |
| 3 | 40 | 60 | 3 | 1.2 | |
| 4 | 20 | 80 | 2 | 0.8 | |
| 5 | 0 | 100 | 1 | 0.4 | |
| Composition | Feed | ||||
|---|---|---|---|---|---|
| DFW | DFW/CM-3 | DFW/WCO-5 | DFW/F-EM-3 | FT-1-2 | |
| Moisture | 9.34% | 10.46% | 8.84% | 9.73% | 11.28% |
| Crude protein | 36.72% | 35.91% | 35.91% | 36.12% | 35.56% |
| Crude fat | 29.50% | 32.03% | 30.85% | 33.00% | 33.87% |
| Crude ash | 11.31% | 7.95% | 10.59% | 8.11% | 8.03% |
| Fatty Acid | Common Name | Unit | Feed | |
|---|---|---|---|---|
| DFW | FT-1-2 | |||
| C4:0 | Butyric acid | g/100 g | n.d | n.d |
| C6:0 | Caproic acid | g/100 g | 0.0001 | 0.0003 |
| C8:0 | Caprylic acid | g/100 g | 0.001 | 0.002 |
| C10:0 | Capric acid | g/100 g | 0.15 | 0.21 |
| C11:0 | Undecanoic acid | g/100 g | 0.002 | 0.003 |
| C12:0 | Lauric acid | g/100 g | 2.6 | 3.4 |
| C13:0 | Tridecanoic acid | g/100 g | 0.002 | 0.003 |
| C14:0 | Myristic acid | g/100 g | 0.46 | 0.55 |
| C14:1 | Myristoleic acid | g/100 g | 0.011 | 0.008 |
| C15:0 | Pentadecanoic acid | g/100 g | 0.012 | 0.024 |
| C15:1 | cis-10-Pentadecenoic acid | g/100 g | n.d | n.d |
| C16:0 | Palmitic acid | g/100 g | 1.46 | 2.36 |
| C16:1 | Palmitoleic acid | g/100 g | 0.18 | 0.15 |
| C17:0 | Heptadecanoic acid | g/100 g | 0.015 | 0.027 |
| C17:1 | Cis-10-Heptadecanoic acid | g/100 g | 0.009 | 0.012 |
| C18:0 | Stearic acid | g/100 g | 0.29 | 0.32 |
| C18:1 | Oleic acid | g/100 g | 1.04 | 1.57 |
| C18:2 | Linoleic acid | g/100 g | 1.02 | 1.64 |
| C18:3n-3 | α-Linolenic acid | g/100 g | 0.001 | 0.007 |
| C18:3n-6 | γ-Linolenic acid | g/100 g | n.d | 0.002 |
| C20:0 | Arachidic acid | g/100 g | n.d | 0.012 |
| C20:1 | cis-11-Eicosenoic acid | g/100 g | n.d | n.d |
| C20:2 | cis-11,14-eicosadienoic acid | g/100 g | 0.001 | 0.002 |
| C20:3n-3 | cis-11,14,17-Eicosadienoic acid | g/100 g | 0.02 | 0.037 |
| C20:3n-6 | cis-8,11,14-Eicosadienoic acid | |||
| C20:4n-6 | Arachidonic acid | g/100 g | 0.018 | 0.025 |
| C20:5n-3 | cis-5,8,11,14,17-Eicosapentaenoic acid | g/100 g | 0.04 | 0.05 |
| C21:0 | Heneicosanoic acid | g/100 g | n.d | n.d |
| Extraction Method | ||
|---|---|---|
| Oil Extractor | Solvent Extraction | |
| Yield (%) | 37.23% | 26.97% |
| Composition | Content (%) | |
|---|---|---|
| Crude Oil | HIL Frass | |
| Moisture | 0.57 ± 0.15 | 1.19 ± 0.24 |
| Carbohydrate | 2.84 ± 0.87 | 20.90 ± 4.66 |
| Crude protein | 8.63 ± 0.98 | 64.00 ± 4.62 |
| Crude fat | 86.66 ± 1.54 | 7.17 ± 0.73 |
| Crude ash | 1.30 ± 1.62 | 10.67 ± 4.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Optimization of Feed Components to Improve Hermetia illucens Growth and Development of Oil Extractor to Produce Biodiesel. Animals 2021, 11, 2573. https://doi.org/10.3390/ani11092573
Lee K-S, Yun E-Y, Goo T-W. Optimization of Feed Components to Improve Hermetia illucens Growth and Development of Oil Extractor to Produce Biodiesel. Animals. 2021; 11(9):2573. https://doi.org/10.3390/ani11092573
Chicago/Turabian StyleLee, Kyu-Shik, Eun-Young Yun, and Tae-Won Goo. 2021. "Optimization of Feed Components to Improve Hermetia illucens Growth and Development of Oil Extractor to Produce Biodiesel" Animals 11, no. 9: 2573. https://doi.org/10.3390/ani11092573
APA StyleLee, K.-S., Yun, E.-Y., & Goo, T.-W. (2021). Optimization of Feed Components to Improve Hermetia illucens Growth and Development of Oil Extractor to Produce Biodiesel. Animals, 11(9), 2573. https://doi.org/10.3390/ani11092573





