Effects of Zinc Sources and Levels on Growth Performance, Zinc Status, Expressions of Zinc Transporters, and Zinc Bioavailability in Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CS–Zn Chelate
2.3. Characterization
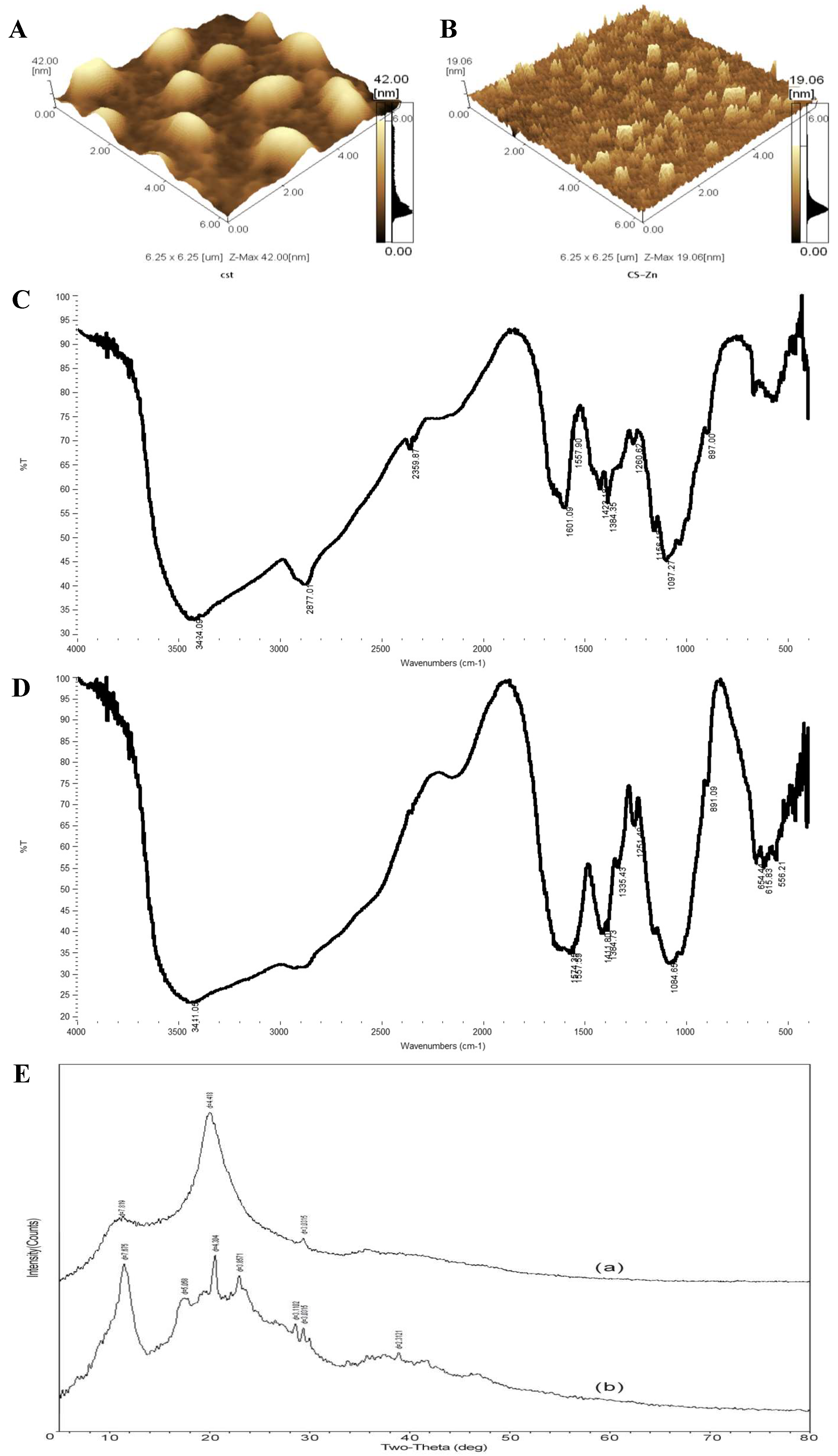
2.3.1. Atomic Force Microscopy Analysis
2.3.2. Fourier Transform Infrared Analysis
2.3.3. X-ray Diffraction Analysis
2.4. Oral Acute Toxicity in Mice
2.5. Bioavailability Analysis in Piglets
2.5.1. Animal Treatment
2.5.2. Sample Collection
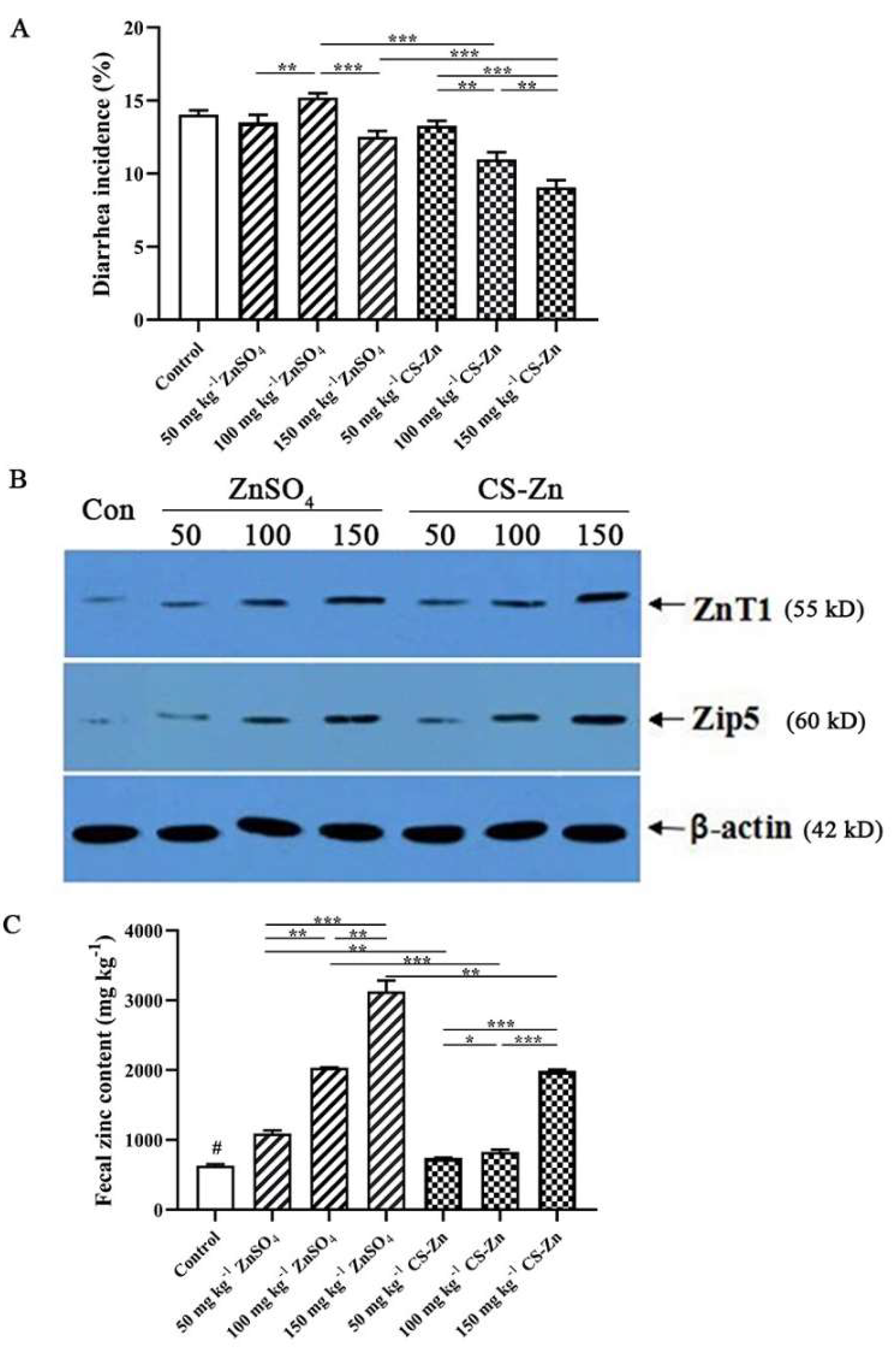
2.5.3. Zinc Content and Transporter Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Atomic Force Microscopy Analysis
3.2. Fourier Transform Infrared Analysis
3.3. X-ray Diffraction Analysis
3.4. Oral Acute Toxicity in Mice
3.5. Bioavailability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| CS | chitosan |
| CS–Zn | chitosan–zinc chelated |
| NRC | National Research Council |
| AFM | atomic force microscopy |
| FT-IR | Fourier transform infrared |
| XRD | X-ray diffraction |
| AR | acetic acid |
| ADG | average daily gain |
| ADFI | average daily feed intake |
| F/G | feed to gain ratio |
| ZIP1 | Slc 30 A 1 zinc transporter |
| ZIP5 | Slc 39 A 5 zinc transporter |
| GLM | general linear model |
| LSD | least significant difference |
| ANOVA | analysis of variance |
References
- Liu, Y.; Espinosa, C.D.; Abeilla, J.J.; Casas, G.A.; Lagos, L.V.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, D.W.; Stein, H.H. Non-antibiotic feed additives in diets for pigs—A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Magdaleno, A.; Velez, C.G.; Wenzel, M.T.; Tell, G. Effects of cadmium, copper and zinc on growth of four isolated algae from a highly polluted argentina river. Bull. Environ. Contam. Toxical. 2014, 92, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.S.; Boren, C.A.; Wu, C.; Huntington, C.E.; Bollinger, D.W.; Veum, T.L. Evaluation of various inclusion rates of organic zinc either as polysaccharide or proteinate complex on the growth performance, plasma, and excretion of nursery pigs. J. Anim. Sci. 2004, 82, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, S.; Rudi, K.; Bernhoft, A. Zinc and copper in animal feed—Development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb. Ecol. Health Dis. 2014, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, C.L.; Carlson, M.S. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 2002, 80, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Sargin, I.; Kaya, M.; Arslan, G.; Baran, T.; Ceter, T. Preparation and characterisation of biodegradable pollen-chitosan microcapsules and its application in heavy metal removal. Bioresour. Technol. 2015, 177, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Aravind, J.; Kamaraj, M.; Sureshbabu, P.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comperhensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Gibson, G.; Formentin, K.; Beer, M.; Rastall, R. Inhibition of the adhesion of enteropathogenic Escherichia colistrains to HT-29 cells in culture by chito-oligosaccharides. Carbohydr. Polym. 2006, 64, 57–59. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Ren, J.M.; Dong, F.; Wang, G.; Li, P.C. Comparative Study of the Influence of Active Groups of Chitosan Derivatives on Antifungal Activity. J. Appl. Polym. Sci. 2013, 127, 2553–2556. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, D.H.; Chung, K.D.; Kim, Y.H.; Lee, Y.S.; Choi, K.C. Antitumor Activity of Trigonelline-Incorporated Chitosan Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 5633–5637. [Google Scholar] [CrossRef]
- Wang, X.H.; Du, Y.M.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohyd. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Han, X.Y.; Ma, Y.F.; Lv, M.Y.; Wu, Z.P.; Qian, L.C. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Br. J. Nutr. 2014, 111, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Kirby, A.R.; MacDougall, A.J.; Morris, V.J. Atomic force microscopy of tomato and sugar beet pectin molecules. Carbohydr. Polym. 2008, 71, 640–647. [Google Scholar] [CrossRef]
- Saeed, S.E.; EI-Molla, M.M.; Hassan, M.L.; Bakir, E.; Abdel-Mottaleb, M.M.S.; Abdel-Mottaleb, M.S.A. Novel chitosan-ZnO based nanocomposites as luminescent tags for cellulosic materials. Carbohydr. Polym. 2014, 99, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Diao, P.; Cai, S. Hydrothermal growth of well-aligned ZnO nanorod arrays: Dependence of morphology and alignment ordering upon preparing conditions. J. Solid State Chem. 2005, 178, 1864–1873. [Google Scholar] [CrossRef]
- AbdElhady, M.M. Preparation and Characterization of Chitosan/Zinc Oxide Nanoparticles for Imparting Antimicrobial and UV Protection to Cotton Fabric. Int. J. Carbohydr. Chem. 2012, 2012, 840591. [Google Scholar] [CrossRef]
- Anadhavelu, S.; Thambidurai, S. Preparation of chitosan-zinc oxide complex during chitin deacetylation. Carbohydr. Polym. 2011, 83, 1565–1569. [Google Scholar] [CrossRef]
- Li, L.H.; Deng, J.C.; Deng, H.R.; Liu, Z.L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 2010, 345, 994–998. [Google Scholar] [CrossRef]
- Trimukhe, K.D.; Varma, A.J. A morphological study of heavy metal complexes of chitosan and crosslinked chitosans by SEM and WAXRD. Carbohydr. Polym. 2008, 71, 698–702. [Google Scholar] [CrossRef]
- Buff, C.E.; Bollinger, D.W.; Ellersieck, M.R.; Brommelsiek, W.A.; Veum, T.L. Comparison of growth performance and zinc absorption, retention, and excretion in weanling pigs fed diets supplemented with zinc-polysaccharide or zinc oxide. J. Anim. Sci. 2005, 83, 2380–2386. [Google Scholar] [CrossRef]
- Madec, F.; Bridoux, N.; Bounaix, S.; Cariolet, R.; Duval-lflah, Y.; Hampson, D.J.; Jestin, A. Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Vet. Microbiol. 2000, 72, 295–310. [Google Scholar] [CrossRef]
- Hill, G.M.; Mahan, D.C.; Jolliff, J.S. Comparison of organic and inorganic zinc sources to maximize growth and meet the zinc needs of the nursery pig. J. Anim. Sci. 2014, 92, 1582–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcon-Perez, J.M.; Dell’Angelica, E.C. Zinc transporter 2 (SLC30A2) can suppress the vesicular zinc defect of adaptor protein 3-depleted fibroblasts by promoting zinc accumulation in lysosomes. Exp. Cell Res. 2007, 313, 1473–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gefeller, E.M.; Martens, H.; Aschenbach, J.R.; Klingspor, S.; Twardziok, S.; Wrede, P.; Pieper, R.; Lodemann, U. Effects of age and zinc supplementation on transport properties in the jejunum of piglets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Kirschke, C.P.; Huang, L. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J. Histochem. Cytochem. 2007, 55, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Liuzzi, J.P.; Cousins, R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004, 24, 151–172. [Google Scholar] [CrossRef]
- Weaver, B.P.; Beattie, J.D.; Kambe, T.; Andrews, G.K. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 2007, 388, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Payne, R.L.; Bidner, T.D.; Fakler, T.M.; Southem, L.L. Growth and intestinal morphology of pigs from sows fed two zinc sources during gestation and lactation. J. Anim. Sci. 2006, 84, 2141–2149. [Google Scholar] [CrossRef]
- Cao, J.; Henry, P.R.; Guo, R.; Holwerda, R.A.; Toth, J.P.; Littell, R.C.; Miles, R.D.; Ammerman, C.B. Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants. J. Anim. Sci. 2000, 78, 2039–2054. [Google Scholar] [CrossRef] [Green Version]
- Pieper, R.; Martin, L.; Schunter, N.; Tudela, C.V.; Weise, C.; Klopfleisch, R.; Zentek, J.; Einspanier, R.; Bondzio, A. Impact of high dietary zinc on zinc accumulation, enzyme activity and proteomic profiles in the pancreas of piglets. J. Trace Elem. Med. Biol. 2015, 30, 30–36. [Google Scholar] [CrossRef]
| Ingredients | % | Nutrient Levels 2 | Content |
|---|---|---|---|
| Corn | 62 | Digestible energy, MJ/kg | 13.75 |
| Soybean meal | 16 | Crude protein, % | 18.12 |
| Extruded soybeans | 7 | Ether extract, % | 3.26 |
| Fish meal | 4 | Calcium, % | 0.93 |
| Whey powder | 5 | Phosphorus, % | 0.79 |
| Bran | 2 | Zinc, mg/kg | 47.30 |
| Salt | 0.4 | ||
| Calcium hydrogen phosphate | 1.1 | ||
| Limestone | 1.5 | ||
| Premix 1 | 1 |
| Item | Added Zn Level (mg kg−1) | ADG (g d−1) | ADFI (g d−1) | F/G (g g−1) |
|---|---|---|---|---|
| Control | 0 | 231.0 ± 55.40 | 480.9 ± 13.6 | 2.08 ± 0.17 |
| ZnSO4 | 50 | 231.3 ± 17.98 Bb | 498.9 ± 20.5 Aa | 2.04 ± 0.08 Aa |
| 100 | 232.5 ± 46.79 Bb | 444.3 ± 23.5 Bc | 1.96 ± 0.21 Ab | |
| 150 | 243.6 ± 25.50 Ba | 473.7 ± 5.6 Bb | 1.94 ± 0.12 Ac | |
| CS–Zn | 50 | 252.9 ± 42.20 Ac | 486.4 ± 26.8 Ba | 1.92 ± 0.11 Ba |
| 100 | 276.3 ± 19.89 Ab | 544.8 ± 24.5 Ab | 1.90 ± 0.13 Bb | |
| 150 | 347.3 ± 34.55 Aa | 622.0 ± 19.5 Ac | 1.76 ± 0.07 Bc | |
| Zn source | ZnSO4 | 235.8 ± 31.03 B | 472.3 ± 25.90 B | 1.98 ± 0.46 A |
| CS–Zn | 292.2 ± 51.93 A | 551.1 ± 22.27 A | 1.86 ± 0.53 B | |
| Added Zn level (mg kg−1) | 50 | 242.1 ± 32.91 b | 492.7 ± 13.21 b | 1.98 ± 0.63 a |
| 100 | 254.4 ± 41.21 b | 494.6 ± 26.51 b | 1.93 ± 0.34 b | |
| 150 | 295.5 ± 61.40 a | 547.9 ± 35.78 a | 1.85 ± 0.85 c | |
| p-value | Zn source | <0.001 | <0.001 | <0.001 |
| Zn level | 0.001 | <0.001 | <0.001 | |
| Interaction | 0.014 | <0.001 | <0.001 |
| Item | Added Zn Level (mg kg−1) | Liver Zn (mg kg−1) | Pancreas Zn (mg kg−1) |
|---|---|---|---|
| Control | 0 | 70.83 ± 9.29 * | 21.02 ± 1.94 * |
| ZnSO4 | 50 | 79.57 ± 0.87 | 38.13 ± 0.88 Bc |
| 100 | 99.52 ± 1.38 | 46.80 ± 4.37 Bb | |
| 150 | 120.52 ± 13.01 | 66.04 ± 4.31 Ba | |
| CS–Zn | 50 | 89.44 ± 3.25 | 43.82 ± 3.76 Ac |
| 100 | 106.53 ± 2.95 | 57.90 ± 4.38 Ab | |
| 150 | 125.68 ± 4.58 | 91.65 ± 3.93 Aa | |
| Zn source | ZnSO4 | 99.87 ± 18.76 B | 52.57 ± 13.04 B |
| CS–Zn | 107.22 ± 15.81 A | 65.93 ± 21.06 A | |
| Added Zn level (mg kg−1) | 50 | 84.51 ± 5.72 c | 40.97 ± 4.00 c |
| 100 | 103.02 ± 4.32 b | 52.97 ± 7.14 b | |
| 150 | 123.10 ± 9.44 a | 77.68 ± 13.94 a | |
| p-value | Zn source | 0.007 | <0.001 |
| Zn level | <0.001 | <0.001 | |
| Interaction | 0.732 | <0.001 | |
| Linear 1 | <0.001 | <0.001 | |
| Quadratic | 0.411 | 0.127 |
| Item | Added Zn Level (mg kg−1) | ZnT1 | ZIP5 |
|---|---|---|---|
| Control | 0 | 3.99 ± 0.30 * | 2.99 ± 0.34 * |
| ZnSO4 | 50 | 7.84 ± 0.56 | 7.73 ± 0.78 c |
| 100 | 13.50 ± 0.84 | 12.84 ± 0.39 Bb | |
| 150 | 19.21 ± 0.78 | 19.71 ± 0.54 Ba | |
| CS–Zn | 50 | 10.68 ± 0.50 | 8.42 ± 0.70 c |
| 100 | 14.73 ± 0.45 | 15.59 ± 0.53 Ab | |
| 150 | 21.15 ± 0.75 | 27.67 ± 0.66 Aa | |
| Zn source | ZnSO4 | 13.52 ± 4.96 B | 13.43 ± 5.23 B |
| CS–Zn | 15.52 ± 4.60 A | 17.23 ± 8.45 A | |
| Added Zn level (mg kg−1) | 50 | 9.26 ± 1.63 c | 8.08 ± 0.76 c |
| 100 | 14.11 ± 0.90 b | 14.21 ± 1.56 b | |
| 150 | 20.18 ± 1.26 a | 23.69 ± 4.39 a | |
| p-value | Zn source | <0.001 | <0.001 |
| Zn level | <0.001 | <0.001 | |
| Interaction | 0.150 | <0.001 | |
| Linear | <0.001 | <0.001 | |
| Quadratic | 0.365 | 0.240 |
| Item | Regression Equation | R2 | p | ZnSO4 | CS–Zn |
|---|---|---|---|---|---|
| Liver zinc content | Y = 68.747 + 0.366X1 + 0.330X2 | 0.899 | 0.000 | 100% | 110.9% |
| Pancreas zinc content | Y = 20.186 + 0.447X1 + 0.300X2 | 0.953 | 0.000 | 100% | 149.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Qian, M.; Yang, Z.; Xu, T.; Han, X. Effects of Zinc Sources and Levels on Growth Performance, Zinc Status, Expressions of Zinc Transporters, and Zinc Bioavailability in Weaned Piglets. Animals 2021, 11, 2515. https://doi.org/10.3390/ani11092515
Ma X, Qian M, Yang Z, Xu T, Han X. Effects of Zinc Sources and Levels on Growth Performance, Zinc Status, Expressions of Zinc Transporters, and Zinc Bioavailability in Weaned Piglets. Animals. 2021; 11(9):2515. https://doi.org/10.3390/ani11092515
Chicago/Turabian StyleMa, Xin, Mengqi Qian, Zhiren Yang, Tingting Xu, and Xinyan Han. 2021. "Effects of Zinc Sources and Levels on Growth Performance, Zinc Status, Expressions of Zinc Transporters, and Zinc Bioavailability in Weaned Piglets" Animals 11, no. 9: 2515. https://doi.org/10.3390/ani11092515
APA StyleMa, X., Qian, M., Yang, Z., Xu, T., & Han, X. (2021). Effects of Zinc Sources and Levels on Growth Performance, Zinc Status, Expressions of Zinc Transporters, and Zinc Bioavailability in Weaned Piglets. Animals, 11(9), 2515. https://doi.org/10.3390/ani11092515






