Pilot Investigation of Anti-Salmonella Antibodies in Oral Fluids from Salmonella Typhimurium Vaccinated and Unvaccinated Swine Herds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms
2.2. Oral Fluid and Pooled Faeces Collection
2.3. Bacteriological Analyses of Salmonella Prevalence in Pooled Faecal Samples
2.4. Detection of Anti-Salmonella IgG and IgA in OF samples
2.5. Statistical Analyses
3. Results
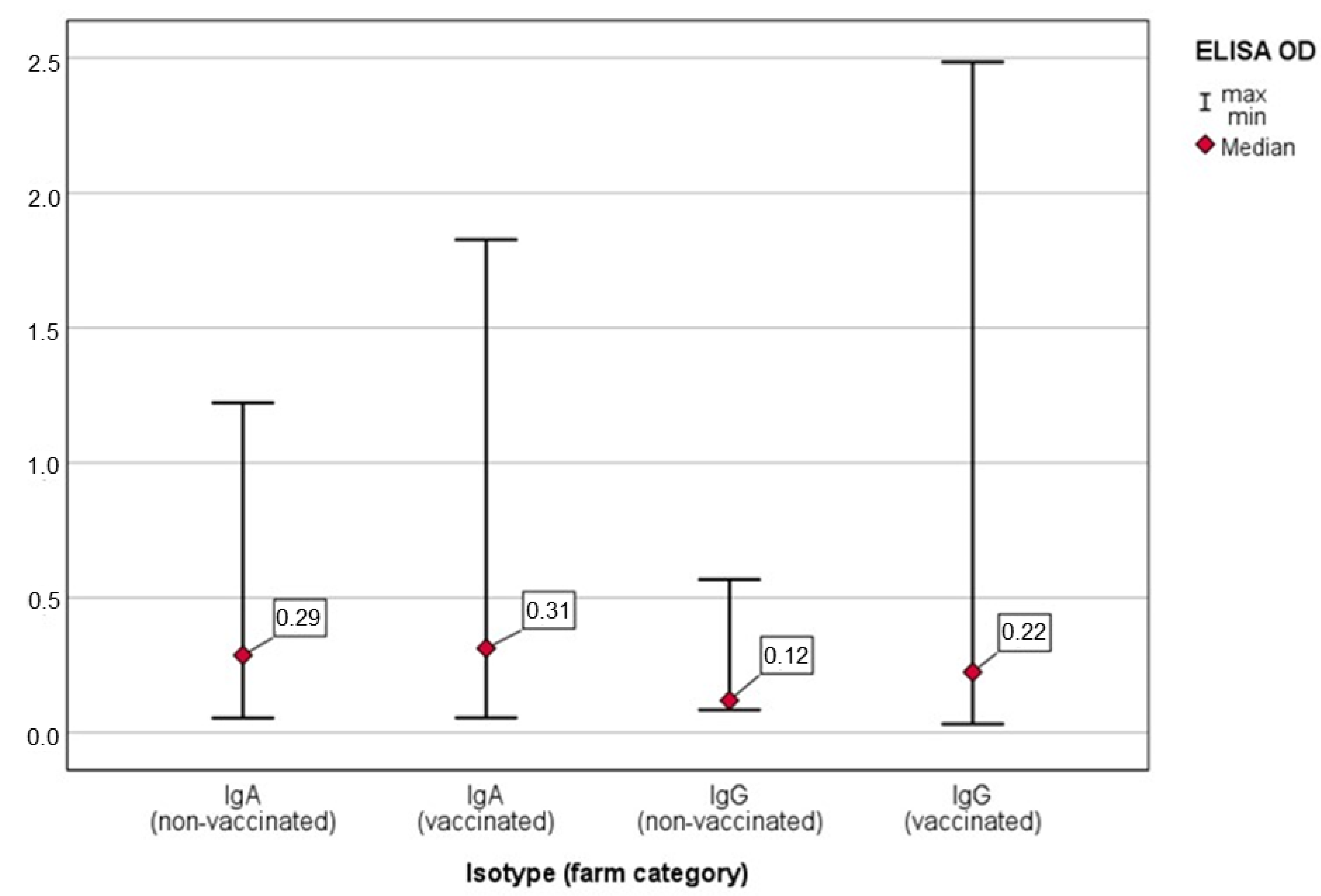
3.1. Detection of Anti-Salmonella IgG and IgA in OF Samples
3.2. Bacteriological Results of Salmonella Prevalence from Pooled Faeces
3.3. Correlation between Salmonella Prevalence in Pooled Faecal Samples and Anti-Salmonella IgG and IgA OD Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- De Jong Skierus, B. Human Salmonellosis-Impact of Travel and Trade from a Swedish Perspective. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2006. [Google Scholar]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Wales, A.; Weaver, J.; McLaren, I.; Smith, R.; Mueller-Doblies, D.; Davies, R. Investigation of the distribution of Salmonella within an integrated pig breeding and production organisation in the United Kingdom. ISRN Vet. Sci. 2013, 2013, 943126. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on a quantitative microbiological risk assessment of Salmonella in slaughter and breeder pigs. EFSA J. 2010, 8, 1–90. [Google Scholar] [CrossRef]
- Alban, L.; Stege, H.; Dahl, J. The new classification system for slaughter-pig herds in the Danish Salmonella surveillance-and-control program. Prev. Vet. Med. 2002, 53, 133–146. [Google Scholar] [CrossRef]
- Alban, L.; Barfod, K.; Petersen, J.V.; Dahl, J.; Ajufo, J.C.; Sandø, G.; Krog, H.H.; Aabo, S. Description of Extended Pre-Harvest Pig Salmonella Surveillance-and-Control Programme and its Estimated Effect on Food Safety Related to Pork. Zoonoses Public Health 2010, 57, 6–15. [Google Scholar] [CrossRef]
- Andres, V.M.; Davies, R.H. Biosecurity measures to control Salmonella and other infectious agents in pig farms: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 317–335. [Google Scholar] [CrossRef]
- Ramirez, A.; Wang, C.; Prickett, J.R.; Pogranichniy, R.; Yoon, K.J.; Main, R.; Johnson, J.K.; Rademacher, C.; Hoogland, M.; Hoffmann, P.; et al. Efficient surveillance of pig populations using oral fluids. Prev. Vet. Med. 2012, 104, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Dron, N.; Doyle, R.; Jover-Hernandez, M.; Holyoake, T. Detection of Actinobacillus Pleuropneumoniae in Pigs Using Pooled Oral Fluids. Available online: http://porkcrc.com.au/wp-content/uploads/2014/05/121209-NDron-Honours-Thesis.pdf (accessed on 1 March 2021).
- McKie, A.; Vyse, A.; Maple, C. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infect. Dis. 2002, 2, 18–24. [Google Scholar] [CrossRef]
- Olsen, C.; Karriker, L.; Wang, C.; Binjawadagi, B.; Renukaradhya, G.; Kittawornrat, A.; Lizano, S.; Coetzee, J.; Main, R.; Meiszberg, A.; et al. Effect of collection material and sample processing on pig oral fluid testing results. Vet. J. 2013, 198, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.D. Salivary diagnosis: Promises, promises. Ann. N. Y. Acad. Sci. 1993, 694, 1–10. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Fjellanger, I.; Gjeruldsen, S.T. Human secretory immunoglobulins. I. Salivary secretions from individuals with normal or low levels of serum immunoglobulins. Scand. J. Haematol. 1970, 12, 1–83. [Google Scholar]
- DeBuysscher, E.V.; Berman, D.T. Secretory immune response in intestinal mucosa and salivary gland after experimental infection of pigs with transmissible gastroenteritis virus. Am. J. Vet. Res. 1980, 41, 1214–1220. [Google Scholar]
- Decorte, I.; Van Breedam, W.; Van der Stede, Y.; Nauwynck, H.J.; De Regge, N.; Cay, A.B. Detection of total and PRRSV-specific antibodies in oral fluids collected with different rope types from PRRSV-vaccinated and experimentally infected pigs. BMC Vet. Res. 2014, 10, 134. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Ceron, J.; Fuentes-Rubio, M.; Tecles, F.; Beeley, J.A. A proteomic approach to porcine saliva. Curr. Protein Pept. Sci. 2014, 15, 56–63. [Google Scholar] [CrossRef]
- Prickett, J.R.; Zimmerman, J.J. The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim. Health Res. Rev. 2010, 11, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Renson, P.; Pol, F.; Dorenlor, V.; Mahe, S.; Eono, F.; Eveno, E.; Le Dimna, M.; Liegard-Vanhecke, D.; Eudier, S.; et al. Oral fluid versus blood sampling in group-housed sows and finishing pigs: Feasibility and performance of antibody detection for porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Microbiol. 2017, 204, 25–34. [Google Scholar] [CrossRef]
- Cameron, S.O.; Carman, W.F. The use of the OraSure® collection device for hepatitis virus testing in health care settings. J. Clin. Virol. 2005, 34, S22–S28. [Google Scholar] [CrossRef]
- Prickett, J.R.; Kim, W.; Simer, R.; Yoon, K.-J.; Zimmerman, J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J. Swine Health Prod. 2008, 16, 86–91. [Google Scholar]
- Dawson, L.L. Oral Fluid as a Non-Invasive Alternative Diagnostic Medium for Disease Monitoring in Pigs. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2015. [Google Scholar]
- Dietze, K.; Tucakov, A.; Engel, T.; Wirtz, S.; Depner, K.; Globig, A.; Kammerer, R.; Mouchantat, S. Rope-based oral fluid sampling for early detection of classical swine fever in domestic pigs at group level. BMC Vet. Res. 2017, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Henao-Diaz, A.; Gimenez-Lirola, L.; Baum, D.H.; Zimmerman, J. Guidelines for oral fluid-based surveillance of viral pathogens in swine. Porc. Health Manag. 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Andres, V.; Martelli, F.; Gosling, B.; Marco-Jimenez, F.; Vaughan, K.; Tchorzewska, M.; Davies, R. Maternal vaccination as a Salmonella Typhimurium reduction strategy on pig farms. J. Appl. Microbiol. 2018, 124, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Gosling, R.; McLaren, I.; Wales, A.; Davies, R. Development and testing of external quality assessment samples for Salmonella detection in poultry samples. Lett. Appl. Microbiol. 2014, 59, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Grimont, P.A.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; WHO Collaborating Centre for Reference and Research on Salmonella: Geneva, Switzerland, 2007; pp. 1–166. Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf (accessed on 1 March 2021).
- Farzan, A.; Friendship, R.M.; Dewey, E. Evaluation of enzyme-linked immunosorbent assay (ELISA) tests and culture for determining Salmonella status of a pig herd. Epidemiol. Infect. 2007, 135, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Vico, J.P.; Mainar-Jaime, R.C. The use of meat juice or blood serum for the diagnosis of Salmonella infection in pigs and its possible implications on Salmonella control programs. J. Vet. Diagn. Investig. 2011, 23, 528–531. [Google Scholar] [CrossRef] [PubMed]
- De Lucia, A.; Cawthraw, S.; Davies, R.; Smith, R.P.; Bianco, C.; Ostanello, F.; Martelli, F. Correlation of Anti-Salmonella Antibodies Between Serum and Saliva Samples Collected From Finisher Pigs. Front. Vet. Sci. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Murray, A.; Lawrence, G.P. How should the repeatability of clinical measurements be analysed? An assessment of analysis techniques with data from cardiovascular autonomic function tests. QJM Int. J. Med. 1993, 86, 831–836. [Google Scholar]
- Atkinson, B.M.; Bearson, B.L.; Loving, C.L.; Zimmerman, J.J.; Kich, J.D.; Bearson, S.M.D. Detection of Salmonella-specific antibody in swine oral fluids. Porc. Health Manag. 2019, 5, 2–5. [Google Scholar] [CrossRef]
- Clouting, C.; Davies, R.H. Evaluation of the Salmonella meat-juice ELISA in the UK situation. In Proceedings of the Fourth International Symposium on the Epidemiology and Control of Salmonella and Other Food Borne Pathogens in Pork, Leipzig, Germany, 2–5 September 2001. [Google Scholar]
- Brito, J.R.; Hinton, M.; Stokes, C.R.; Pearson, G.R. The humoral and cell mediated immune response of young chicks to Salmonella typhimurium and S. Kedougou. Br. Vet. J. 1993, 149, 225–234. [Google Scholar] [CrossRef]
- Garrido, V.; Sanchez, S.; Roman, B.S.; Zabalza-Barangua, A.; Diaz-Tendero, Y.; de Frutos, C.; Mainar-Jaime, R.C.; Grillo, M.J. Simultaneous infections by different Salmonella strains in mesenteric lymph nodes of finishing pigs. BMC Vet. Res. 2014, 10, 59. [Google Scholar] [CrossRef][Green Version]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. The distribution and functions of immunoglobulin isotypes. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Roesler, U.; Heller, P.; Waldmann, K.H.; Truyen, U.; Hensel, A. Immunization of Sows in an Integrated Pig-breeding Herd using a Homologous Inactivated Salmonella Vaccine Decreases the Prevalence of Salmonella typhimurium Infection in the Offspring. Zoonoses Public Health 2006, 53, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Cawthraw, S.A.; Feldman, R.A.; Sayers, A.R.; Newell, D.G. Long-term antibody responses following human infection with Campylobacter jejuni. Clin. Exp. Immunol. 2002, 130, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Luzza, F.; Maletta, M.; Imeneo, M.; Doldo, P.; Marasco, R.; Biancone, L.; Pallone, F. Salivary specific IgG is a sensitive indicator of the humoral immune response to Helicobacter pylori. FEMS Immunol. Med. Mic. 1995, 10, 281–283. [Google Scholar] [CrossRef]
- Van Winsen, R.; Van Nes, A.; Keuzenkamp, D.; Urlings, H.; Lipman, L.; Biesterveld, S.; Snijders, J.; Verheijden, J.; Van Knapen, F. Monitoring of transmission of Salmonella enterica serovars in pigs using bacteriological and serological detection methods. Vet. Microbiol. 2001, 80, 267–274. [Google Scholar] [CrossRef]
- Peeters, L.; Dewulf, J.; Boyen, F.; Brossé, C.; Vandersmissen, T.; Rasschaert, G.; Heyndrickx, M.; Cargnel, M.; Pasmans, F.; Maes, D. Effects of attenuated vaccine protocols against Salmonella Typhimurium on Salmonella serology in subclinically infected pig herds. Vet. J. 2019, 249, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mainar-Jaime, R.C.; Casanova-Higes, A.; Andrés-Barranco, S.; Vico, J.P. Looking for new approaches for the use of serology in the context of control programmes against pig salmonellosis. Zoonoses Public Health 2018, 65, e222–e228. [Google Scholar] [CrossRef]
- Pepin, B.; Liu, F.; Main, R.; Ramirez, A.; Zimmerman, J. Collection of oral fluid from individually housed sows. J. Swine Health Prod. 2015, 23, 35–37. [Google Scholar]
- Kittawornrat, A.; Prickett, J.; Chittick, W.; Wang, C.; Engle, M.; Johnson, J.; Patnayak, D.; Schwartz, T.; Whitney, D.; Olsen, C.; et al. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: Will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010, 154, 170–176. [Google Scholar] [CrossRef] [PubMed]


| Pig Category | Farms 1 and 2 (V) | Farms 3 and 4 (NV) | Total |
|---|---|---|---|
| Weaners | 13 | 16 | 29 |
| Growers | 11 | 19 | 30 |
| Sows | 13 | 9 | 22 |
| Total OF samples | 37 | 44 | 81 |
| Isotype | Farm Category | Farm id. | Sows | Weaners | Growers | Offspring (Weaners and Growers) | All Animals | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | V | 1 | 0.13 (0.07–0.82) | (4) a | 0.30 (0.19–0.34) | (7) | 0.23 (0.17–0.48) | (8) | 0.26 (0.17–0.48) | 0.24 (0.07–0.82) | (19) |
| 2 | 0.39 (0.07–2.49) | (9) | 0.18 (0.03–0.32) | (6) | 0.09 (0.08–0.09) | (2) b | 0.14 (0.03–0.32) | 0.22 (0.03–2.49) | (17) | ||
| NV | 3 | 0.10 (0.09–0.14) | (6) | 0.11 (0.09–0.28) | (7) | 0.11 (0.09–0.57) | (10) | 0.11 (0.09–0.57) | 0.11 (0.09–0.57) | (23) | |
| 4 | 0.17 (0.08–0.21) | (3) | 0.14 (0.11–0.37) | (9) | 0.13 (0.10–0.34) | (9) | 0.14 (0.10–0.37) | 0.14 (0.08–0.37) | (21) | ||
| V | 1 + 2 | 0.22 (0.07–2.49) | (13) | 0.23 (0.03–0.34) | (13) | 0.22 (0.08–0.48) | (10) | 0.23 (0.03–0.48) | 0.22 (0.03–2.49) | (36) | |
| NV | 3 + 4 | 0.11 (0.08–0.21) | (9) | 0.12 (0.09–0.37) | (16) | 0.13 (0.09–0.57) | (19) | 0.12 (0.09–0.57) | 0.12 (0.08–0.57) | (44) | |
| V + NV | all farms | 0.16 (0.07–2.49) | (22) | 0.15 (0.03–0.37) | (29) | 0.17 (0.08–0.57) | (29) | 0.16 (0.03–0.57) | 0.16 (0.03–2.49) | (80) | |
| IgA | V | 1 | 0.32 (0.31–0.66) | (4) | 0.28 (0.09–0.51) | (7) | 0.44 (0.21–1.83) | (8) | 0.35 (0.09–1.83) | 0.33 (0.09–1.83) | (19) |
| 2 | 0.53 (0.06–1.38) | (9) | 0.08 (0.06–0.14) | (6) | 0.12 (0.10–0.20) | (3) | 0.10 (0.06–0.20) | 0.15 (0.06–1.38) | (18) | ||
| NV | 3 | 0.28 (0.20–0.42) | (6) | 0.07 (0.05–0.20) | (7) | 0.16 (0.08–0.51) | (10) | 0.13 (0.05–0.51) | 0.18 (0.05–0.51) | (23) | |
| 4 | 0.62 (0.17–1.08) | (3) | 0.35 (0.20–0.47) | (9) | 0.53 (0.25–1.22) | (9) | 0.46 (0.20–1.22) | 0.47 (0.17–1.22) | (21) | ||
| V | 1 + 2 | 0.46 (0.06–1.38) | (13) | 0.14 (0.06–0.51) | (13) | 0.43 (0.10–1.83) | (11) | 0.22 (0.06–1.83) | 0.31 (0.06–1.83) | (37) | |
| NV | 3 + 4 | 0.30 (0.17–1.08) | (9) | 0.23 (0.05–0.47) | (16) | 0.47 (0.08–1.22) | (19) | 0.27 (0.05–1.22) | 0.29 (0.05–1.22) | (44) | |
| V + NV | all farms | 0.35 (0.06–1.38) | (22) | 0.20 (0.05–0.51) | (29) | 0.43 (0.08–1.83) | (30) | 0.25 (0.05–1.83) | 0.30 (0.05–1.83) | (81) | |
| IgG | Negative control c | 0.05 (0.05–0.06) | (4) | - | - | - | - | ||||
| IgA | 0.54 (0.26–1.12) | (4) | - | - | - | - | |||||
| Farm Category | Pig Category | Farm | No. of Positive/Examined (%) | p | Serotype | |
|---|---|---|---|---|---|---|
| V | Weaners | 1 | 7/7 | (100) | <0.01 | 4,5,12:i:- (7) |
| 2 | 0/6 | (0.0) | - | |||
| Growers | 1 | 4/8 | (50.0) | 0.24 | 4,5,12:i:- (4) | |
| 2 | 0/3 | (0.0) | - | |||
| Offspring a | 1 | 11/15 | (73.3) | <0.001 | 4,5,12:i:- (11) | |
| 2 | 0/9 | (0.0) | - | |||
| Sows | 1 | 0/4 | (0.0) | 1 | - | |
| 2 | 2/9 | (22.2) | Typhimurium (2) | |||
| All pig categories | 1 | 11/19 | (57.9) | <0.01 | 4,5,12:i:- (11) | |
| 2 | 2/18 | (11.1) | Typhimurium (2) | |||
| NV | Weaners | 3 | 7/7 | (100) | 1 | Kedougou (7) |
| 4 | 9/9 | (100) | 4,5,12:i:- (9) | |||
| Growers | 3 | 10/10 | (100) | 0.47 | Kedougou (10) | |
| 4 | 8/9 | (88.9) | 4,5,12:i:- (8) | |||
| Offspring | 3 | 17/17 | (100) | 1 | Kedougou (17) | |
| 4 | 17/18 | (94.4) | 4,5,12:i:- (17) | |||
| Sows | 3 | 3/6 | (50.0) | 0.46 | Kedougou (1); 4,5,12:i:- (2) | |
| 4 | 3/3 | (100) | 4,5,12:i:- (3) | |||
| All pig categories | 3 | 20/23 | (87.0) | 0.61 | Kedougou (18); 4,5,12:i:- (2) | |
| 4 | 20/21 | (95.2) | 4,5,12:i:- (20) | |||
| V | Weaners | 1 and 2 | 7/13 | (53.8) | <0.01 | 4,5,12:i:- (7) |
| NV | Weaners | 3 and 4 | 16/16 | (100) | Kedougou (7); 4,5,12:i:- (9) | |
| V | Growers | 1 and 2 | 4/11 | (36.4) | <0.01 | 4,5,12:i:- (4) |
| NV | Growers | 3 and 4 | 18/19 | (94.7) | Kedougou (10); 4,5,12:i:- (8) | |
| V | Offspring | 1 and 2 | 11/24 | (45.8) | <0.001 | 4,5,12:i:- (11) |
| NV | Offspring | 3 and 4 | 34/35 | (97.1) | Kedougou (17); 4,5,12:i:- (17) | |
| V | Sows | 1 and 2 | 2/13 | (15.4) | <0.05 | Typhimurium (2) |
| NV | Sows | 3 and 4 | 6/9 | (66.7) | Kedougou (1); 4,5,12:i:- (5) | |
| V | All pig categories | 1 and 2 | 13/37 | (35.1) | <0.001 | Typhimurium (2); 4,5,12:i:- (11) |
| NV | All pig categories | 3 and 4 | 40/44 | (90.9) | Kedougou (18); 4,5,12:i:- (22) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lucia, A.; Cawthraw, S.A.; Smith, R.P.; Davies, R.; Bianco, C.; Ostanello, F.; Martelli, F. Pilot Investigation of Anti-Salmonella Antibodies in Oral Fluids from Salmonella Typhimurium Vaccinated and Unvaccinated Swine Herds. Animals 2021, 11, 2408. https://doi.org/10.3390/ani11082408
De Lucia A, Cawthraw SA, Smith RP, Davies R, Bianco C, Ostanello F, Martelli F. Pilot Investigation of Anti-Salmonella Antibodies in Oral Fluids from Salmonella Typhimurium Vaccinated and Unvaccinated Swine Herds. Animals. 2021; 11(8):2408. https://doi.org/10.3390/ani11082408
Chicago/Turabian StyleDe Lucia, Alessia, Shaun A. Cawthraw, Richard Piers Smith, Rob Davies, Carlo Bianco, Fabio Ostanello, and Francesca Martelli. 2021. "Pilot Investigation of Anti-Salmonella Antibodies in Oral Fluids from Salmonella Typhimurium Vaccinated and Unvaccinated Swine Herds" Animals 11, no. 8: 2408. https://doi.org/10.3390/ani11082408
APA StyleDe Lucia, A., Cawthraw, S. A., Smith, R. P., Davies, R., Bianco, C., Ostanello, F., & Martelli, F. (2021). Pilot Investigation of Anti-Salmonella Antibodies in Oral Fluids from Salmonella Typhimurium Vaccinated and Unvaccinated Swine Herds. Animals, 11(8), 2408. https://doi.org/10.3390/ani11082408





