Microbiological Safety and Presence of Major Mycotoxins in Animal Feed for Laboratory Animals in a Developing Country: The Case of Costa Rica
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Study Objects
2.1.1. Sampling for Mycotoxin Analyses
2.1.2. Sampling for Microbiological Analysis
2.2. Safety and Quality in Animal Feed
2.2.1. Mycotoxin Analysis
2.2.2. Water Activity (aw)
2.2.3. Microbiological Analysis
2.3. Contaminants Behavior in Animal Feed during Storage
2.3.1. Mycotoxins and Molds Growth during Storage
2.3.2. Bacterial Strains for Animal Feed Contamination
2.3.3. Salmonella Growth in Animal Feed during Storage
2.3.4. Salmonella Survival in Animal Feed during Storage and UV Resistance
2.4. Salmonella Thermal Resistance in Animal Feed
2.5. Statistical Analysis
2.5.1. For Mycotoxins Analysis
2.5.2. For Salmonella Behavior Analysis
3. Results
3.1. Water Activity between Different Feed Batches
3.2. Safety and Quality of Animal Feed
3.2.1. Mycotoxins of Animal Feed
3.2.2. Indicator Microorganisms and Pathogens in Animal Feed
3.3. Microbiological and Mycotoxin Analysis of Lab Animal Feed during Storage
3.3.1. Mycotoxin, Water Activity and Mold Analysis on a Feed Batch over Time
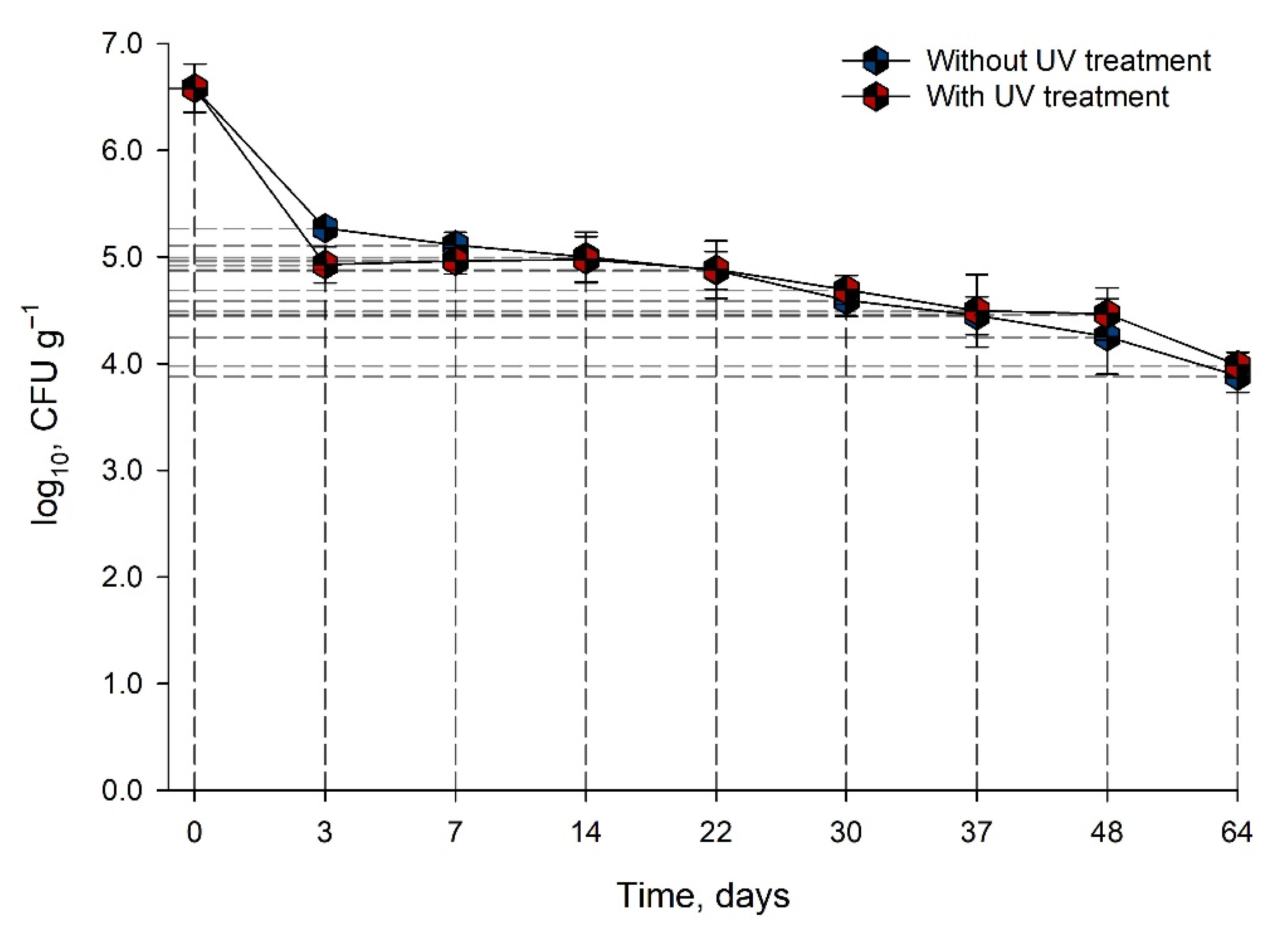
3.3.2. Salmonella Behavior during Storage after Inoculation
Bacterial Growth
Bacterial Survival
3.4. Salmonella Resistance in Animal Feed
Salmonella Thermal Resistance in Animal Feed
4. Discussion
4.1. Safety and Quality in Animal Feed
4.1.1. Mycotoxin Analysis between Different Feed Batches
4.1.2. Microbiological Quality of Samples
4.2. Water Activity between Different Feed Batches
4.3. Contaminants Behavior in Lab Animal Feed during Storage
4.3.1. Water Activity and Toxin Behavior on a Feed Batch over Time
4.3.2. Salmonella Behavior during Storage
4.4. UV and Thermal Resistance of Salmonella
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, R.L.; Lipman, N.S. Feed and Bedding. In Management of Animal Care and Use Programs in Research, Education, and Testing, 2nd ed.; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 639–653. [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; pp. 11–41. [Google Scholar]
- Turner, P.V.; Pekow, C.; Clark, J.M.; Vergara, P.; Bayne, K.; White, W.J.; Kurosawa, T.M.; Seok, S.-H.; Baneux, P. Roles of the International Council for Laboratory Animal Science (ICLAS) and International Association of Colleges of Laboratory Animal Medicine (IACLAM) in the Global Organization and Support of 3Rs Advances in Laboratory Science. J. Am. Assoc. Lab. Anim. Sci. 2005, 39, 230–235. [Google Scholar]
- Mesnage, R.; Defarge, N.; Rocque, L.-M.; de Vendômois, J.S.; Séralini, G.-E. Laboratory rodent diet contain toxic levels of environmental contaminants: Implications for regulatory tests. PLoS ONE 2015, 10, e0128429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, A.; Lee, A.; Farber, J. Methods for the Control of Foodborne Pathogens in Low-Moisture Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 177–208. [Google Scholar]
- Molina, A.; Granados-Chinchilla, F.; Jiménez, M.; Acuña-Calvo, M.; Alfaro, M.; Chavarría, G. Vigilance for Salmonella in feedstuffs available in Costa Rica: Prevalence, Serotyping and Tetracycline Resistance of Isolates obtained from 2009 to 2014. Foodborne Pathog. Dis. 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.; Granados-Chinchilla, F.; Redondo-Solano, M.; Arrieta-González, M.; Pineda-Salazar, E.; Molina, A. Characterization of the animal by-product meal industry in Costa Rica: Manufacturing practices through the production chain and food safety. Poult. Sci. 2018, 6, 2159–2169. [Google Scholar] [CrossRef]
- Finn, S.; Condell, O.; McClure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habermann, R.T.; Williams, F.P. Salmonellosis in Laboratory Animals. J. Natl. Cancer Inst. 1958, 20, 933–947. [Google Scholar] [PubMed]
- Shek, W.R.; Smith, A.L.; Pritchett-Corning, K.R. Microbiological Quality Control for Laboratory Rodents and Lagomorphs. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Otto, G.M., Whary, M.T., Anderson, L.C., Pritchett-Corning, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 463–510. [Google Scholar]
- Habimana, O.; Nesse, L.L.; Møretrø, T.; Berg, K.; Heir, E.; Vestby, L.K.; Langsrud, S. The persistence of Salmonella following desiccation under feed processing environmental conditions: A subject of relevance. Lett. Appl. Microbiol. 2014, 59, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Santillana, S.; Frank, J.; Schaffner, D. Modeling the influence of temperature, water activity and water mobility on the persistence of Salmonella in low-moisture foods. Int. J. Food Microbiol. 2013, 166, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Snoeyenbos, H.; Carlson, V. Thermal resistance of Salmonella Senftenberg 775W in dry animal feeds. Avian Dis. 1969, 13, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bethune, L.; Jia, Y.; Lovell, R.; Proescholdt, T.; Benz, S.A.; Schell, T.C.; Kaplan, G.; McChesney, D. Surveillance of Salmonella Prevalence in Animal Feeds and Characterization of the Salmonella Isolates by Serotyping and Antimicrobial Susceptibility. Foodborne Pathog. Dis. 2012, 9, 692–698. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.; Lee, K.; Poole, T.; Runyon, M.; Jones, B.; Herrman, T. Detection and isolation of Salmonella spp. in animal feeds from 2007–2011. J. Regul. Sci. 2014, 2, 14–27. [Google Scholar]
- Verma, N.S.; Sharma, S.P. Salmonellosis in laboratory animals. Indian Vet. J. 1969, 46, 1101–1102. [Google Scholar] [PubMed]
- Koopman, J.P.; Janssen, F.G. The occurrence of salmonellas in laboratory animals and a comparison of three enrichment methods used in their isolation. Z. Vers. 1975, 17, 155–158. [Google Scholar]
- Koopman, J.P.; Janssen, F.G. Occurrence and treatment of Salmonella infections in experimental dogs, cats and some other animal species. DTW. Dtsch. Tierarztl. Wochenschr. 1972, 79, 218–220. [Google Scholar]
- Fox, J.G.; Lipman, N.S. Infections transmitted by large and small laboratory animals. Infect. Dis. Clin. N. Am. 1991, 5, 131–163. [Google Scholar] [CrossRef]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; Giraudi, G. Mycotoxins in food and feed: Extraction, analysis and emerging technologies for rapid and on-field detection. Recent Pat. Food Nutr. Agric. 2014, 2, 140–153. [Google Scholar]
- Abrunhosa, L.; Morales, H.; Soares, C.; Calado, T.; Vila-Chã, A.S.; Pereira, M.; Venâncio, A. A Review of mycotoxins in food and feed products in Portugal and estimation of probable daily intake. Crit. Rev. Food Sci. Nutr. 2016, 56, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Bullerman, L.; Bianchini, A. Stability of mycotoxins during food processing. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Leiva, A.; Méndez, G.; Rodríguez, C.; Molina, A.; Granados-Chinchilla, F. Chemical assessment of mycotoxin contaminants and veterinary residues in Costa Rican animal feed. Int. J. Food Contam. 2019, 6, 5. [Google Scholar] [CrossRef]
- Molina, A.; Chavarría, G.; Alfaro-Cascante, M.; Leiva, A.; Granados-Chinchilla, F. Mycotoxins at the start of the food chain in Costa Rica: Analysis of Six Fusarium toxins and Ochratoxin A between 2013 and 2017 in animal feed and Aflatoxin M1 in dairy products. Toxins 2019, 11, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldemarson, A.H.; Hedenqvist, P.; Salomonsson, A.-C.; Häggblom, P. Mycotoxins in laboratory rodent feed. Lab. Anim. 2005, 39, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escrivá, L.; Font, G.; Berrada, H.; Manyes, L. Mycotoxin contamination in laboratory rat feeds and their implications in animal research. Toxicol. Mech. Methods 2016, 7, 529–537. [Google Scholar] [CrossRef]
- Islam, M.R.; Kim, J.W.; Roh, Y.-S.; Kim, J.-H.; Min, H.K.; Kwon, H.-J.; Lim, C.W.; Kim, B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oplatowska-Stachowiak, M.; Haughey, S.A.; Chevalier, O.P.; Galvin-King, P.; Campbell, K.; Magowan, E.; Adam, G.; Berthiller, F.; Krska, R.; Elliot, C.T. Determination of the mycotoxin content in distiller’s dried grain with solubles using a multianalyte UHPLC-MS/MS method. J. Agric. Food Chem. 2015, 63, 9441–9451. [Google Scholar] [CrossRef] [PubMed]
- El Kontar, W.; Santiago Silva, B.; Ruiz, J.; De Jesús de Durán, R.; Vit Olivier, P. Explorando la calidad del alimento concentrado para ratas (ACR) utilizado en el bioterio de la Universidad de Los Andes. Fuerza Farm. 2008, 1, 26–29. [Google Scholar]
- Association of American Feed Control Officials (AAFCO). Feed Inspector’s Manual, 5th ed.; Association of American Feed Control Officials Inspection and Sampling Committee: Champaign, IL, USA, 2014; 220p. [Google Scholar]
- Leiva, A.; Molina, A.; Redondo-Solano, M.; Artavia, G.; Rojas-Bogantes, L.; Granados Chinchilla, F. Pet food quality assurance and safety and quality survey within the Costa Rican pet food industry. Animals 2019, 9, 980. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Bacteriological Analytical Manual, Chapter 5 Salmonella. 2014. Available online: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm (accessed on 28 April 2021).
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German biodiversity exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal by-products. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. Part A 2010, 27, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Granados-Chinchilla, F.; Molina, A.; Chavarría, G.; Alfaro-Cascante, M.; Bogantes-Ledezma, D.; Murillo-Williams, A. Aflatoxins occurrence through the food chain in Costa Rica: Applying the One Health approach to mycotoxin surveillance. Food Control 2017, 82, 217–226. [Google Scholar] [CrossRef]
- Castells, M.; Marín, S.; Sanchis, V.; Ramos, A. Fate of mycotoxins in cereals during extrusion cooking: A review. Food Addit. Contam. 2005, 22, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-mycotoxin screening of feed and feed raw materials from Africa. World Mycotoxin J. 2017, 11, 369–383. [Google Scholar] [CrossRef]
- Wareing, P.; Stuart, F.; Fernandes, R. Food-Spoilage Fungi, Micro-Facts: The Working Companion for Food Microbiologists, 7th ed.; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 313–318. [Google Scholar]
- Abbès, S.; Salah-Abbès, J.B.; Jebali, R.; Younes, R.B.; Oueslati, R. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress: Possible protective role using lactic acid. J. Immunotoxicol. 2016, 13, 46–54. [Google Scholar] [CrossRef]
- Chawanthayatham, S.; Valentine, C.C.; Fedeles, B.I.; Fox, E.J.; Loeb, L.A.; Levine, S.S.; Slocum, S.L.; Wogan, G.N.; Croy, R.G.; Essigmann, J.M. Mutational spectra of aflatoxin B1 in vivo establish biomarkers of exposure for human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2017, 114, E3101–E3109. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Yamasaki, C.; Iwanari, H.; Yamashita, H.; Ogawa, Y.; Yanagi, A.; Furukawa, S.; Kojima, Y.; Chayama, K.; Kamiie, J.; et al. Detection of acute toxicity of aflatoxin B1 to human hepatocytes in vitro and in vivo using chimeric mice with humanized livers. PLoS ONE 2020, 15, e0239540. [Google Scholar]
- Ministerio de Agricultura y Ganadería. Reglamento para el Control de la Elaboración y Expendio de Alimentos para Animales. N° 16899. 1985. Available online: http://www.mag.go.cr/legislacion/1986/de-16899.pdf (accessed on 28 April 2021).
- Ministerio de Agricultura y Ganadería. Reforma Reglamento para el Control de la Elaboración y Expendio de Alimentos para Animales. N° 17413. 1987. Available online: http://www.mag.go.cr/legislacion/1987/de-17413.pdf (accessed on 28 April 2021).
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef]
- Somoskői, B.; Kovács, M.; Cseh, S. Effects of T-2 and Fumonisin B1 combined treatment on in vitro mouse embryo development and blastocyst quality. Toxicol. Ind. Health 2018, 34, 353–360. [Google Scholar] [CrossRef]
- Prosperini, A.; Font, G.; Ruiz, M.J. Interaction effects of Fusarium enniatins (A, A1, B, B1) combinations on in vitro cytotoxicity of Caco-2 cells. Toxicology In Vitro 2014, 28, 88–94. [Google Scholar] [CrossRef]
- Krug, I.; Behrens, M.; Esselen, M.; Humpf, H.-U. Transport of enniatin B and enniatin B1 across the blood-brain barrier and hints for neurotoxic effects in cerebral cells. PLoS ONE 2018, 13, e0197406. [Google Scholar] [CrossRef]
- Maranghi, F.; Tassinari, R.; Narciso, L.; Tait, S.; La Rocca, C.; Di Felice, G.; Butteroni, C.; Corinti, S.; Barletta, B.; Cordelli, E.; et al. In vivo toxicity and genotoxicity of beauvericin and enniatins. Combined approach to study in vivo toxicity and genotoxicity of mycotoxins beauvericin (BEA) and enniatin B (ENNB). EFSA J. 2018, 15, 1406E. [Google Scholar] [CrossRef]
- Bertero, A.; Fossatti, P.; Angela Tedesco, D.E.; Caloni, F. Beauvericin and enniatins: In vitro intestinal effects. Toxins 2020, 12, 686. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, G.X.; Liu, J.L.; Fan, J.J.; Cui, S. Toxic effects of zearalenone and its derivatives α-zearalenol on male reproductive system in mice. Reprod. Toxicol. 2007, 24, 381–387. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals’ granulosa cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchini, A.; Stratton, J.; Weier, S.; Hartter, T.; Plattner, B.; Rokey, G.; Hertzel, G.; Gompa, L.; Martinez, B.; Eskridge, K.M. Use of Enterococcus faecium as a surrogate for Salmonella enterica during extrusion of a balanced carbohydrate-protein meal. J. Food Prot. 2014, 77, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.; Elliott, P. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef] [PubMed]
- Granados-Chinchilla, F.; Alfaro, M.; Chavarría, G.; Rodríguez, C. Unravelling a vicious circle: Animal feed marketed in Costa Rica contains irregular concentrations of tetracyclines and abundant oxytetracycline-resistant Gram-positive bacteria. Food Addit. Contam. Part A 2014, 31, 1017–1025. [Google Scholar] [CrossRef]
- Jin, Y.; Pickens, S.; Hildebrandt, I.; Burbick, S.; Grasso, K.; Keller, S.; Anderson, N. Thermal inactivation of Salmonella Agona in low–water activity. Foods: Predictive models for the combined effect of temperature, water activity, and food component. J. Food Prot. 2018, 81, 1411–1417. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the safety and efficacy of propionic acid, sodium propionate, calcium propionate and ammonium propionate for all animal species. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA J. 2011, 9, 2446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Nan, X.; Wang, H.; Guo, Y.; Xiong, B. Research on the applications of calcium propionate in dairy cows: A review. Animals 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- US FDA [Food and Drug Administration]. Inspection Technical Guides: Water Activity (aw) in Foods; FDA: Rockville, MD, USA, 1984. Available online: https://www.fda.gov/ICECI/Inspections/InspectionGuides/InspectionTechnicalGuides/ucm072916.htm (accessed on 30 August 2020).
- Han, J.H.; Scanlon, M.G. Mass Transfer of gas and solute through packaging materials. In Innovations in Food Packaging, 1st ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 12–23. [Google Scholar]
- Cheli, F.; Campagnoli, A.; Pinotti, L.; Fusi, E.; Dell’Orto, V. Sampling feed for mycotoxins: Acquiring knowledge from food. Ital. J. Anim. Sci. 2009, 8, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Streit, E.; Schartzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of mycotoxin contamination and co-occurrence in animal feed-Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [Green Version]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in conversation with bacteria and fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- Lund, B.M.; Eklund, T. Control of pH and use of organic acids. In Microbiological Safety and Quality of Food, 1st ed.; Lund, B.M., Baird-Parker, T.C., Gould, G.W., Eds.; Aspen Publishers: Gaithersburg, MD, USA, 2000; pp. 175–199. [Google Scholar]
- Beuchat, L.; Mann, D. Factors affecting infiltration and survival of Salmonella on in-shell pecans and pecan nutmeats. J. Food Prot. 2010, 73, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Bustos Pino, C. Calidad microbiológica de alimentos para perros comercializados a granel. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2006. [Google Scholar]
- Jones, F.T. A review of practical Salmonella control measures in animal feed. Appl. Poult. Res. 2011, 20, 102–113. [Google Scholar] [CrossRef]
- Cheon, H.; Shin, J.; Park, K.; Chung, M.; Kang, D. Inactivation of foodborne pathogens in powdered red pepper (Capsicum annuum L.) using combined UV-C irradiation and mild heat treatment. Food Control 2015, 50, 441–445. [Google Scholar] [CrossRef]
- Yaun, R.; Sumner, S.; Eifert, J.; Marcy, J. Response of Salmonella and Escherichia coli O157:H7 to UV Energy. J. Food Prot. 2003, 66, 1071–1073. [Google Scholar] [CrossRef]
- Gayán, E.; Serrano, M.J.; Raso, J.; Alvarez, I.; Condón, S. Inactivation of Salmonella enterica by UV-C light alone and in combination with mild temperatures. Appl. Environ. Microbiol. 2012, 78, 8353–8361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lound, L.; Aleu, H.; Broggi, L.; Genaro, V.; Tesouro, R.; Favre, L. Resistencia térmica de Salmonella. Efecto del pH y la actividad del agua. Cienc. Docencia Tecnol. Supl. 2017, 7, 1–17. [Google Scholar]
- Syamaladevi, R.; Tang, J.; Villa, R.; Sablani, S.; Carter, B.; Campbell, G. Influence of Water Activity on Thermal Resistance of Microorganisms in Low-Moisture Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 353–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Tang, J.; Kiran, R.; Yang, R.; Jun, M. Exponentially increased thermal resistance of Salmonella spp. and Enterococcus faecium at reduced water activity. Am. Soc. Microbiol. 2018, 84, 1–12. [Google Scholar]
- Ramesh, M.N. Canning and Sterilization of Foods. In Handbook of Food Preservation, 2nd ed.; Rahman, M.S., Ed.; CRC Press: Boca Ratón, FL, USA, 2007; pp. 584–587. [Google Scholar]
 NIV (95%),
NIV (95%),  15-ADON(4%),
15-ADON(4%),  AFB1 (1%). Feed batch S1F.
AFB1 (1%). Feed batch S1F.  DON (66%),
DON (66%),  ENNB (24%), 15-ADON (4%),
ENNB (24%), 15-ADON (4%),  ENNB1 (4%),
ENNB1 (4%),  3-ADON (1%),
3-ADON (1%),  ZEA (1 %). Feed batch S1G. ENNB (57%),
ZEA (1 %). Feed batch S1G. ENNB (57%),  β-ZON (26%),
β-ZON (26%),  FB1 (9%), ENNB1 (8%). Feed batch S1H. ENNB (50%), ENNB1 (43%), FB1 (5%),
FB1 (9%), ENNB1 (8%). Feed batch S1H. ENNB (50%), ENNB1 (43%), FB1 (5%),  FB2 (2%). Feed batch S1I. ENNB1 (50%), ENNB (38%), FB1 (6%), β-ZON (3%), FB2 (2%), ZEA (1%) and (B) (n = 7 samples for supplier 2). Key (from inner to outer ring): Feed batch S2A.
FB2 (2%). Feed batch S1I. ENNB1 (50%), ENNB (38%), FB1 (6%), β-ZON (3%), FB2 (2%), ZEA (1%) and (B) (n = 7 samples for supplier 2). Key (from inner to outer ring): Feed batch S2A.  DON (90%),
DON (90%),  FB1 (4%),
FB1 (4%),  ENNB1 (3%),
ENNB1 (3%),  FB2 (1%),
FB2 (1%),  β-ZON (1%),
β-ZON (1%),  ENNB (1%). Feed batch S2B.
ENNB (1%). Feed batch S2B.  HT-2 (26%),
HT-2 (26%),  OTA (17%), FB1 (14%), ENNB (14%),
OTA (17%), FB1 (14%), ENNB (14%),  NEO (10%), ENNB1 (2%), FB2 (1%),
NEO (10%), ENNB1 (2%), FB2 (1%),  STE (1%) Feed batch S2C. DON (90%), FB1 (5%), β-ZON (4%). Feed batch S2D. ENNB (41%), FB1 (26%), β-ZON (22%), ENNB1 (6%), FB2 (3%),
STE (1%) Feed batch S2C. DON (90%), FB1 (5%), β-ZON (4%). Feed batch S2D. ENNB (41%), FB1 (26%), β-ZON (22%), ENNB1 (6%), FB2 (3%),  ZEA (3%). Feed batch S2E.
ZEA (3%). Feed batch S2E.  FSX (44%), ENNB (24%), β-ZON (18%), FB1 (12%), FB2 (3%). Feed batch S2F. ENNB (36%), FB1 (30%), β-ZON (23%), FB2 (5%), ZEA (4%) G. HT-2 (87%), FB1 (5%), β-ZON (4%), ENNB (3%),
FSX (44%), ENNB (24%), β-ZON (18%), FB1 (12%), FB2 (3%). Feed batch S2F. ENNB (36%), FB1 (30%), β-ZON (23%), FB2 (5%), ZEA (4%) G. HT-2 (87%), FB1 (5%), β-ZON (4%), ENNB (3%),  3-ADON (1%), FB2 (1%). Each ring represents a different batch of rodent feed tested. Values within brackets are relative to the total concentration found for each sample (toxins < 1% not shown).
3-ADON (1%), FB2 (1%). Each ring represents a different batch of rodent feed tested. Values within brackets are relative to the total concentration found for each sample (toxins < 1% not shown).
 NIV (95%),
NIV (95%),  15-ADON(4%),
15-ADON(4%),  AFB1 (1%). Feed batch S1F.
AFB1 (1%). Feed batch S1F.  DON (66%),
DON (66%),  ENNB (24%), 15-ADON (4%),
ENNB (24%), 15-ADON (4%),  ENNB1 (4%),
ENNB1 (4%),  3-ADON (1%),
3-ADON (1%),  ZEA (1 %). Feed batch S1G. ENNB (57%),
ZEA (1 %). Feed batch S1G. ENNB (57%),  β-ZON (26%),
β-ZON (26%),  FB1 (9%), ENNB1 (8%). Feed batch S1H. ENNB (50%), ENNB1 (43%), FB1 (5%),
FB1 (9%), ENNB1 (8%). Feed batch S1H. ENNB (50%), ENNB1 (43%), FB1 (5%),  FB2 (2%). Feed batch S1I. ENNB1 (50%), ENNB (38%), FB1 (6%), β-ZON (3%), FB2 (2%), ZEA (1%) and (B) (n = 7 samples for supplier 2). Key (from inner to outer ring): Feed batch S2A.
FB2 (2%). Feed batch S1I. ENNB1 (50%), ENNB (38%), FB1 (6%), β-ZON (3%), FB2 (2%), ZEA (1%) and (B) (n = 7 samples for supplier 2). Key (from inner to outer ring): Feed batch S2A.  DON (90%),
DON (90%),  FB1 (4%),
FB1 (4%),  ENNB1 (3%),
ENNB1 (3%),  FB2 (1%),
FB2 (1%),  β-ZON (1%),
β-ZON (1%),  ENNB (1%). Feed batch S2B.
ENNB (1%). Feed batch S2B.  HT-2 (26%),
HT-2 (26%),  OTA (17%), FB1 (14%), ENNB (14%),
OTA (17%), FB1 (14%), ENNB (14%),  NEO (10%), ENNB1 (2%), FB2 (1%),
NEO (10%), ENNB1 (2%), FB2 (1%),  STE (1%) Feed batch S2C. DON (90%), FB1 (5%), β-ZON (4%). Feed batch S2D. ENNB (41%), FB1 (26%), β-ZON (22%), ENNB1 (6%), FB2 (3%),
STE (1%) Feed batch S2C. DON (90%), FB1 (5%), β-ZON (4%). Feed batch S2D. ENNB (41%), FB1 (26%), β-ZON (22%), ENNB1 (6%), FB2 (3%),  ZEA (3%). Feed batch S2E.
ZEA (3%). Feed batch S2E.  FSX (44%), ENNB (24%), β-ZON (18%), FB1 (12%), FB2 (3%). Feed batch S2F. ENNB (36%), FB1 (30%), β-ZON (23%), FB2 (5%), ZEA (4%) G. HT-2 (87%), FB1 (5%), β-ZON (4%), ENNB (3%),
FSX (44%), ENNB (24%), β-ZON (18%), FB1 (12%), FB2 (3%). Feed batch S2F. ENNB (36%), FB1 (30%), β-ZON (23%), FB2 (5%), ZEA (4%) G. HT-2 (87%), FB1 (5%), β-ZON (4%), ENNB (3%),  3-ADON (1%), FB2 (1%). Each ring represents a different batch of rodent feed tested. Values within brackets are relative to the total concentration found for each sample (toxins < 1% not shown).
3-ADON (1%), FB2 (1%). Each ring represents a different batch of rodent feed tested. Values within brackets are relative to the total concentration found for each sample (toxins < 1% not shown).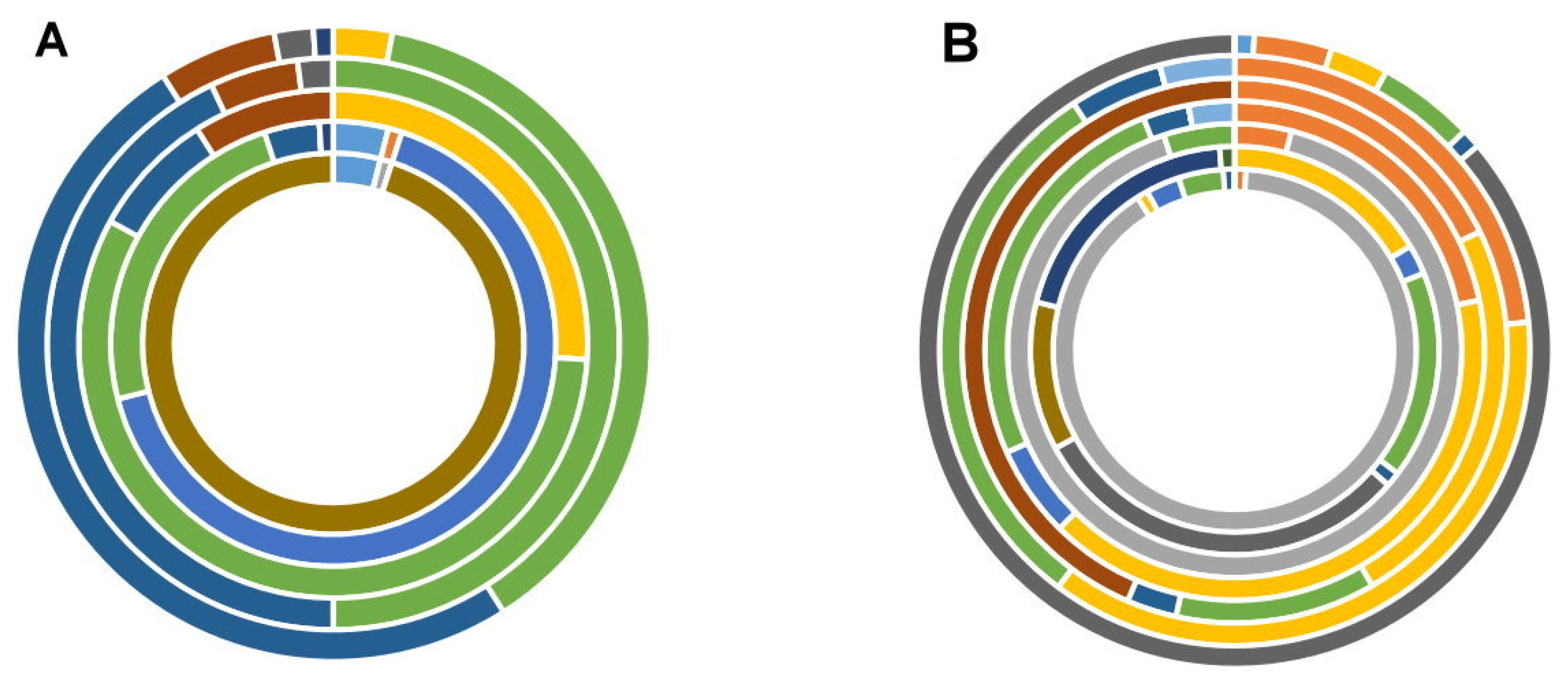

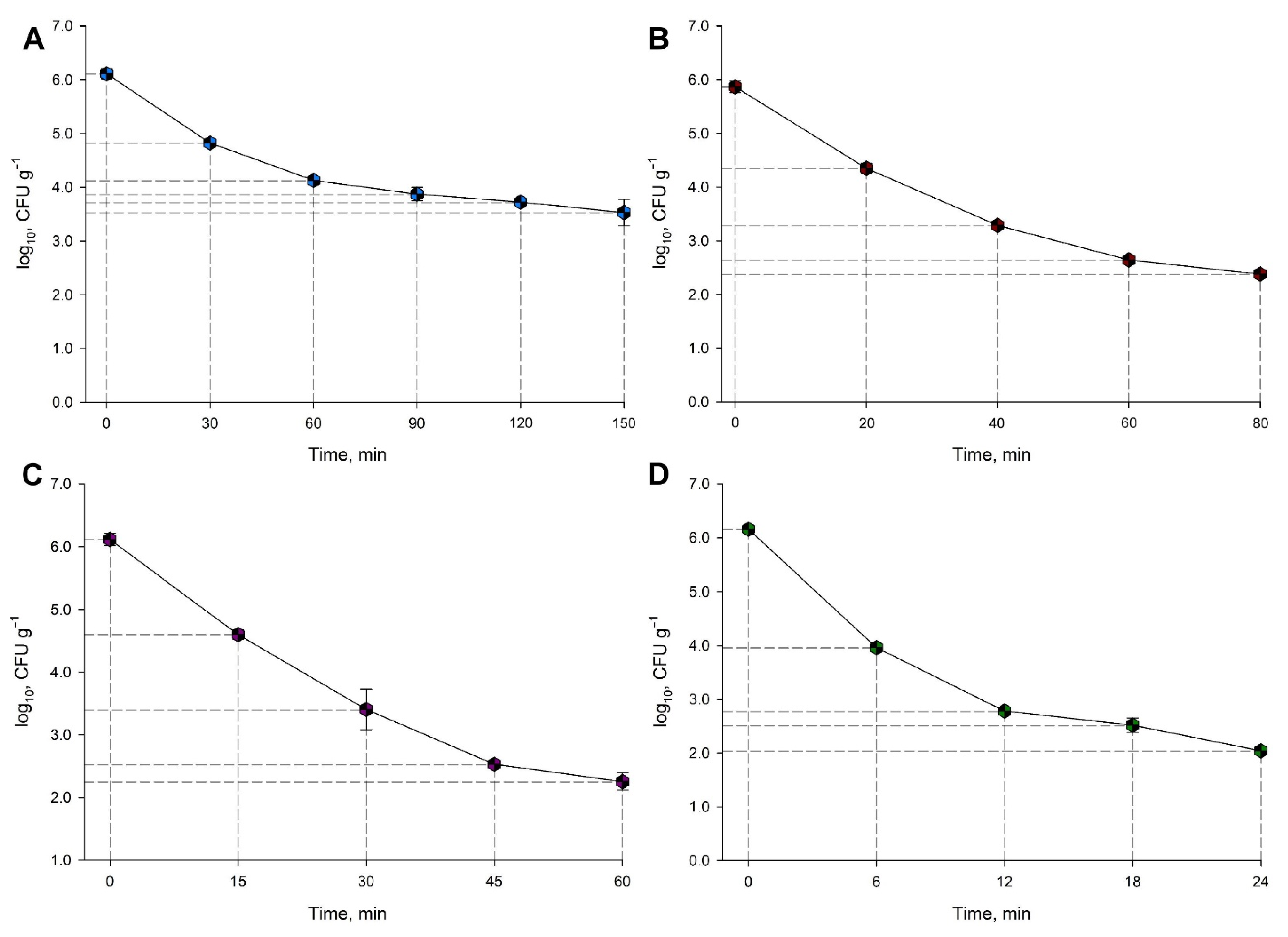
| Temperature, °C | Time, min | |||||
|---|---|---|---|---|---|---|
| 65 | 0 | 30 | 60 | 90 | 120 | 150 |
| 70 | 0 | 20 | 40 | 60 | 80 | - |
| 75 | 0 | 15 | 30 | 45 | 60 | - |
| 80 | 0 | 6 | 12 | 18 | 24 | - |
| Feed Supplier | Mean ± Standard Deviation * | Median | Maximum | Minimum |
|---|---|---|---|---|
| 1 | 0.5120 ± 0.0993 a | 0.5103 | 0.6582 | 0.3846 |
| 2 | 0.5232 ± 0.0579 a | 0.5024 | 0.6183 | 0.4674 |
| Toxin | Mean ± Standard Deviation * | Median | Maximum | Minimum |
|---|---|---|---|---|
| Concentration, μg kg−1 | ||||
| Supplier 1 | ||||
| 15-ADON (n = 2/9, 22.2%) | 398.45 ± 349.09 | 389.45 | 747.55 | 49.36 |
| ZEA (n = 2) | 92.55 ± 42.93 | 92.55 | 135.49 | 49.62 |
| β-ZON (n = 3, 33.3%) a | 42.07 ± 6.76 | 43.39 | 49.62 | 33.22 |
| FB2 (n = 3) a | 31.35 ± 10.80 | 23.95 | 46.62 | 23.48 |
| FB1 (n = 4, 44.4%) a | 60.50 ± 26.53 | 69.78 | 84.71 | 17.74 |
| ENNB1 (n = 4) a | 539.39 ± 333.37 | 612.27 | 917.47 | 15.56 |
| AFB1 (n = 4) | 3.30 ± 5.38 | 0.24 | 12.61 | 0.09 |
| ENNB (n = 5, 55.5%) a | 1296.05 ± 1907.42 | 539.43 | 5090.18 | 110.50 |
| Supplier 2 | ||||
| DON (n = 2/7, 28.6%) | 32.02 ± 2.89 | 32.02 | 34.91 | 29.13 |
| HT-2 (n = 2) | 28.64 ± 17.02 | 28.64 | 45.67 | 11.62 |
| OTA (n = 2) | 84.90 ± 8.66 | 84.90 | 93.56 | 76.24 |
| STE (n = 2) | 36.00 ± 22.52 | 36.00 | 58.52 | 13.47 |
| ZEA (n = 2) | 6.80 ± 2.69 | 6.80 | 9.50 | 4.10 |
| AFG2 (n = 3, 43.8%) | 7.47 ± 10.53 | 0.043 | 22.37 | 0.022 |
| ENNB1 (n = 4, 57.1%) a | 167.45 ± 136.82 | 160.00 | 330.14 | 19.66 |
| ENNB (n = 6, 85.7%) a | 225.65 ± 156.30 | 233.89 | 503.64 | 25.44 |
| β-ZON (n = 7, 100%) b | 268.71 ± 527.85 | 78.62 | 1559.62 | 10.08 |
| FB1 (n = 7) | 958.22 ± 2080.44 | 29.99 | 6044.04 | 0.76 |
| FB2 (n = 7) | 147.37 ± 147.94 | 64.05 | 421.53 | 0.54 |
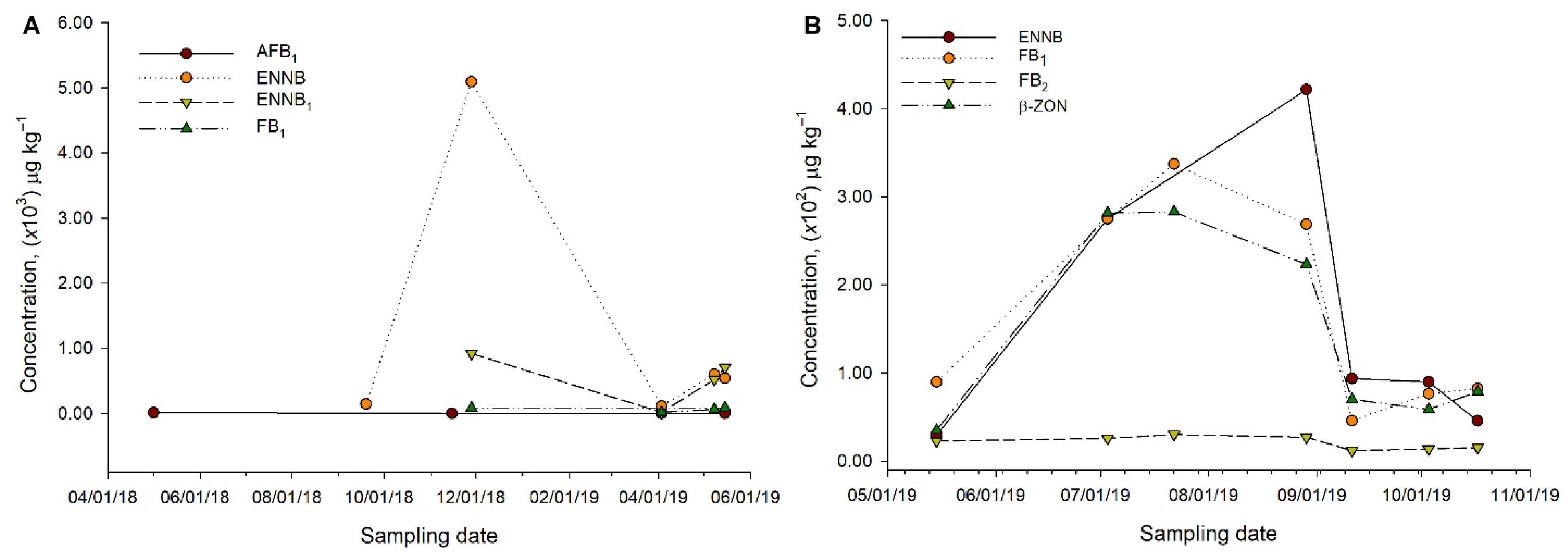
| Toxin * | Mean ± Standard Deviation § | Median | Maximum | Minimum |
|---|---|---|---|---|
| Concentration, μg kg−1 | ||||
| Supplier 1, sample S1I | ||||
| AFG2 (n = 4) | 0.41 ± 0.48 | 0.16 | 1.35 | 0.05 |
| ENNB1 (n = 5) | 157.47 ± 97.16 | 111.01 | 329.25 | 58.81 |
| ENNB (n = 6) | 279.46 ± 230.49 | 211.64 | 778.65 | 64.77 |
| β-ZON (n = 6) | 157.71 ± 93.95 | 144.58 | 328.01 | 13.75 |
| FB2 (n = 7) | 14.85 ± 8.27 | 11.53 | 31.11 | 6.08 |
| FB1 (n = 9) | 159.90 ± 137.44 | 94.53 | 380.93 | 14.67 |
| Supplier 2, sample S2A | ||||
| AFG2 (n = 4) | 0.39 ± 0.50 | 0.14 | 1.24 | 0.03 |
| DON (n = 4) | 50.21 ± 42.33 | 28.79 | 123.92 | 22.00 |
| ENNB (n = 7) | 331.22 ± 177.41 | 335.98 | 693.98 | 50.24 |
| ENNB1 (n = 5) | 83.19 ± 62.49 | 72.05 | 200.01 | 25.80 |
| FB1 (n = 9) | 263.14 ± 132.46 | 235.79 | 446.80 | 86.34 |
| FB2 (n = 9) | 24.33 ± 9.03 | 24.61 | 38.34 | 10.96 |
| STE (n = 3) | 145.97 ± 180.50 | 29.11 | 400.94 | 7.86 |
| β-ZON (n = 5) | 156.58 ± 154.08 | 94.75 | 462.12 | 41.71 |
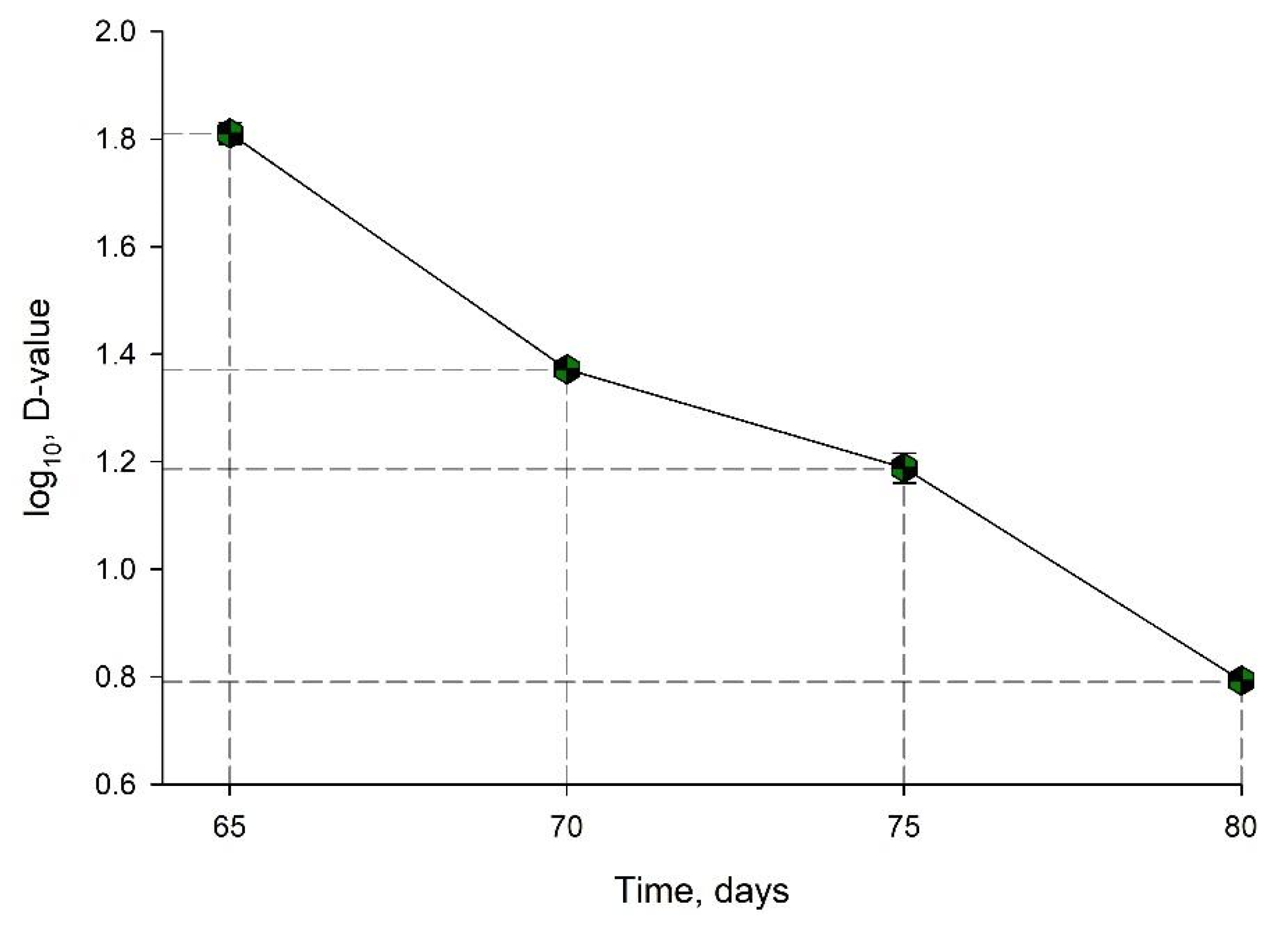
| Temperature, °C | D-Value ± Standard Deviation, min | log (D-Value) ± Standard Deviation, min |
|---|---|---|
| 65 | 65.58 ± 2.95 | 1.8101 ± 0.0198 |
| 70 | 23.53 ± 0.39 | 1.3717 ± 0.0072 |
| 75 | 15.4 ± 21.00 | 1.1881 ± 0.2830 |
| 80 | 6.21 ± 0.11 | 0.7931 ± 0.0079 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados-Chinchilla, F.; Valenzuela-Martínez, C.; García-Murillo, B.; Aguilar-Madrigal, D.; Redondo-Solano, M.; Molina, A. Microbiological Safety and Presence of Major Mycotoxins in Animal Feed for Laboratory Animals in a Developing Country: The Case of Costa Rica. Animals 2021, 11, 2389. https://doi.org/10.3390/ani11082389
Granados-Chinchilla F, Valenzuela-Martínez C, García-Murillo B, Aguilar-Madrigal D, Redondo-Solano M, Molina A. Microbiological Safety and Presence of Major Mycotoxins in Animal Feed for Laboratory Animals in a Developing Country: The Case of Costa Rica. Animals. 2021; 11(8):2389. https://doi.org/10.3390/ani11082389
Chicago/Turabian StyleGranados-Chinchilla, Fabio, Carol Valenzuela-Martínez, Berny García-Murillo, David Aguilar-Madrigal, Mauricio Redondo-Solano, and Andrea Molina. 2021. "Microbiological Safety and Presence of Major Mycotoxins in Animal Feed for Laboratory Animals in a Developing Country: The Case of Costa Rica" Animals 11, no. 8: 2389. https://doi.org/10.3390/ani11082389
APA StyleGranados-Chinchilla, F., Valenzuela-Martínez, C., García-Murillo, B., Aguilar-Madrigal, D., Redondo-Solano, M., & Molina, A. (2021). Microbiological Safety and Presence of Major Mycotoxins in Animal Feed for Laboratory Animals in a Developing Country: The Case of Costa Rica. Animals, 11(8), 2389. https://doi.org/10.3390/ani11082389






