Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Design of Experiment
2.2. Growth Performance
2.3. Meat Quality
2.3.1. pH and Meat Color
2.3.2. Drip Loss
2.3.3. Cooking Loss
2.3.4. Shear Force
2.4. Antioxidant Indices in Breast Muscle and Plasma
2.5. Dipeptide Content in Breast and Gene Expression of Carnosine Synthesis-Related Enzymes
2.5.1. Dipeptide Content
2.5.2. Total RNA Extraction and cDNA Synthesis
2.5.3. Quantitative of Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Meat Quality
3.3. Antioxidant Indices in Breast Muscle and Plasma
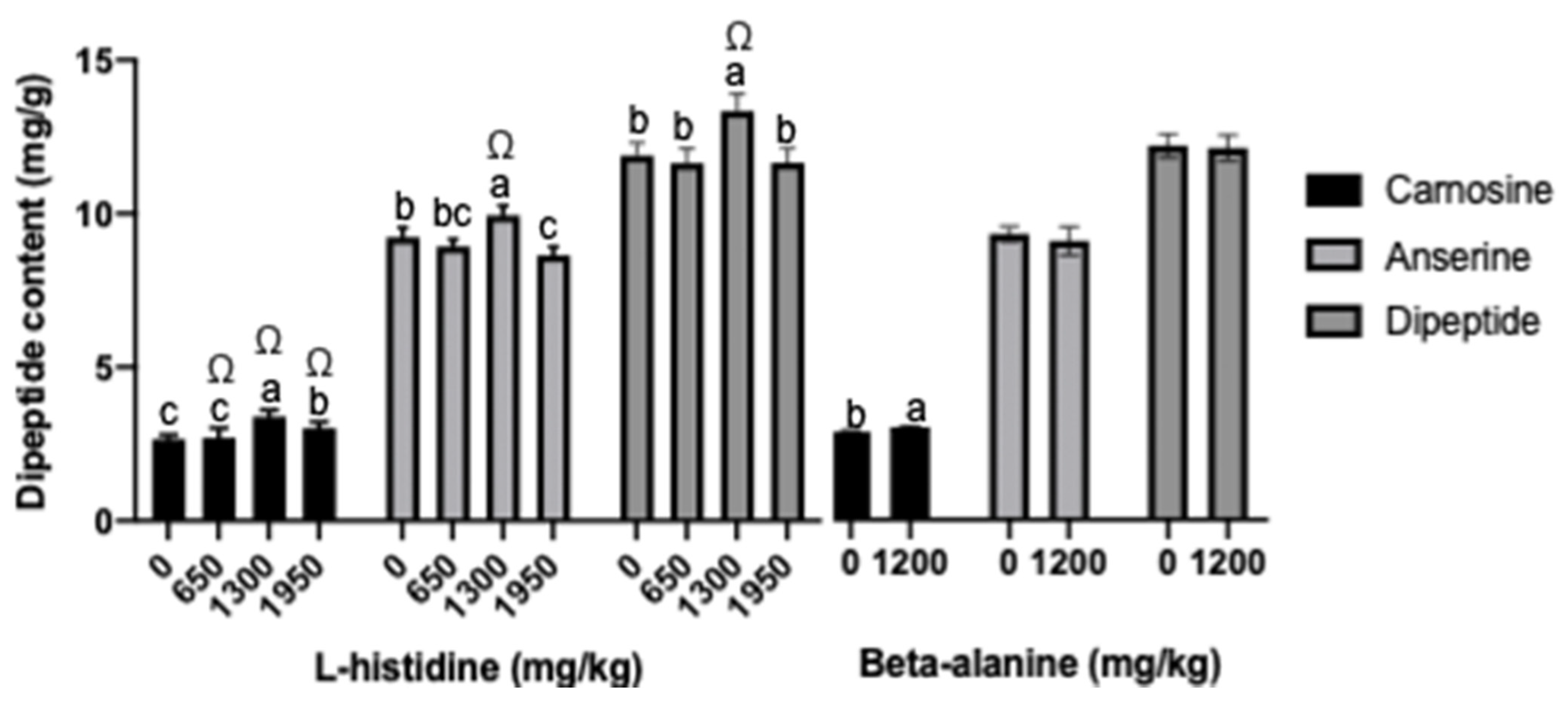
3.4. Dipeptide Content in Breast and Gene Expression of Carnosine Synthesis-Related Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monteverde, V.; Congiu, F.; Vazzana, I.; Dara, S.; Dipietro, S.; Piccione, G. Serum lipid profile modification related to polyunsaturated fatty acid supplementation in thoroughbred horses. J. Appl. Anim. Sci. 2017, 45, 615–618. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C.; Bruschetta, D.; Congiu, F.; Arfuso, F.; Giudice, E. Influence of exercise and dietary omega-3 oil supplementation on interleukin 1-Ra serum concentrations in Standardbred horses. Anim. Prod. Sci. 2019, 59, 232–235. [Google Scholar] [CrossRef]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and carnosine-related antioxidants: A review. Curr. Med. Chem. 2005, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Severin, S.E. The histidine-containing dipeptides, carnosine and anserine: Distribution, properties and biological significance. Adv. Enzym. Regul. 1990, 30, 175–188. [Google Scholar] [CrossRef]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B. De Courten, B. The potential of carnosine in brain-related disorders: A comprehensive review of current evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [Green Version]
- Everaert, I.; De Naeyer, H.; Taes, Y.; Derave, W. Gene expression of carnosine-related enzymes and transporters in skeletal muscle. Eur. J. Appl. Physiol. 2013, 113, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, S.; Kaji, Y.; Tachibana, T.; Denbow, D.M.; Furuse, M. Oral administration of β-alanine modifies carnosine concentrations in the muscles and brains of chickens. Anim. Sci. J. 2005, 76, 249–254. [Google Scholar] [CrossRef]
- Tomonaga, S.; Kaneko, K.; Kaji, Y.; Kido, Y.; Denbow, D.M.; Furuse, M. Dietary β-alanine enhances brain, but not muscle, carnosine and anserine concentrations in broilers. Anim. Sci. J. 2006, 77, 79–86. [Google Scholar] [CrossRef]
- Boldyrev, A.A. Carnosine and Oxidative Stress in Cells and Tissue, 4th ed.; Nova Science Publishers Inc.: New York, NY, USA, 2006. [Google Scholar]
- Harris, R.C.; Wise, J.A.; Price, K.A.; Kim, H.J.; Kim, C.K.; Sale, C. Determinants of muscle carnosine content. Amino Acids 2012, 43, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralik, G.; Kralik, Z.; Djurkin Kušec, I.; Škrtić, Z.; Kralik, I. Effect of dietary histidine, hybrid line and gender on chicken meat quality and concentration of carnosine. Poult. Sci. 2015, 52, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Tomonaga, S.; Matsumoto, M.; Furuse, M. β-Alanine enhances brain and muscle carnosine levels in broiler chicks. Poul. Sci. 2012, 49, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Kim, C.H.; Namgung, N.; Jung, B.Y.; Paik, I.K.; Kil, D.Y. Effects of dietary supplementation of histidine, β-alanine, magnesium oxide, and blood meal on carnosine and anserine concentrations of broiler breast meat. Poult. Sci. 2013, 50, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Qi, B.; Wang, J.; Ma, Y.B.; Wu, S.G.; Qi, G.H.; Zhang, H.J. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult. Sci. 2018, 97, 1220–1228. [Google Scholar] [CrossRef]
- Aviagen. Arbor Acres Broiler Management Guide; Aviagen Inc.: Huntsville, AL, USA, 2009. [Google Scholar]
- Zhang, L.; Yue, H.Y.; Zhang, H.J.; Xu, L.; Wu, S.G.; Yan, H.J.; Gong, Y.S.; Qi, G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 2009, 88, 2033–2041. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef]
- Xue, C.; Li, Y.; Lv, H.; Zhang, L.; Bi, C.; Dong, N.; Shan, A.; Wang, J. Oleanolic Acid Targets the Gut-Liver Axis to Alleviate Metabolic Disorders and Hepatic Steatosis. J. Agric. Food Chem. 2021. [Google Scholar] [CrossRef]
- Kai, S.; Watanabe, G.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary histidine on contents of carnosine and anserine in muscles of broilers. Anim. Sci. J. 2015, 86, 541–546. [Google Scholar] [CrossRef]
- Haug, A.; Rodbotten, R.; Mydland, L.T.; Christophersen, O.A. Increased broiler muscle carnosine and anserine following histidine supplementation of commercial broiler feed concentrate. Acta Agr. Scand. A 2008, 58, 71–77. [Google Scholar] [CrossRef]
- Kopec, W.; Wiliczkiewicz, A.; Jamroz, D.; Biazik, E.; Pudlo, A.; Hikawczuk, T.; Skiba, T.; Korzeniowska, M. Antioxidant status of turkey breast meat and blood after feeding a diet enriched with histidine. Poult. Sci. 2016, 95, 53–61. [Google Scholar] [CrossRef]
- Mei, L.; Cromwell, G.L.; Crum, A.D.; Decker, E.A. Influence of dietary β-alanine and histidine on the oxidative stability of pork. Meat Sci. 1998, 49, 55–64. [Google Scholar] [CrossRef]
- Hitomi-Ohmura, E.; Amano, N.; Aoyama, Y.; Yoshida, A. The effect of a histidine-excess diet on cholesterol synthesis and degradation in rats. Lipids 1992, 27, 755–760. [Google Scholar] [CrossRef]
- Solomon, J.K.; Geison, R.L. Effect of excess dietary L-histidine on plasma cholesterol levels in weanling rats. J. Nutr. 1978, 108, 936–943. [Google Scholar] [CrossRef]
- Yoshimatsu, H.; Chiba, S.; Tajima, D.; Akehi, Y.; Sakata, T. Histidine suppresses food intake through its conversion into neuronal histamine. Exp. Biol. Med. 2002, 227, 63–68. [Google Scholar] [CrossRef]
- Harry, E.G.; Tucker, J.F. The effect of orally administered histamine on the weight gain and development of gizzard lesions in chicks. Vet. Rec. 1976, 99, 206–207. [Google Scholar] [CrossRef]
- Baker, R.C.; Bruce, C.A. Further processing of poultry. In Processing of Poultry, 4th ed.; Springer: Boston, MA, USA, 1995; pp. 251–282. [Google Scholar]
- Barbut, S. Estimates and detection of the PSE problem in young turkey breast meat. Can. J. Anim. Sci 1996, 76, 455–457. [Google Scholar] [CrossRef]
- Santiago, H.L. Biological, Nutritional, and Processing Factors Affecting Breast Meat Quality of Broilers. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Virginia, VA, USA, 2002. [Google Scholar]
- Jochem, J. Haematological, blood gas and acid-base effects of central histamine-induced reversal of critical haemorrhagic hypotension in rats. J. Physiol. Pharmacol. 2001, 52, 447–458. [Google Scholar]
- Cong, J.; Zhang, L.; Li, J.; Wang, S.; Gao, F.; Zhou, G. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 2017, 97, 3733–3741. [Google Scholar] [CrossRef]
- Harris, R.C.; Marlin, D.J.; Dunnett, M.; Snow, D.H.; Hultman, E. Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp. Biochem. Physiol. A. 1990, 97, 249–251. [Google Scholar] [CrossRef]
- Kopeć, W.; Jamroz, D.; Wiliczkiewicz, A.; Biazik, E.; Pudlo, A.; Hikawczuk, T.; Skiba, T.; Korzeniowska, M. Influence of different histidine sources and zinc supplementation of broiler diets on dipeptide content and antioxidant status of blood and meat. Br. Poult. Sci. 2013, 54, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Hu, X.; Hongtrakul, K.; Ji, C.; Ma, Q.; Guan, S.; Song, C.; Zhang, Y.; Zhao, L. Effect of carnosine on growth performance, carcass characteristics, meat quality and oxidative stability in broiler chickens. Poult. Sci. 2009, 46, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Kralik, G.; Sak-Bosnar, M.; Kralik, Z.; Galović, O. Effects of β-alanine dietary supplementation on concentration of carnosine and quality of broiler muscle tissue. Poult. Sci. 2014, 51, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.; Bulygina, E.; Leinsoo, T.; Petrushanko, I.; Tsubone, S.; Abe, H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 137, 81–88. [Google Scholar] [CrossRef]
- Stenesh, J.J.; Winnick, T. Carnosine–anserine synthetase of muscle. 4. Partial purification of the enzyme and further studies of β-alanyl peptide synthesis. Biochem. J. 1960, 77, 575–581. [Google Scholar] [CrossRef]
- Jones, N.R. The free amino acids of fish. 1-Methylhistidine and β-alanine liberation by skeletal muscle anserinase of codling (Gadus callarias). Biochem. J. 1955, 60, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Blancquaert, L.; Everaert, I.; Missinne, M.; Baguet, A.; Stegen, S.; Volkaert, A.; Petrovic, M.; Vervaet, C.; Achten, E.; De Maeyer, M.; et al. Effects of histidine and β-alanine supplementation on human muscle carnosine storage. Med. Sci. Sports Exerc. 2017, 49, 602–609. [Google Scholar] [CrossRef]
- Saunders, B.; de Salles Painelli, V.; De Oliveira, L.F.; da Eira Silva, V.; Da Silva, R.P.; Riani, L.; Franchi, M.; Goncalves, L.D.; Harris, R.C.; Roschel, H.; et al. Twenty-four weeks of β-alanine supplementation on carnosine content, related genes, and exercise. Med. Sci. Sports Exerc. 2017, 49, 896–906. [Google Scholar] [CrossRef] [Green Version]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef] [Green Version]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef]
| Item | Starter Period (1–21 Days) | Grower Period (22–42 Days) |
|---|---|---|
| Components, % | ||
| Corn | 59.22 | 62.20 |
| Soybean meal (47%) | 34.37 | 30.84 |
| Vegetable oil | 2.27 | 3.26 |
| Dicalcium phosphate | 1.82 | 1.55 |
| Limestone | 1.31 | 1.23 |
| Salt | 0.30 | 0.30 |
| dl-Methionine (98%) | 0.24 | 0.19 |
| l-Lysine-HCl (78%) | 0.09 | 0.07 |
| l-Threonine (98) | 0.06 | 0.04 |
| Vitamin premix 1 | 0.02 | 0.02 |
| Mineral premix 2 | 0.20 | 0.20 |
| Choline chloride (50 %) | 0.10 | 0.10 |
| Total | 100 | 100 |
| Calculated nutrient levels | ||
| AME, MJ/kg | 12.35 | 12.77 |
| Crude protein, % | 21.5 | 20.00 |
| Calcium, % | 1.00 | 0.90 |
| Total phosphorus, % | 0.69 | 0.63 |
| Available phosphorus, % | 0.45 | 0.40 |
| Lysine, % | 1.21 | 1.10 |
| Methionine, % | 0.55 | 0.48 |
| Methionine + cysteine, % | 0.88 | 0.80 |
| Threonine, % | 0.86 | 0.78 |
| Genes | Forward Primer (5′–3′) | Reserve Primer (3′–5′) | Tm (°C) | Gene ID |
|---|---|---|---|---|
| CARNS | CTGGAGGGGTCAGCAAGAG | CTGTCGTAGGGCAGGAAGGT | 62 | 100359387 |
| CNDP2 | CACCTCACCTTCTGGCTTGT | ACATGCTTCCCTCTTCTCCA | 62 | 421013 |
| PEPT1 | TGTCACTGGGCTGGAGTTTT | AGCAAGGCAGCAAAGAGAAC | 60 | 378789 |
| PEPT2 | GTGGGGTTCAGACATGGAAG | GGCCAGACCTGTAATGGAGA | 62 | 424244 |
| PHT1 | CTGGCAGAGGACAAACACAA | ACTCGCTGCACTCAATTTCC | 60 | 416808 |
| HDC | GGCAGGCTCTTCCTTATTCC | GCAGTGCGTTGAATGATGTT | 62 | 425454 |
| β-actin | TGACAATGGCTCCGGTATGT | TCTTTCTGGCCCATACCAAC | 60 | 396526 |
| L-histidine (mg/kg) | Beta-alanine (mg/kg) | BW | ADFI | ADG | FCR | BW | ADFI | ADG | FCR | ADFI | ADG | FCR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–21 Days | 22–42 Days | 1–42 Days | ||||||||||||
| 0 | 0 | 819.66 | 53.52 | 37.71 | 1.42 | 2411.86 | 146.26 | 75.87 | 1.94 | 90.32 | 52.81 | 1.71 | ||
| 0 | 1200 | 848.57 | 53.23 | 39.07 | 1.40 | 2562.31 | 150.97 | 79.18 | 1.91 | 92.59 | 52.99 | 1.68 | ||
| 650 | 0 | 843.37 | 54.61 | 38.88 | 1.41 | 2416.83 | 148.21 | 73.27 | 2.04 | 92.87 | 52.92 | 1.76 | ||
| 650 | 1200 | 825.00 | 53.91 | 37.86 | 1.45 | 2510.90 | 147.24 | 78.73 | 1.87 | 92.08 | 54.13 | 1.70 | ||
| 1300 | 0 | 809.61 | 54.27 | 36.93 | 1.47 | 2429.88 | 143.93 | 73.58 | 1.96 | 90.88 | 51.77 | 1.75 | ||
| 1300 | 1200 | 801.53 | 53.51 | 35.99 | 1.46 | 2391.71 | 141.65 | 71.75 | 1.98 | 87.59 | 50.13 | 1.75 | ||
| 1950 | 0 | 815.89 | 53.28 | 37.51 | 1.42 | 2553.80 | 146.97 | 78.61 | 1.88 | 91.98 | 54.41 | 1.69 | ||
| 1950 | 1200 | 786.40 | 52.26 | 34.69 | 1.52 | 2438.17 | 138.10 | 73.94 | 1.89 | 86.29 | 50.14 | 1.72 | ||
| Pooled SEM | 5.860 | 0.475 | 0.332 | 0.010 | 25.497 | 1.353 | 1.076 | 0.017 | 0.858 | 0.523 | 0.009 | |||
| Source | p-value | |||||||||||||
| l-histidine | 0.085 | 0.708 | 0.024 | 0.076 | 0.642 | 0.252 | 0.432 | 0.279 | 0.430 | 0.353 | 0.119 | |||
| Beta-alanine | 0.567 | 0.469 | 0.204 | 0.129 | 0.658 | 0.497 | 0.794 | 0.214 | 0.279 | 0.285 | 0.355 | |||
| l-histidine × beta-alanine | 0.330 | 0.995 | 0.185 | 0.135 | 0.247 | 0.373 | 0.332 | 0.197 | 0.404 | 0.277 | 0.365 | |||
| Main Effect | l-histidine | 0 | 834.11 | 53.38 | 38.39 a | 1.41 | 2487.09 | 148.62 | 77.52 | 1.93 | 91.46 | 52.9 | 1.69 | |
| 650 | 834.19 | 54.26 | 38.37 a | 1.43 | 2463.87 | 147.73 | 76.00 | 1.96 | 92.48 | 53.52 | 1.73 | |||
| 1300 | 805.57 | 53.89 | 36.46 ab | 1.47 | 2410.79 | 142.79 | 72.67 | 1.97 | 89.24 | 50.95 | 1.75 | |||
| 1950 | 801.15 | 52.77 | 36.10 b | 1.47 | 2495.99 | 142.54 | 76.28 | 1.89 | 89.14 | 52.27 | 1.71 | |||
| Beta-alanine | 0 | 822.13 | 53.92 | 37.76 | 1.43 | 2453.09 | 146.34 | 75.33 | 1.96 | 91.51 | 52.97 | 1.73 | ||
| 1200 | 815.38 | 53.23 | 36.90 | 1.46 | 2475.77 | 144.49 | 75.90 | 1.91 | 89.64 | 51.85 | 1.71 | |||
| l-histidine (mg/kg) | Beta-alanine (mg/kg) | L | a | b | pH | L | a | b | pH | △pH | Shear Force | Drip Loss | Cooking Loss | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | 24 h | ||||||||||||||
| 0 | 0 | 49.49 | 8.35 | 16.11 | 6.13 | 56.97 | 8.21 | 11.58 | 5.72 | 0.41 | 17.45 | 5.75 | 8.04 | ||
| 0 | 1200 | 52.00 | 9.85 | 16.10 | 6.29 | 58.72 | 7.89 | 12.23 | 5.77 | 0.59 | 16.87 | 5.60 | 8.17 | ||
| 650 | 0 | 53.58 | 8.12 | 14.18 | 6.19 | 58.64 | 7.31 | 11.88 | 5.89 | 0.30 | 19.17 | 5.21 | 8.59 | ||
| 650 | 1200 | 51.20 | 7.73 | 15.47 | 6.35 | 58.60 | 7.98 | 11.98 | 5.77 | 0.58 | 18.27 | 7.23 | 8.51 | ||
| 1300 | 0 | 49.85 | 6.02 | 14.33 | 6.36 | 56.54 | 7.76 | 11.94 | 5.85 | 0.50 | 20.58 | 6.88 | 8.68 | ||
| 1300 | 1200 | 49.48 | 6.41 | 11.61 | 6.27 | 55.97 | 8.18 | 12.04 | 5.83 | 0.43 | 20.57 | 5.79 | 8.29 | ||
| 1950 | 0 | 49.55 | 6.13 | 14.03 | 6.31 | 56.00 | 8.15 | 13.23 | 5.89 | 0.48 | 21.46 | 5.52 | 8.52 | ||
| 1950 | 1200 | 50.56 | 5.60 | 13.49 | 6.32 | 58.26 | 8.03 | 13.46 | 5.84 | 0.48 | 18.29 | 3.80 | 7.91 | ||
| Pooled SEM | 0.244 | 0.013 | 0.302 | 0.016 | 0.317 | 0.052 | 0.160 | 0.012 | 0.018 | 0.644 | 0.335 | 0.067 | |||
| Source | p-value | ||||||||||||||
| l-histidine | 0.001 | <0.001 | 0.004 | 0.095 | 0.065 | 0.016 | 0.005 | 0.005 | 0.760 | 0.348 | 0.066 | 0.602 | |||
| Beta-alanine | 0.691 | 0.433 | 0.419 | 0.058 | 0.186 | 0.127 | 0.400 | 0.144 | 0.010 | 0.412 | 0.633 | 0.374 | |||
| l-histidine × beta-alanine | 0.007 | 0.086 | 0.138 | 0.021 | 0.331 | 0.004 | 0.917 | 0.089 | 0.005 | 0.872 | 0.054 | 0.768 | |||
| Main effect | l-histidine | 0 | 50.74 ab | 9.10 a | 16.10 a | 6.21 | 57.85 | 8.05 a | 11.90 b | 5.75 b | 0.50 | 17.09 | 5.67 | 8.11 | |
| 650 | 52.39 a | 7.92 b | 14.83 ab | 6.27 | 58.62 | 7.65 b | 11.93 b | 5.83 ab | 0.44 | 18.72 | 6.08 | 8.54 | |||
| 1300 | 49.67 b | 6.22 c | 12.97 b | 6.31 | 56.25 | 7.97 ab | 11.99 b | 5.84 a | 0.47 | 20.58 | 6.37 | 8.51 | |||
| 1950 | 50.06 b | 5.87 c | 13.76 b | 6.32 | 57.13 | 8.09 a | 13.35 a | 5.86 a | 0.48 | 19.35 | 4.66 | 8.25 | |||
| Beta- alanine | 0 | 50.62 | 7.16 | 14.66 | 6.25 | 57.04 | 7.86 | 12.16 | 5.84 | 0.42 ** | 19.55 | 5.84 | 8.52 | ||
| 1200 | 50.81 | 7.40 | 14.17 | 6.31 | 57.89 | 8.02 | 12.43 | 5.80 | 0.52 * | 18.19 | 5.49 | 8.24 | |||
| l-histidine (mg/kg) | Beta-alanine (mg/kg) | Breast | Plasma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T-AOC | MDA | T-SOD | T-AOC | MDA | T-SOD | ||||
| 0 | 0 | 0.22 | 0.92 | 45.94 | 3.55 | 2.26 | 149.48 | ||
| 0 | 1200 | 0.30 | 0.70 | 49.48 | 3.92 | 2.12 | 169.86 | ||
| 650 | 0 | 0.26 | 0.75 | 48.71 | 3.62 | 2.22 | 177.87 | ||
| 650 | 1200 | 0.26 | 0.76 | 45.31 | 4.93 | 2.10 | 167.61 | ||
| 1300 | 0 | 0.28 | 0.72 | 49.17 | 4.99 | 2.28 | 177.30 | ||
| 1300 | 1200 | 0.32 | 0.69 | 47.07 | 6.20 | 2.15 | 186.17 | ||
| 1950 | 0 | 0.29 | 0.60 | 51.37 | 5.63 | 2.28 | 206.40 | ||
| 1950 | 1200 | 0.26 | 0.64 | 51.26 | 4.67 | 2.23 | 158.79 | ||
| Pooled SEM | 0.005 | 0.008 | 0.335 | 0.127 | 0.017 | 2.343 | |||
| Source | p-value | ||||||||
| l-histidine | 0.010 | <0.001 | <0.001 | <0.001 | 0.253 | 0.004 | |||
| Beta-alanine | 0.024 | 0.003 | 0.444 | 0.063 | 0.002 | 0.133 | |||
| l-histidine × beta-alanine | 0.001 | 0.003 | 0.004 | 0.009 | 0.708 | <0.001 | |||
| Main effect | l-histidine | 0 | 0.26 b | 0.81 a | 47.71 b | 3.74 c | 2.19 | 159.67 b | |
| 650 | 0.26 b | 0.75 b | 47.01 b | 4.28 bc | 2.16 | 172.74 a | |||
| 1300 | 0.30 a | 0.71 b | 48.12 b | 5.59 a | 2.22 | 181.74 a | |||
| 1950 | 0.28 ab | 0.62 c | 51.31 a | 5.15 ab | 2.25 | 182.59 a | |||
| Beta-alanine | 0 | 0.26 ** | 0.75 * | 48.80 | 4.45 | 2.26 * | 177.76 | ||
| 1200 | 0.29 * | 0.70 ** | 48.28 | 4.93 | 2.15 ** | 170.61 | |||
| l-histidine (mg/kg) | Beta-alanine (mg/kg) | HDC | PHT1 | PEPT1 | PEPT2 | CNDP2 | CARNS | ||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 0 | 1200 | 0.94 | 1.41 | 1.09 | 1.41 | 1.02 | 1.07 | ||
| 650 | 0 | 1.06 | 1.62 | 1.58 | 1.13 | 1.44 | 1.00 | ||
| 650 | 1200 | 1.42 | 1.69 | 1.57 | 1.30 | 1.87 | 1.60 | ||
| 1300 | 0 | 2.24 | 1.92 | 1.60 | 1.30 | 1.51 | 1.57 | ||
| 1300 | 1200 | 1.61 | 1.48 | 1.51 | 1.48 | 2.30 | 1.95 | ||
| 1950 | 0 | 1.32 | 1.21 | 1.42 | 1.34 | 1.64 | 1.34 | ||
| 1950 | 1200 | 1.52 | 1.87 | 1.50 | 1.38 | 1.55 | 1.78 | ||
| Pooled SEM | 0.088 | 0.068 | 0.063 | 0.076 | 0.127 | 0.127 | |||
| Source | p-value | ||||||||
| l-histidine | 0.004 | 0.060 | 0.017 | 0.743 | 0.100 | <0.001 | |||
| Beta-alanine | 0.853 | 0.201 | 0.897 | 0.201 | 0.268 | 0.002 | |||
| l-histidine × beta-alanine | 0.232 | 0.038 | 0.951 | 0.863 | 0.609 | 0.397 | |||
| Main effect | l-histidine | 0 | 0.97 b | 1.21 | 1.04 b | 1.20 | 1.01 | 1.04 c | |
| 650 | 1.24 b | 1.65 | 1.57 a | 1.21 | 1.66 | 1.30 bc | |||
| 1300 | 1.93 a | 1.70 | 1.55 a | 1.39 | 1.91 | 1.76 a | |||
| 1950 | 1.42 ab | 1.54 | 1.46 ab | 1.36 | 1.60 | 1.56 ab | |||
| Beta-alanine | 0 | 1.41 | 1.44 | 1.40 | 1.19 | 1.40 | 1.23 ** | ||
| 1200 | 1.37 | 1.61 | 1.42 | 1.39 | 1.69 | 1.60 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, B.; Wang, J.; Hu, M.; Ma, Y.; Wu, S.; Qi, G.; Qiu, K.; Zhang, H. Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers. Animals 2021, 11, 2265. https://doi.org/10.3390/ani11082265
Qi B, Wang J, Hu M, Ma Y, Wu S, Qi G, Qiu K, Zhang H. Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers. Animals. 2021; 11(8):2265. https://doi.org/10.3390/ani11082265
Chicago/Turabian StyleQi, Bo, Jing Wang, Meng Hu, Youbiao Ma, Shugeng Wu, Guanghai Qi, Kai Qiu, and Haijun Zhang. 2021. "Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers" Animals 11, no. 8: 2265. https://doi.org/10.3390/ani11082265
APA StyleQi, B., Wang, J., Hu, M., Ma, Y., Wu, S., Qi, G., Qiu, K., & Zhang, H. (2021). Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers. Animals, 11(8), 2265. https://doi.org/10.3390/ani11082265







