The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries
Abstract
:Simple Summary
Abstract
1. Introduction
2. Properties of AgNPs Based on Size and Shape
2.1. Size of AgNPs (Size Dependent)
2.2. Shape of AgNPs (Shape Dependent)
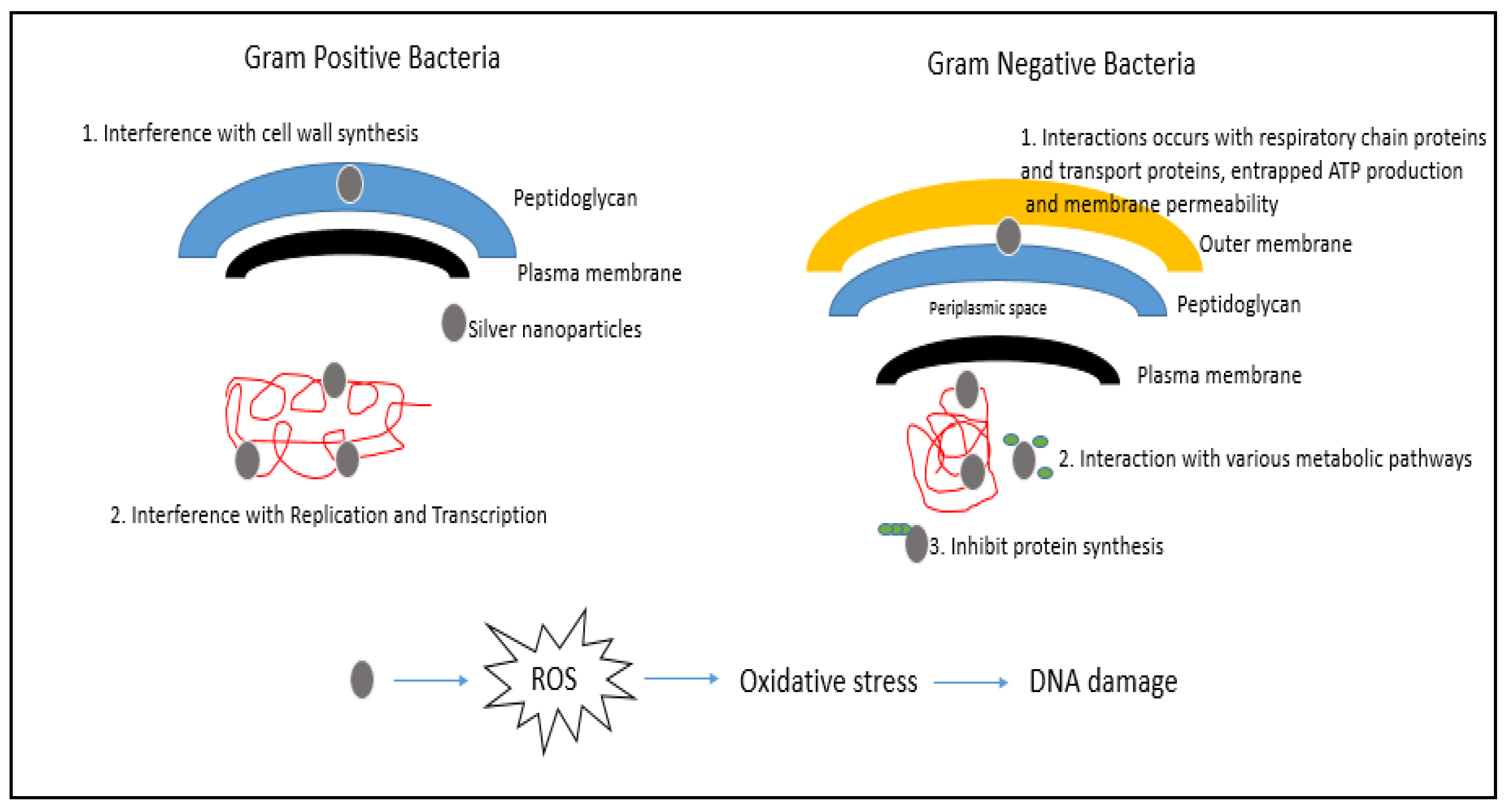
3. Mechanism of Action of AgNPs
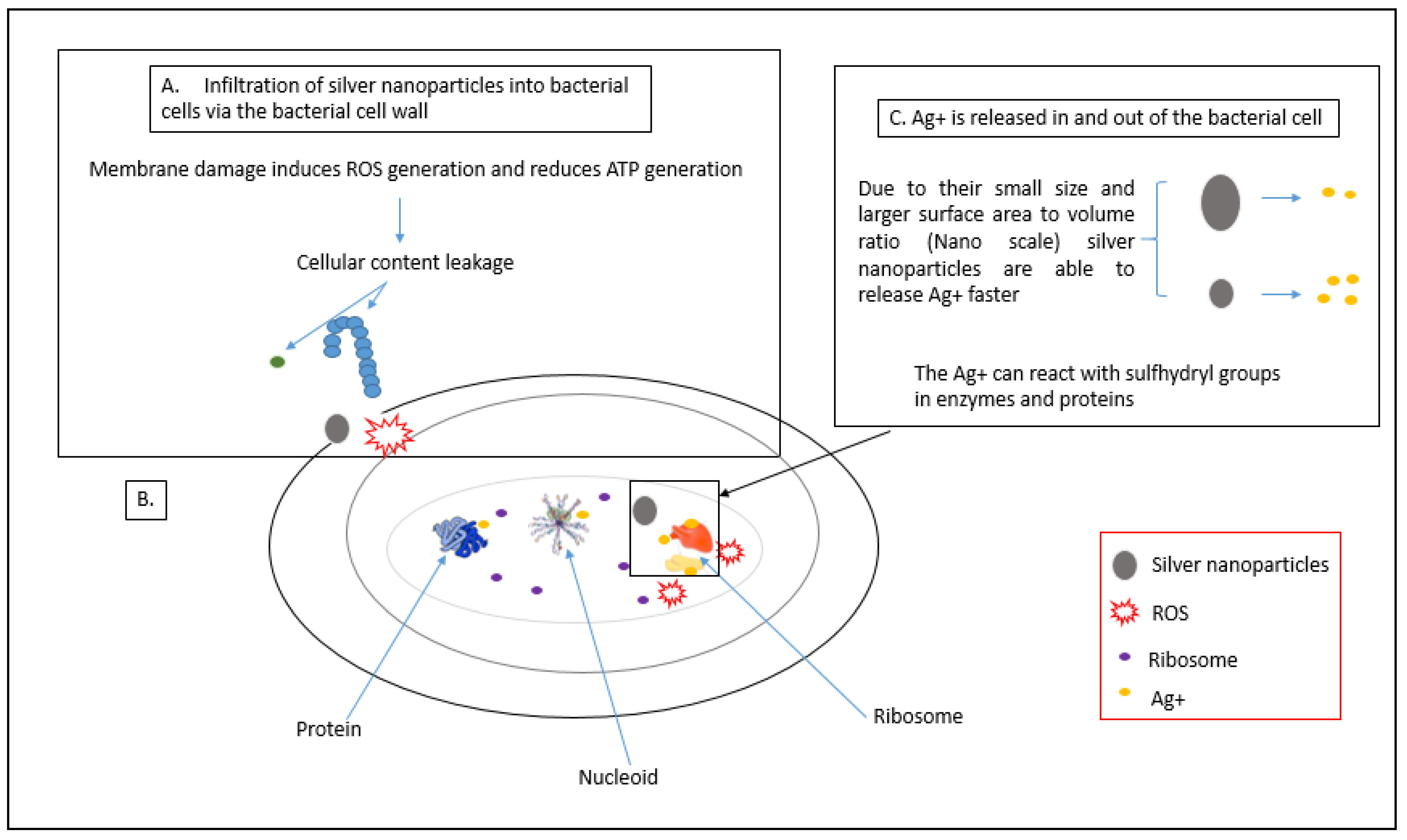
3.1. Production of Reactive Oxygen Species (ROS)
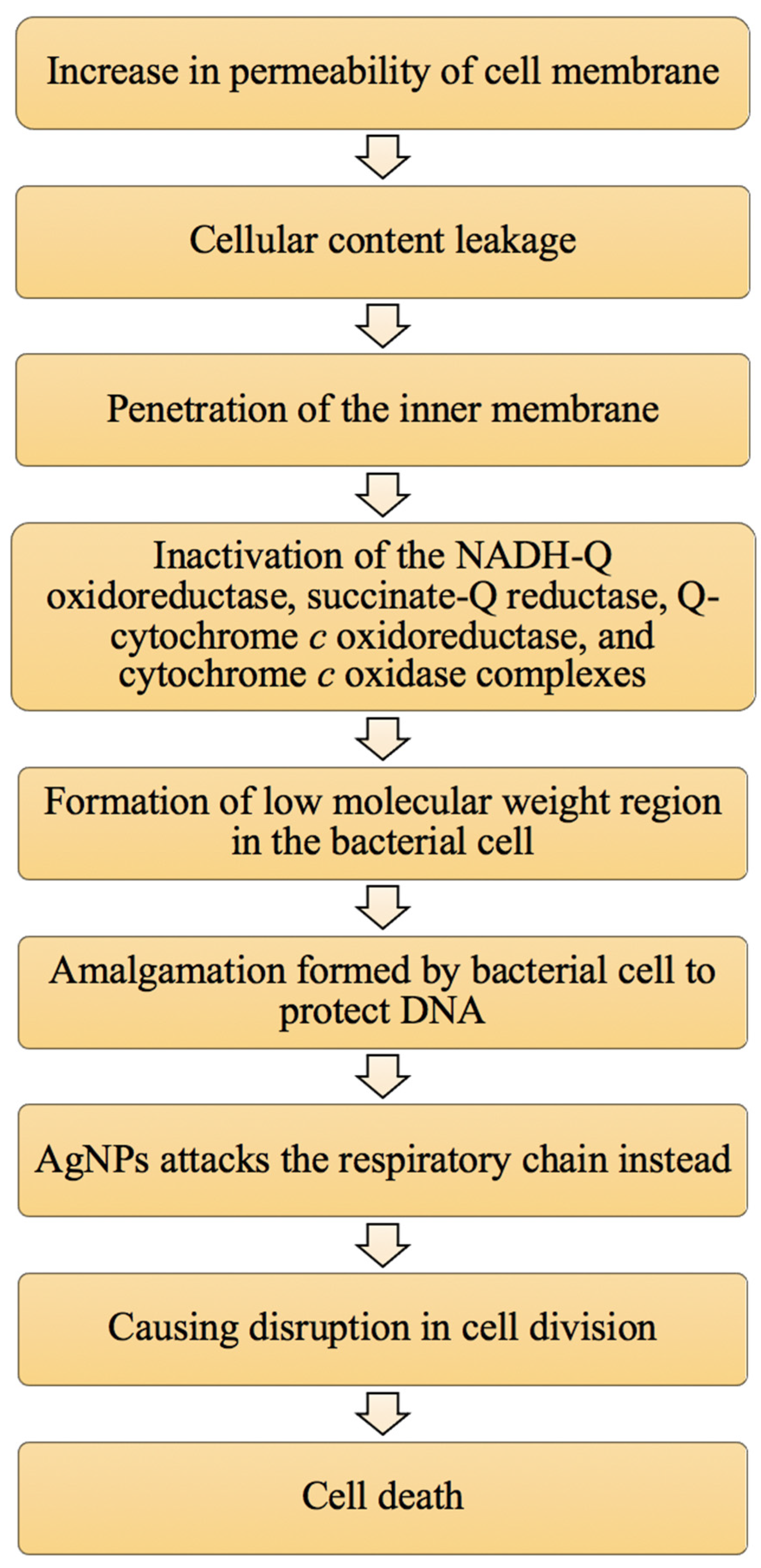
3.2. Attachment of AgNPs to the Cell Membrane of Bacterial Cells
3.3. Damage to Intracellular Components of Bacterial Cells
3.4. Inducing the Viable But Non-Culturable (VBNC) State
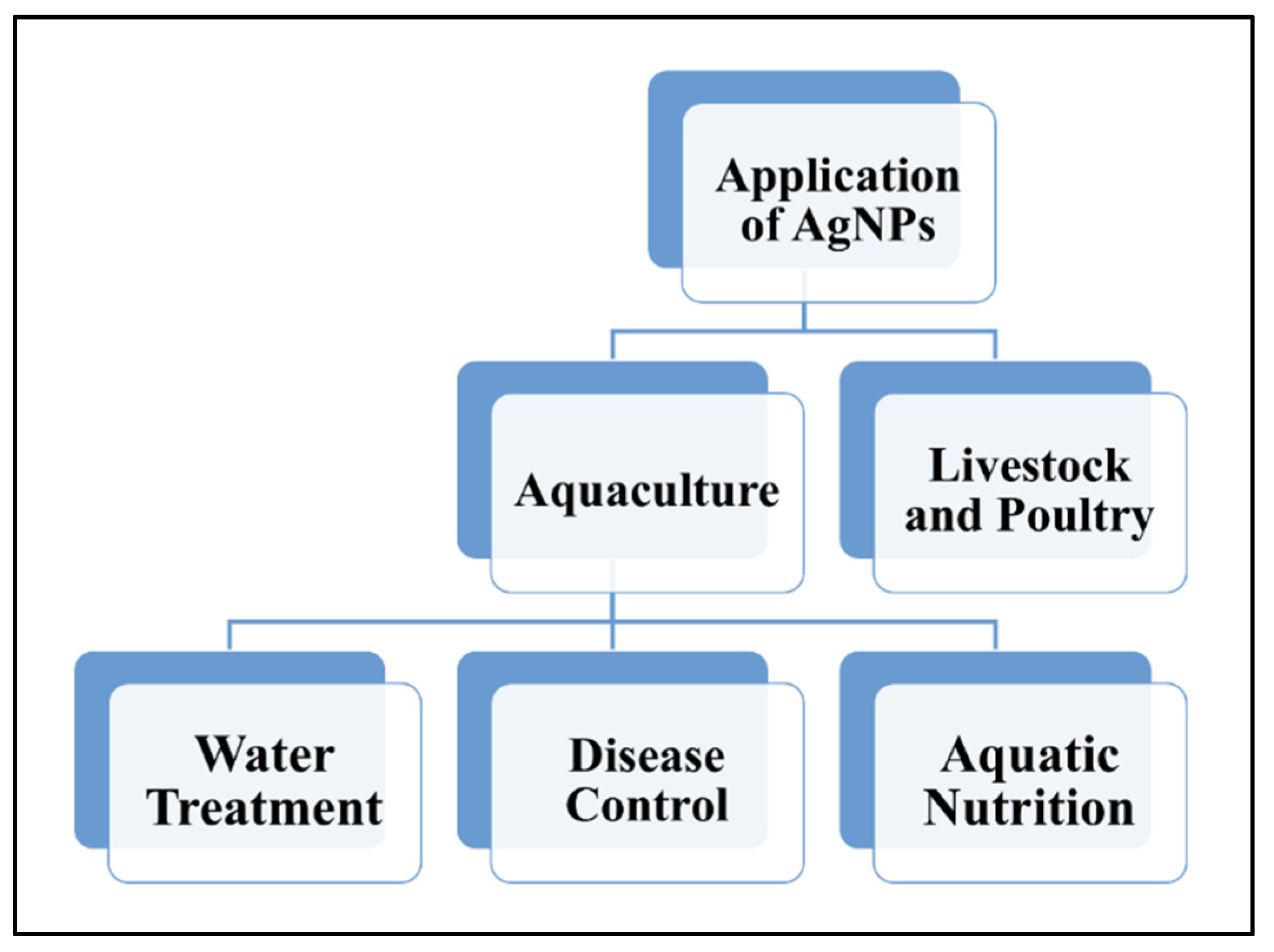
4. Application of AgNPs in Aquaculture, Livestock and Poultry Industries
4.1. Application of AgNPs in Aquaculture
4.1.1. AgNPs in Water Treatment
4.1.2. AgNPs in Disease Control
4.1.3. AgNPs in Aquatic Nutrition
4.2. Application of AgNPs in Livestock and Poultry
4.2.1. Application of Silver Nanoparticles in the Livestock Industry
4.2.2. Application of Silver Nanoparticles in the Poultry Industry
5. Toxicity of AgNPs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Wang, L.; Liu, L.; Hou, Y.; Li, L. Nanotechnology in agriculture, livestock, and aquaculture in China. A review. Agron. Sustain. Dev. 2015, 35, 369–400. [Google Scholar] [CrossRef] [Green Version]
- Ghidan, A.Y.; Al-Antary, T.M.; Awwad, A.M. Aphidicidal potential of green synthesized magnesium hydroxide nanoparticles using Olea europaea leaves extract. J. Agric. Biol. Sci. 2017, 12, 293–301. [Google Scholar]
- Ghidan, A.Y.; Al-Antary, T.M.; Salem, N.M.; Awwad, A.M. Facile green synthetic route to the zinc oxide (ZnONPs) nanoparticles: Effect on green peach aphid and antibacterial activity. J. Agric. Biol. Sci. 2017, 9, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Jiang, X.; Yang, L.; Landfester, K.; Mailänder, V.; Simmet, T.; Nienhaus, G.U. Nanoparticle interactions with live cells: Quantitative fluorescence microscopy of nanoparticle size effects. Beilstein J. Nanotechnol. 2014, 5, 2388–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef] [Green Version]
- El-Nour, K.M.A.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Castañón, G.A.; Niño, N.; Martinez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wen, J.; Xiong, X.; Hu, Y. Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ. Sci. Pollut. Res. 2016, 23, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.-H. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Soliman, H.; Elsayed, A.; Dyaa, A. Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egypt. J. Basic Appl. Sci. 2018, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Mathivanan, K.; Selva, R.; Chandirika, J.U.; Govindarajan, R.; Srinivasan, R.; Annadurai, G.; Duc, P.A. Biologically synthesized silver nanoparticles against pathogenic bacteria: Synthesis, calcination and characterization. Biocatal. Agric. Biotechnol. 2019, 22, 101373. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Li, H.; Duan, H.; Bi, Y.; Wu, D.; Yu, H. Optimized biosynthesis and antifungal activity of Osmanthus fragrans leaf extract-mediated silver nanoparticles. Int. J. Agric. Biol. 2017, 19, 668–672. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Walters, G.; Parkin, I.P. The incorporation of noble metal nanoparticles into host matrix thin films: Synthesis, characterisation and applications. J. Mater. Chem. 2009, 19, 574–590. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Wang, Y.; Xie, S. Shape-controlled synthesis of metal nanocrystals. MRS Bull. 2013, 38, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Helmlinger, J.; Heise, M.; Heggen, M.; Ruck, M.; Epple, M. A rapid, high-yield and large-scale synthesis of uniform spherical silver nanoparticles by a microwave-assisted polyol process. RSC Adv. 2015, 5, 92144–92150. [Google Scholar] [CrossRef]
- Actis, L.; Srinivasan, A.; Lopez-Ribot, J.; Ramasubramanian, A.K.; Ong, J. Effect of silver nanoparticle geometry on methicillin susceptible and resistant Staphylococcus aureus, and osteoblast viability. J. Mater. Sci. Mater. Med. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [Green Version]
- Raman, G.; Park, S.J.; Sakthivel, N.; Suresh, A.K. Physico-cultural parameters during AgNPs biotransformation with bactericidal activity against human pathogens. Enzym. Microb. Technol. 2017, 100, 45–51. [Google Scholar] [CrossRef]
- Mokhena, T.; Luyt, A. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312. [Google Scholar] [CrossRef]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.-M.; Ma, J.; Alvarez, P.J.J. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ. Sci. Technol. 2011, 45, 9003–9008. [Google Scholar] [CrossRef]
- Fadeel, B.; Ahlin, A.; Henter, J.I.; Orrenius, S.; Hampton, M.B. Involvement of caspases in neutrophil apoptosis: Regulation by reactive oxygen species. Blood 1998, 92, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahiminezhad, A.; Davaran, S.; Rasoul-Amini, S.; Barar, J.; Moghadam, M.; Ghasemi, Y. Synthesis, characterization and anti-listeria monocytogenes effect of amino acid coated magnetite nanoparticles. Curr. Nanosci. 2012, 8, 868–874. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Kouhpayeh, A.; Davaran, S.; Barar, J.; Ghasemi, Y. Impacts of amine functionalized iron oxide nanoparticles on HepG2 cell line. Curr. Nanosci. 2014, 11, 113–119. [Google Scholar] [CrossRef]
- Gurr, J.-R.; Wang, A.S.S.; Chen, C.-H.; Jan, K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Tasat, D.R.; Guglielmotti, M.; Cabrini, R. Effect of titanium dioxide on the oxidative metabolism of alveolar macrophages: An experimental study in rats. J. Biomed. Mater. Res. Part A 2005, 73, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.-I.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Jahromi, A.S.; Ghasemi, Y. Ancient and novel forms of silver in medicine and biomedicine. J. Adv. Med Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Matés, J.M.; Jiménez, F.M.S. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000, 32, 157–170. [Google Scholar] [CrossRef]
- Messner, K.; Imlay, J.A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 1999, 274, 10119–10128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K.; Dwivedi, P.; Singh, M.K.; Sundaram, S.; Gopal, R. Silver nanoparticles synthesized by pulsed laser ablation: As a potent antibacterial agent for human enteropathogenic Gram-positive and Gram-negative bacterial strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef]
- Nalwade, A.R.; Jadhav, A.A. Biosynthesis of silver nanoparticles using leaf extract of Datura alba Nees. and evaluation of their antibacterial activity. Arch. Appl. Sci. Res. 2013, 5, 45–49. [Google Scholar]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial characterization of silver nanoparticles against E. Coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar] [CrossRef]
- Li, J.J.; Muralikrishnan, S.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Li, J.; Rong, K.; Zhao, H.; Li, F.; Lu, Z.; Chen, R. Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013, 13, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob. Agents Chemother. 2014, 58, 5818–5830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.A.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Abadi, A.J.N.; Abasi, E.; Akbarzadeh, A. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res. (Stuttg) 2016, 67, 70–76. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J.D. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J. Biomed. Mater. Res. 2000, 53, 621–631. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Lin, K.-S.; Ke, W.-J.; Hsieh, C.-T.; Chiang, C.-L.; Tzou, D.-Y.; Liu, S.-T. The antimicrobial properties of silver nanoparticles in Bacillus subtilis are mediated by released Ag+ ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Das, S.; Jyoti, A.; Kaushik, S. Synergistic effect of silver nanoparticles with doxycycline against Klebsiella pneumoniae. Int. J. Pharm. Sci. 2016, 8, 183–186. [Google Scholar]
- Oliver, J.D. The viable but non-culturable state in bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, Y.; Gu, Q.; Li, W. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81. [Google Scholar] [CrossRef]
- Meneses, J.; Hamdan, A.; Del Carmen Monroy Dosta, M.; Mejía, J.; Bustos Martinez, J. Silver nanoparticles applications (AgNPS) in aquaculture. Int. J. Fish. Aquat. 2018, 6, 5–11. [Google Scholar]
- Martinez-Porchas, M.; Martinez-Cordova, L.R. World Aquaculture: Environmental Impacts and Troubleshooting Alternatives. Sci. World J. 2012, 2012, 389623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, T.V. R Aquaculture and the Environment, 2nd ed.; Blackwell Publishing Ltd.: Oxford, UK, 2008; p. 208. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Johari, S.A.; Kalbassi, M.R.; Soltani, M.; Yu, I.J. Toxicity comparison of colloidal silver nanoparticles in various life stages of rainbow trout (Oncorhynchus mykiss). Iran. J. Fish. Sci. 2013, 12, 76–95. [Google Scholar]
- Sarkheil, M.; Sourinejad, I.; Mirbakhsh, M.; Kordestani, D.; Johari, S.A. Application of silver nanoparticles immobilized on TEPA-Den-SiO2 as water filter media for bacterial disinfection in culture of Penaeid shrimp larvae. Aquac. Eng. 2016, 74, 17–29. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Ramasamy, P.; Chen, J.C. Antibacterial activity of silver nanoparticles (AgNps) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Lett. Appl. Microbiol. 2010, 50, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sivaramasamy, E.; Zhiwei, W.; Li, F.; Xiang, J. Enhancement of vibriosis resistance in Litopenaeus vannamei by supplementation of biomastered silver nanoparticles by Bacillus subtilis. J. Nanomed. Nanotechnol. 2016, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Barakat, K.M.; El-Sayed, H.S.; Gohar, Y.M. Protective effect of squilla chitosan–silver nanoparticles for Dicentrarchus labrax larvae infected with Vibrio anguillarum. Int. Aquat. Res. 2016, 8, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Daniel, S.C.G.K.; Sironmani, T.A.; Dinakaran, S. Nano formulations as curative and protective agent for fish diseases: Studies on red spot and white spot diseases of ornamental gold fish Carassius auratus. Int. J. Fish. Aquat. 2016, 4, 255–261. [Google Scholar]
- Rajeshkumar, S.; Venkatesan, C.; Sarathi, M.; Sarathbabu, V.; Thomas, J.; Basha, K.A.; Hameed, A.S. Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish. Immunol. 2009, 26, 429–437. [Google Scholar] [CrossRef]
- Juarez-Moreno, K.; Mejia-Ruiz, C.H.; Díaz, F.; Reyna-Verdugo, H.; Re, A.D.; Vazquez-Felix, E.F.; Sánchez-Castrejón, E.; Mota-Morales, J.D.; Pestryakov, A.; Bogdanchikova, N. Effect of silver nanoparticles on the metabolic rate, hematological response, and survival of juvenile white shrimp Litopenaeus vannamei. Chemosphere 2017, 169, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Meza, A.R.; Álvarez-Sánchez, A.R.; Romo-Quiñonez, C.R.; Barraza, A.; Magallón-Barajas, F.J.; Chávez-Sánchez, A.; García-Ramos, J.C.; Toledano-Magaña, Y.; Bogdanchikova, N.; Pestryakov, A.; et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish. Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef]
- Romo-Quiñonez, C.R.; Sanchez, A.R.A.; Álvarez-Ruiz, P.; Chávez-Sánchez, M.C.; Bogdanchikova, N.; Pestryakov, A.; Mejia-Ruiz, C.H. Evaluation of a new Argovit as an antiviral agent included in feed to protect the shrimp Litopenaeus vannamei against White Spot Syndrome Virus infection. PeerJ 2020, 8, e8446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rather, M.A.; Sharma, R.; Gupta, S.; Ferosekhan, S.; Ramya, V.L.; Jadhao, S.B. Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PLoS ONE 2013, 8, e57094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.; Farahmand, H.; Koshio, S.; Dorkoosh, F.; Elsabee, M.Z. Chitosan nanoparticle to carry vitamin C through the gastrointestinal tract and induce the non-specific immunity system of rainbow trout (Oncorhynchus mykiss). Carbohydr. Polym. 2011, 86, 142–146. [Google Scholar] [CrossRef]
- Fondevila, M. Potential Use of Silver Nanoparticles as an Additive in Animal Feeding. In Silver Nanoparticles; Perez, D.P., Ed.; InTech: London, UK, 2010; Available online: https://www.intechopen.com/books/silver-nanoparticles/potential-use-of-silver-nanoparticles-as-an-additive-in-animal-feeding (accessed on 13 July 2021).
- El-Gohary, F.A.; Abdel-Hafez, L.J.M.; Zakaria, A.I.; Shata, R.R.; Tahoun, A.; El-Mleeh, A.; Elfadl, E.A.A.; Elmahallawy, E.K. Enhanced antibacterial activity of silver nanoparticles combined with hydrogen peroxide against multidrug-resistant pathogens isolated from dairy farms and beef slaughterhouses in Egypt. Infect. Drug Resist. 2020, 13, 3485–3499. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, M.; Herrer, R.; Casallas, M.; Abecia, L.; Ducha, J. Silver nanoparticles as a potential antimicrobial additive for weaned pigs. Anim. Feed. Sci. Technol. 2009, 150, 259–269. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and copper nanoparticles—an alternative in future mastitis treatment and prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [Green Version]
- Sawosz, F.; Marta, G.; Marlena, Z.; Niemiec, T.; Boena, O.; Chwalibog, A. Nanoparticles of silver do not effect growth, development and DNA oxidative damage in chicken embryo. Arch. Geflugelkd. 2009, 73, 208–213. [Google Scholar]
- Sawosz, F.; Pineda, L.; Hotowy, A.; Hyttel, P.; Sawosz, E.; Szmidt, M.; Niemiec, T.; Chwalibog, A. Nano-nutrition of chicken embryos. The effect of silver nanoparticles and glutamine on molecular responses, and the morphology of pectoral muscle. Baltic J. Comp. Clin. Syst. Biol. 2012, 2, 29–45. [Google Scholar] [CrossRef]
- Raieszadeh, H.; Noaman, V.; Yadegari, M. Echocardiographic assessment of cardiac structural and functional indices in broiler chickens treated with silver nanoparticles. Sci. World J. 2013, 2013, 931432. [Google Scholar] [CrossRef] [PubMed]
- Chmielowiec-Korzeniowska, A.; Tymczyna, L.; Dobrowolska, M.; Banach, M.; Nowakowicz-Debek, B.; Bryl, M.; Drabik, A.; Tymczyna-Sobotka, M.; Kolejko, M. Silver (Ag) in tissues and eggshells, biochemical parameters and oxidative stress in chickens. Open Chem. 2015, 13, 1269–1274. [Google Scholar] [CrossRef]
- Kumar, I.; Bhattacharya, J.; Das, B.K.; Lahiri, P. Growth, serum biochemical, and histopathological responses of broilers administered with silver nanoparticles as a drinking water disinfectant. 3 Biotech 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Jamil, B.; Javed, R.; Qazi, A.S.; Syed, M.A. Nanomaterials: Toxicity, risk managment and public perception. In Nanomaterials: Ecotoxicity, Safety, and Public Perception; Rai, M., Biswas, J.K., Eds.; Springer: Cham, Switzerland, 2018; pp. 283–304. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [Green Version]



| Bacterial Strain | AgNP Size | Author |
|---|---|---|
| Escherichia coli | 7 nm | [13] |
| Oral pathogenic bacteria: Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Streptococcus mitis, Streptococcus mutans, Streptococcus sanguinis. Aerobic: Escherichia coli | 5 nm | [14] |
| Escherichia coli | 55 nm | [15] |
| Foodborne pathogenic bacteria: Bacillus cereus ATCC 13061, Listeria monocytogenes ATCC 19115, Staphylococcus aureus ATCC 49444, Escherichia coli ATCC 43890, and Salmonella Typhimurium ATCC 43174 Candida species: C. albicans KACC 30003 and KACC 30062, C. glabrata KBNO6P00368, C. geochares KACC 30061, and C. saitoana KACC 41238 | 31.18 nm, 35.74 nm and 69.14 nm | [16] |
| Gram-positive bacteria: Streptococcus sp., Bacillus sp., Staphylococcus sp. Gram-negative bacteria: Shigella sp., Escherichia coli, Pseudomonas aeruginosa and Klebsiella sp. Fungus: Candida sp. | 8.8 nm to 21.4 nm | [17] |
| Pseudomonas fluorescens MTCC 1749, Proteus mirabilis MTCC 425, Escherichia coli MTCC 1610, Bacillus cereus and Staphylococcus aureus MTCC 2940 | 18 nm to 100 nm and 49 nm to 153 nm | [18] |
| Gram-negative :Escherichia coli O157:H7 Gram-positive: Listeria monocytogenes | 8 nm to 15 nm | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Silva, C.; Nawawi, N.M.; Abd Karim, M.M.; Abd Gani, S.; Masarudin, M.J.; Gunasekaran, B.; Ahmad, S.A. The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries. Animals 2021, 11, 2097. https://doi.org/10.3390/ani11072097
De Silva C, Nawawi NM, Abd Karim MM, Abd Gani S, Masarudin MJ, Gunasekaran B, Ahmad SA. The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries. Animals. 2021; 11(7):2097. https://doi.org/10.3390/ani11072097
Chicago/Turabian StyleDe Silva, Catrenar, Norazah Mohammad Nawawi, Murni Marlina Abd Karim, Shafinaz Abd Gani, Mas Jaffri Masarudin, Baskaran Gunasekaran, and Siti Aqlima Ahmad. 2021. "The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries" Animals 11, no. 7: 2097. https://doi.org/10.3390/ani11072097
APA StyleDe Silva, C., Nawawi, N. M., Abd Karim, M. M., Abd Gani, S., Masarudin, M. J., Gunasekaran, B., & Ahmad, S. A. (2021). The Mechanistic Action of Biosynthesised Silver Nanoparticles and Its Application in Aquaculture and Livestock Industries. Animals, 11(7), 2097. https://doi.org/10.3390/ani11072097







