Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances
Abstract
:Simple Summary
Abstract
1. Introduction
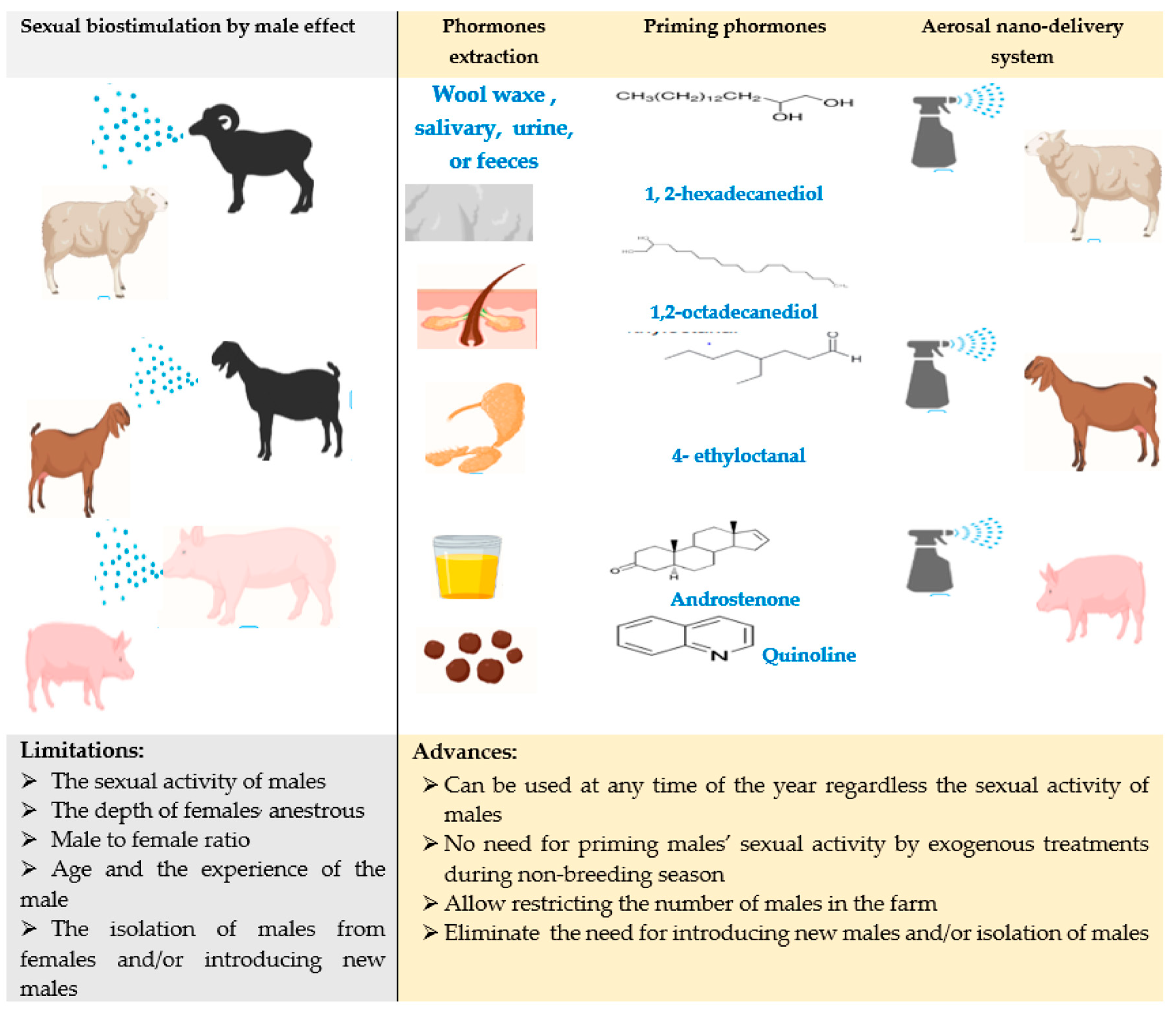
2. Biological Stimulation Management, Male Effect
2.1. Challenges of Male Effect Applications
2.2. Nanotechnology Approaches for Developing Male Effect Procedure
3. Hormonal Based-Treatments
3.1. Importance and Challenges of Hormonal Based-Treatments
3.2. Nanotechnology Approaches for Developing Hormonal Based-Treatments
4. Nutritional Management
4.1. Importance and Challenges of Nutritional Management
4.2. Nanotechnology Approaches for Improving Nutritional Management Outputs
5. Management of Reproductive-Related Diseases
5.1. Importance and Challenges of Antibiotic Applications
5.2. Nanotechnology Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Olynk, N.; Wolf, C. Economic analysis of reproductive management strategies on US commercial dairy farms. J. Dairy Sci. 2008, 91, 4082–4091. [Google Scholar] [CrossRef]
- Smith, M.F.; Geisert, R.D.; Parrish, J.J. Reproduction in domestic ruminants during the past 50 yr: Discovery to application. J. Anim. Sci. 2018, 96, 2952–2970. [Google Scholar] [CrossRef]
- Caraviello, D.; Weigel, K.; Fricke, P.; Wiltbank, M.; Florent, M.; Cook, N.; Nordlund, K.; Zwald, N.; Rawson, C. Survey of management practices on reproductive performance of dairy cattle on large US commercial farms. J. Dairy Sci. 2006, 89, 4723–4735. [Google Scholar] [CrossRef] [Green Version]
- Delgadillo, J.A.; Martin, G.B. Alternative methods for control of reproduction in small ruminants: A focus on the needs of grazing industries. Anim. Front. 2015, 5, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Hashem, N.M.; Hassanein, E.M.; Simal-Gandara, J. Improving reproductive performance and health of mammals using honeybee products. Antioxidants 2021, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.M.; Gonzalez-Bulnes, A. State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals 2020, 10, 840. [Google Scholar] [CrossRef]
- Hassanein, E.; Hashem, N.; El-Azrak, K.; Gonzalez-Bulnes, A.; Hassan, G.; Salem, M. Efficiency of GnRH–Loaded Chitosan Nanoparticles for Inducing LH Secretion and Fertile Ovulations in Protocols for Artificial Insemination in Rabbit Does. Animals 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Tejada, L.M.; Meza-Herrera, C.A.; Rivas-Munoz, R.; Rodriguez-Martinez, R.; Carrillo, E.; Mellado, M.; Veliz-Deras, F.G. Appetitive and consummatory sexual behaviors of rams treated with exogenous testosterone and exposed to anestrus dorper ewes: Efficacy of the male effect. Arch. Sex Behav. 2017, 46, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, R. Socio-sexual signalling and gonadal function: Opportunities for reproductive management in domestic ruminants. Soc. Reprod. Fertil. Suppl. 2007, 64, 207. [Google Scholar] [CrossRef]
- Chasles, M.; Chesneau, D.; Moussu, C.; Delgadillo, J.A.; Chemineau, P.; Keller, M. Sexually active bucks are efficient to stimulate female ovulatory activity during the anestrous season also undertemperate latitudes. Anim. Reprod. Sci. 2016, 168, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.C.; Gutiérrez, J.P.; Hernández, W.M.; Mancera, A.V.; Crispín, R.H. Obtención, evaluación y manipulación del semen de verraco en una unidad de producción mexicana. Rev. Vet. 2016, 26, 69–74. [Google Scholar] [CrossRef]
- Hashem, N.; El-Zarkouny, S. Effect of short-term supplementation with rumen-protected fat during the late luteal phase on reproduction and metabolism of ewes. J. Anim. Physiol. Anim. Nutr. 2014, 98, 65–71. [Google Scholar] [CrossRef]
- Yang, X.; Ouyang, W.; Sun, J.; Li, X. Post-antibiotic effect of Amoxicillin nanoparticles against main pathogenic bacteria of Bovine mastitis in vitro. J. Northwest A F Univ. Nat. Sci. Ed. 2009, 37, 1–6. [Google Scholar]
- Cerbu, C.; Kah, M.; White, J.C.; Astete, C.E.; Sabliov, C.M. Fate of biodegradable engineered nanoparticles used in veterinary medicine as delivery systems from a one health perspective. Molecules 2021, 26, 523. [Google Scholar] [CrossRef] [PubMed]
- Algharib, S.A.; Dawood, A.; Xie, S. Nanoparticles for treatment of bovine Staphylococcus aureus mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef] [Green Version]
- Osama, E.; El-Sheikh, S.M.; Khairy, M.H.; Galal, A.A. Nanoparticles and their potential applications in veterinary medicine. J. Adv. Vet. Res. 2020, 10, 268–273. [Google Scholar]
- Signoret, J.; Lindsay, D. The male effect in domestic mammals: Effect on LH secretion and ovulation—importance of olfactory cues. Olfaction Endocr. Regul. 1982, 63–72. [Google Scholar]
- Murata, K.; Tamogami, S.; Itou, M.; Ohkubo, Y.; Wakabayashi, Y.; Watanabe, H.; Okamura, H.; Takeuchi, Y.; Mori, Y. Identification of an olfactory signal molecule that activates the central regulator of reproduction in goats. Curr. Biol. 2014, 24, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Silva, J.C.; Burnett, T.A.; Souto, P.F.M.P.; Filho, P.C.B.G.; Pereira, L.C.; Araujo, M.V.; Moura, M.T.; Oliveira, M.A.L. Progesterone (P4), luteinizing hormone (LH) levels and ovarian activity in postpartum Santa Inês ewes subject to a male effect. Arch. Anim. Breed. 2017, 60, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Meza-Herrera, C.A.; Cano-Villegas, O.; Flores-Hernandez, A.; Veliz-Deras, F.G.; Calderon-Leyva, G.; Guillen-Munoz, J.M.; de la Pena, C.G.; Rosales-Nieto, C.A.; Macias-Cruz, U.; Avendano-Reyes, L. Reproductive outcomes of anestrous goats supplemented with spineless Opuntia megacantha Salm-Dyck protein-enriched cladodes and exposed to the male effect. Trop. Anim. Health Prod. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Rekwot, P.; Ogwu, D.; Oyedipe, E.; Sekoni, V. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 2001, 65, 157–170. [Google Scholar] [CrossRef]
- De Santiago-Miramontes, M.; Rivas-Muñoz, R.; Muñoz-Gutiérrez, M.; Malpaux, B.; Scaramuzzi, R.; Delgadillo, J. The ovulation rate in anoestrous female goats managed under grazing conditions and exposed to the male effect is increased by nutritional supplementation. Anim. Reprod. Sci. 2008, 105, 409–416. [Google Scholar] [CrossRef]
- Delgadillo, J.A.; Gelez, H.; Ungerfeld, R.; Hawken, P.A.; Martin, G.B. The ‘male effect’in sheep and goats—Revisiting the dogmas. Behav. Brain Res. 2009, 200, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Milton, J.; Davidson, R.; Hunzicker, G.B.; Lindsay, D.; Blache, D. Natural methods for increasing reproductive efficiency in small ruminants. Anim. Reprod. Sci. 2004, 82, 231–245. [Google Scholar] [CrossRef]
- Hashem, N.; El-Zarkouny, S.; Taha, T.; Abo-Elezz, Z. Effect of season, month of parturition and lactation on estrus behavior and ovarian activity in Barki x Rahmani crossbred ewes under subtropical conditions. Theriogenology 2011, 75, 1327–1335. [Google Scholar] [CrossRef]
- Rosa, H.; Bryant, M. The ‘ram effect’as a way of modifying the reproductive activity in the ewe. Small Rumin. Res. 2002, 45, 1–16. [Google Scholar] [CrossRef]
- Zarazaga, L.; Gatica, M.; Hernández, H.; Gallego-Calvo, L.; Delgadillo, J.; Guzmán, J. The isolation of females from males to promote a later male effect is unnecessary if the bucks used are sexually active. Theriogenology 2017, 95, 42–47. [Google Scholar] [CrossRef]
- Zarazaga, L.; Gatica, M.; Hernández, H.; Chemineau, P.; Delgadillo, J.; Guzmán, J. Photoperiod-treated bucks are equal to melatonin-treated bucks for inducing reproductive behaviour and physiological functions via the “male effect” in Mediterranean goats. Anim. Reprod. Sci. 2019, 202, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zarazaga, L.A.; Gatica, M.C.; Hernandez, H.; Keller, M.; Chemineau, P.; Delgadillo, J.A.; Guzman, J.L. The reproductive response to the male effect of 7- or 10-month-old female goats is improved when photostimulated males are used. Animal 2019, 13, 1658–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, J.; Velázquez, H.; Duarte, G.; Bedos, M.; Hernández, H.; Keller, M.; Chemineau, P.; Delgadillo, J. Reducing exposure to long days from 75 to 30 days of extra-light treatment does not decrease the capacity of male goats to stimulate ovulatory activity in seasonally anovulatory females. Domest. Anim. Endocrinol. 2014, 48, 119–125. [Google Scholar] [CrossRef]
- Knight, T.; Lynch, P. Source of ram pheromones that stimulate ovulation in the ewe. Anim. Reprod. Sci. 1980, 3, 133–136. [Google Scholar] [CrossRef]
- Izard, M. Pheromones and reproduction in domestic animals. Pheromones Reprod. Mamm. 1983, 253. [Google Scholar]
- McGlone, J.J.; Devaraj, S.; Garcia, A. A novel boar pheromone mixture induces sow estrus behaviors and reproductive success. Appl. Anim. Behav. Sci. 2019, 219, 104832. [Google Scholar] [CrossRef]
- Dugasa, H.L.; Williams, R.O., III. Nanotechnology for pulmonary and nasal drug delivery. Nanotechnol. Drug Deliv. Vol. Two Nano Eng. Strateg. Nanomed. Against Sev. Dis. 2016, 102. [Google Scholar]
- Pamungkas, F.A.; Sianturi, R.S.G.; Wina, E.; Kusumaningrum, D.A. Chitosan nanoparticle of hCG (Human Chorionic Gonadotrophin) hormone in increasing induction of dairy cattle ovulation. Indones. J. Anim. Vet. Sci. 2016, 21, 34–40. [Google Scholar]
- Kekan, P.M.; Ingole, S.D.; Sirsat, S.D.; Bharucha, S.V.; Kharde, S.D.; Nagvekar, A.S. The role of pheromones in farm animals—A review. Agric. Rev. 2017, 38. [Google Scholar] [CrossRef]
- Archunan, G. Reproductive enhancement in buffalo: Looking at urinary pheromones and hormones. Iran. J. Vet. Res. 2020, 21, 163. [Google Scholar]
- Higgins, H.M.; Ferguson, E.; Smith, R.F.; Green, M.J. Using hormones to manage dairy cow fertility: The clinical and ethical beliefs of veterinary practitioners. PLoS ONE 2013, 8, e62993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, N.; El-Azrak, K.; El-Din, A.N.; Taha, T.; Salem, M. Effect of GnRH treatment on ovarian activity and reproductive performance of low-prolific Rahmani ewes. Theriogenology 2015, 83, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.; El-Zarkouny, S.; Taha, T.; Abo-Elezz, Z. Oestrous response and characterization of the ovulatory wave following oestrous synchronization using PGF2α alone or combined with GnRH in ewes. Small Rumin. Res. 2015, 129, 84–87. [Google Scholar] [CrossRef]
- Hashem, N.; Aboul-Ezz, Z. Effects of a single administration of different gonadotropins on day 7 post-insemination on pregnancy outcomes of rabbit does. Theriogenology 2018, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-C.; Li, H.-Y.; Li, X.-Y.; Yu, K.; Deng, S.-L.; Tian, L. Protective effects of melatonin on male fertility preservation and reproductive system. Cryobiology 2020, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.V.; Cortell, C.; Mocé, E.; Marco-Jiménez, F.; Joly, T.; Vicente, J. Effect of recombinant gonadotropins on embryo quality in superovulated rabbit does and immune response after repeated treatments. Theriogenology 2009, 72, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Forcada, F.; Ait Amer-Meziane, M.; Abecia, J.A.; Maurel, M.C.; Cebrian-Perez, J.A.; Muino-Blanco, T.; Asenjo, B.; Vazquez, M.I.; Casao, A. Repeated superovulation using a simplified FSH/eCG treatment for in vivo embryo production in sheep. Theriogenology 2011, 75, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Herve, V.; Roy, F.; Bertin, J.; Guillou, F.; Maurel, M.C. Antiequine chorionic gonadotropin (eCG) antibodies generated in goats treated with eCG for the induction of ovulation modulate the luteinizing hormone and follicle-stimulating hormone bioactivities of eCG differently. Endocrinology 2004, 145, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, E.; Dupuy, L.; Bouillon, C.; Casteret, S.; Maurel, M.-C. Modulation of gonadotropins activity by antibodies. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Roy, F.; Maurel, M.-C.; Combes, B.; Vaiman, D.; Cribiu, E.P.; Lantier, I.; Pobel, T.; Delétang, F.; Combarnous, Y.; Guillou, F. The negative effect of repeated equine chorionic gonadotropin treatment on subsequent fertility in Alpine goats is due to a humoral immune response involving the major histocompatibility complex. Biol. Reprod. 1999, 60, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Manteca Vilanova, X.; De Briyne, N.; Beaver, B.; Turner, P.V. Horse welfare during equine Chorionic Gonadotropin (eCG) production. Animals 2019, 9, 1053. [Google Scholar] [CrossRef] [Green Version]
- Rathbone, M.J.; Burke, C.R. Controlled release intravaginal veterinary drug delivery. In Long Acting Animal Health Drug Products; Springer: New York, NY, USA, 2013; pp. 247–270. [Google Scholar]
- De Graaff, W.; Grimard, B. Progesterone-releasing devices for cattle estrus induction and synchronization: Device optimization to anticipate shorter treatment durations and new device developments. Theriogenology 2018, 112, 34–43. [Google Scholar] [CrossRef]
- Fernández-Serrano, P.; Casares-Crespo, L.; Viudes-de-Castro, M.P. Chitosan–dextran sulphate nanoparticles for Gn RH release in rabbit insemination extenders. Reprod. Domest. Anim. 2017, 52, 72–74. [Google Scholar] [CrossRef] [Green Version]
- Casares-Crespo, L.; Fernández-Serrano, P.; Viudes-De-Castro, M.P. Protection of GnRH analogue by chitosan-dextran sulfate nanoparticles for intravaginal application in rabbit artificial insemination. Theriogenology 2018, 116, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Hashem, N.; Sallam, S. Reproductive performance of goats treated with free gonadorelin or nanoconjugated gonadorelin at estrus. Domest. Anim. Endocrinol. 2020, 71, 106390. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Sharma, R.; Gupta, S.; Ferosekhan, S.; Ramya, V.; Jadhao, S.B. Chitosan-nanoconjugated hormone nanoparticles for sustained surge of gonadotropins and enhanced reproductive output in female fish. PLoS ONE 2013, 8, e57094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.E.; Medeiros, E.S.; Cardozo, L.; Voll, F.; Madureira, E.H.; Mattoso, L.H.C.; Assis, O.B.G. Development of poly (lactic acid) nanostructured membranes for the controlled delivery of progesterone to livestock animals. Mater. Sci. Eng. C 2013, 33, 844–849. [Google Scholar] [CrossRef]
- Helbling, I.M.; Ibarra, J.C.; Luna, J.A. The optimization of an intravaginal ring releasing progesterone using a mathematical model. Pharm. Res. 2014, 31, 795–808. [Google Scholar] [CrossRef]
- Fogolari, O.; Felippe, A.C.; Leimann, F.V.; Gonçalves, O.H.; Sayer, C.; Araújo, P.H.H.D. Method validation for progesterone determination in poly (Methyl methacrylate) nanoparticles synthesized via miniemulsion polymerization. Int. J. Polym. Sci. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Remião, M.H.; Lucas, C.G.; Domingues, W.B.; Silveira, T.; Barther, N.N.; Komninou, E.R.; Basso, A.C.; Jornada, D.S.; Beck, R.C.R.; Pohlmann, A.R. Melatonin delivery by nanocapsules during in vitro bovine oocyte maturation decreased the reactive oxygen species of oocytes and embryos. Reprod. Toxicol. 2016, 63, 70–81. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Wang, L.; Liu, Y.; Wu, W.; Zhong, C.; Zhang, Q.; Yang, J. Preparation, characterization and in vitro evaluation of melatonin-loaded porous starch for enhanced bioavailability. Carbohydr. Polym. 2018, 202, 125–133. [Google Scholar] [CrossRef]
- Siahdasht, F.N.; Farhadian, N.; Karimi, M.; Hafizi, L. Enhanced delivery of melatonin loaded nanostructured lipid carriers during in vitro fertilization: NLC formulation, optimization and IVF efficacy. RSC Adv. 2020, 10, 9462–9475. [Google Scholar] [CrossRef] [Green Version]
- Helbling, I.M.; Busatto, C.A.; Fioramonti, S.A.; Pesoa, J.I.; Santiago, L.; Estenoz, D.A.; Luna, J.A. Preparation of TPP-crosslinked chitosan microparticles by spray drying for the controlled delivery of progesterone intended for estrus synchronization in cattle. Pharm. Res. 2018, 35, 1–15. [Google Scholar] [CrossRef]
- Combarnous, Y.; Salesse, R.; Garnier, J. Physico-chemical properties of pregnant mare serum gonadotropin. Biochim. et Biophys. Acta BBA Protein Struct. 1981, 667, 267–276. [Google Scholar] [CrossRef]
- STEWART, F.; Allen, W.; Moor, R.M. Pregnant mare serum gonadotrophin: Ratio of follicle-stimulating hormone and luteinizing hormone activities measured by radioreceptor assay. J. Endocrinol. 1976, 71, 371–382. [Google Scholar] [CrossRef]
- Santos-Jimenez, Z.; Guillen-Gargallo, S.; Encinas, T.; Berlinguer, F.; Veliz-Deras, F.G.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Use of propylene-glycol as a cosolvent for gnrh in synchronization of estrus and ovulation in sheep. Animals 2020, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Izquierdo, A. Best Practices in Animal Reproduction: Impact of Nutrition on Reproductive Performance Livestock. J. Adv. Dairy Res. 2016, 4, 152. [Google Scholar] [CrossRef] [Green Version]
- Cannas, A.; Tedeschi, L.O.; Atzori, A.S.; Lunesu, M.F. How can nutrition models increase the production efficiency of sheep and goat operations? Anim. Front. 2019, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bai, Y.; Fu, S.; Wu, L.; Xu, C.; Xia, C. Follicular fluid proteomic profiling of dairy cows with anestrus caused by negative energy balance. Ital. J. Anim. Sci. 2021, 20, 650–663. [Google Scholar] [CrossRef]
- Scaramuzzi, R.; Martin, G. The importance of interactions among nutrition, seasonality and socio-sexual factors in the development of hormone-free methods for controlling fertility. Reprod. Dom. Anim. 2008, 43, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adva. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Peñagaricano, F.; Souza, A.H.; Carvalho, P.D.; Driver, A.M.; Gambra, R.; Kropp, J.; Hackbart, K.S.; Luchini, D.; Shaver, R.D.; Wiltbank, M.C. Effect of maternal methionine supplementation on the transcriptome of bovine preimplantation embryos. PLoS ONE 2013, 8, e72302. [Google Scholar] [CrossRef] [Green Version]
- Abdelnour, S.A.; Al-Gabri, N.A.; Hashem, N.M.; Gonzalez-Bulnes, A. Supplementation with proline improves haemato-biochemical and reproductive indicators in male rabbits affected by environmental heat-stress. Animals 2021, 11, 373. [Google Scholar] [CrossRef]
- Wiltbank, M.; Shaver, R.; Toledo, M.; Carvalho, P.; Baez, G.; Follendorf, T.; Lobos, N.; Luchini, D.; Souza, A. Potential benefits of feeding methionine on reproductive efficiency of lactating dairy cows. Four State Dairy Nutr. Manag. 2014, 4, 19–26. [Google Scholar]
- Toledo, M.Z.; Baez, G.M.; Garcia-Guerra, A.; Lobos, N.E.; Guenther, J.N.; Trevisol, E.; Luchini, D.; Shaver, R.D.; Wiltbank, M.C. Effect of feeding rumen-protected methionine on productive and reproductive performance of dairy cows. PLoS ONE 2017, 12, e0189117. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, W.; Santos, J.E.; Staples, C.R. Dietary manipulations to improve embryonic survival in cattle. Theriogenology 2011, 76, 1619–1631. [Google Scholar] [CrossRef]
- Sturmey, R.; Reis, A.; Leese, H.; McEvoy, T. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Dom. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef]
- Castro, T.; Martinez, D.; Isabel, B.; Cabezas, A.; Jimeno, V. Vegetable oils rich in polyunsaturated fatty acids supplementation of dairy cows’ diets: Effects on productive and reproductive performance. Animals 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, N.S.; Hashem, N.M.; Morsy, A.S.; Abo-Elezz, Z.R. Effects of Organic Selenium on the Physiological Response, Blood Metabolites, Redox Status, Semen Quality, and Fertility of Rabbit Bucks Kept Under Natural Heat Stress Conditions. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Zereu, G. Factors affecting feed intake and its regulation mechanisms in ruminants a review. Int. J. Livest. Res. 2016, 6, 19–40. [Google Scholar]
- Hippen, A.R.; DeFrain, J.M.; Linke, P.L. Glycerol and other energy sources for metabolism and production of transition dairy cows. In Proceedings of the Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 29–30 January 2008. [Google Scholar]
- Shin, J.; Wang, D.; Kim, S.; Adesogan, A.; Staples, C. Effects of feeding crude glycerin on performance and ruminal kinetics of lactating Holstein cows fed corn silage-or cottonseed hull-based, low-fiber diets. J. Dairy Sci. 2012, 95, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.; Cieslak, A.; Pers-Kamczyc, E.; Szczechowiak, J.; Kowalczyk, D.; Szumacher-Strabel, M. A nanoemulsified form of oil blends positively affects the fatty acid proportion in ruminal batch cultures. J. Dairy Sci. 2016, 99, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Hackmann, T.J.; Firkins, J.L. Maximizing efficiency of rumen microbial protein production. Front. Microbiol. 2015, 6, 465. [Google Scholar] [CrossRef] [Green Version]
- Hammon, D.; Holyoak, G.; Dhiman, T. Association between blood plasma urea nitrogen levels and reproductive fluid urea nitrogen and ammonia concentrations in early lactation dairy cows. Anim. Reprod. Sci. 2005, 86, 195–204. [Google Scholar] [CrossRef]
- Boerman, J.; Lock, A. Effect of unsaturated fatty acids and triglycerides from soybeans on milk fat synthesis and biohydrogenation intermediates in dairy cattle. J. Dairy Sci. 2014, 97, 7031–7042. [Google Scholar] [CrossRef] [Green Version]
- Gawad, R.; Fellner, V. Evaluation of glycerol encapsulated with alginate and alginate-chitosan polymers in gut environment and its resistance to rumen microbial degradation. Asian Australas. J. Anim. Sci. 2019, 32, 72. [Google Scholar] [CrossRef]
- Kachuee, R.; Abdi-Benemar, H.; Mansoori, Y.; Sánchez-Aparicio, P.; Seifdavati, J.; Elghandour, M.M.; Guillén, R.J.; Salem, A.Z. Effects of sodium selenite, L-selenomethionine, and selenium nanoparticles during late pregnancy on selenium, zinc, copper, and iron concentrations in Khalkhali Goats and their kids. Biol. Trace Elem. Res. 2019, 191, 389–402. [Google Scholar] [CrossRef]
- Jahanbin, R.; Yazdanshenas, P.; Amin Afshar, M.; Mohammadi Sangcheshmeh, A.; Varnaseri, H.; Chamani, M.; Nazaran, M.H.; Bakhtiyarizadeh, M.R. Effect of zinc nano-complex on bull semen quality after freeze-thawing process. Anim. Prod. 2015, 17, 371–380. [Google Scholar]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.; Hassan, M.A. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127. [Google Scholar] [CrossRef]
- Shahin, M.A.; Khalil, W.A.; Saadeldin, I.M.; Swelum, A.A.-A.; El-Harairy, M.A. Comparison between the effects of adding vitamins, trace elements, and nanoparticles to shotor extender on the cryopreservation of dromedary camel epididymal spermatozoa. Animals 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, J.; Casal, S.; de Jorge Páscoa, R.N.M.; Van Dorpe, I.; Fonseca, A.J.M.; Cabrita, A.R.J.; Neves, A.R.; Reis, S. Applying nanotechnology to increase the rumen protection of amino acids in dairy cows. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Choi, Y.-J.; Kim, J.-H. Antibacterial efficacy of silver nanoparticles on endometritis caused by Prevotella melaninogenica and Arcanobacterum pyogenes in dairy cattle. Int. J. Mol. Sci. 2018, 19, 1210. [Google Scholar] [CrossRef] [Green Version]
- Vallejo-Timaran, D.A.; Arango-Sabogal, J.C.; Reyes-Velez, J.; Maldonado-Estrada, J.G. Postpartum uterine diseases negatively impact the time to pregnancy in grazing dairy cows from high-altitude tropical herds. Prev. Vet. Med. 2020, 185, 105202. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, R.; Vázquez, P.; Ferre, I.; Ortega-Mora, L.M. Treatment of toxoplasmosis and neosporosis in farm ruminants: State of knowledge and future trends. Curr. Top. Med. Chem. 2018, 18, 1304–1323. [Google Scholar] [CrossRef]
- Zhou, K.; Li, C.; Chen, D.; Pan, Y.; Tao, Y.; Qu, W.; Liu, Z.; Wang, X.; Xie, S. A review on nanosystems as an effective approach against infections of Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 7333–7347. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.E.; Christensen, H.; Aarestrup, F.M. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 57, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Piotr, S.; Marta, S.; Aneta, F.; Barbara, K.; Magdalena, Z. Antibiotic resistance in Staphylococcus aureus strains isolated from cows with mastitis in the eastern Poland and analysis of susceptibility of resistant strains to alternative non-antibiotic agents: Lysostaphin, nisin and polymyxin B. J. Vet. Med Sci. 2013. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholipourmalekabadi, M.; Mobaraki, M.; Ghaffari, M.; Zarebkohan, A.; Omrani, V.F.; Urbanska, A.M.; Seifalian, A. Targeted drug delivery based on gold nanoparticle derivatives. Cur. Pharmac. Des. 2017, 23, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Cerbu, C.; Astete, C.E.; Louie, S.M.; Sabliov, C.; Rodrigues, D.F. Enrofloxacin-impregnated PLGA nanocarriers for efficient therapeutics and diminished generation of reactive oxygen species. ACS Appl. Nano Mat. 2019, 2, 5035–5043. [Google Scholar] [CrossRef]
- El-Zawawy, L.A.; El-Said, D.; Mossallam, S.F.; Ramadan, H.S.; Younis, S.S. Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Experime. Parasitol. 2015, 149, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Shubar, H.M.; Lachenmaier, S.; Heimesaat, M.M.; Lohman, U.; Mauludin, R.; Mueller, R.H.; Fitzner, R.; Borner, K.; Liesenfeld, O. SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood–brain barriers. J. Drug Target. 2011, 19, 114–124. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhu, L.; Xie, S.; Dong, Z.; Wang, Y.; Zhou, W. Enhancement of antibacterial activity of tilmicosin against Staphylococcus aureus by solid lipid nanoparticles in vitro and in vivo. Vet. J. 2012, 191, 115–120. [Google Scholar] [CrossRef]
- Mozafari, M.; Torkaman, S.; Karamouzian, F.M.; Rasti, B.; Baral, B. Antimicrobial applications of nanoliposome encapsulated silver nanoparticles: A potential strategy to overcome bacterial resistance. Curr. Nanosci. 2021, 17, 26–40. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Barra, F.; Casarin, J.; Cromi, A.; Raffaelli, R.; Uccella, S.; Franchi, M.; Ghezzi, F.; Ferrero, S. Novel drug delivery methods for improving efficacy of endometriosis treatments. Expert Opin. Drug Deliv. 2020, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chakravarty, B.; Chaudhury, K. Nanoparticle-Assisted Combinatorial Therapy for Effective Treatment of Endometriosis. J. Biomed. Nanotechnol. 2015, 11, 789–804. [Google Scholar] [CrossRef]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcon Godoy, C.; Cayupe Rivas, B.; Gonzalez-Casanova, J.; Rojas-Gomez, D.; Caro Fuentes, N. Antimicrobial and antibiofilm capacity of chitosan nanoparticles against wild type strain of Pseudomonas sp. isolated from milk of cows diagnosed with bovine mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Das, C.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Govindaraju, K.; Joselin, J.M.; Baalamurugan, J. Antibacterial activity of silver nanoparticles (biosynthesis): A short review on recent advances. Biocatal. Agric. Biotechnol. 2020, 101593. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef] [Green Version]
- Radzikowski, D.; Kalińska, A.; Ostaszewska, U.; Gołębiewski, M. Alternative solutions to antibiotics in mastitis treatment for dairy cows-a review. Anim. Sci. Pap. Rep. 2020, 38, 117–133. [Google Scholar]
- Cardozo, V.F.; Lancheros, C.A.; Narciso, A.M.; Valereto, E.C.; Kobayashi, R.K.; Seabra, A.B.; Nakazato, G. Evaluation of antibacterial activity of nitric oxide-releasing polymeric particles against Staphylococcus aureus and Escherichia coli from bovine mastitis. Int. J. Pharm. 2014, 473, 20–29. [Google Scholar] [CrossRef]
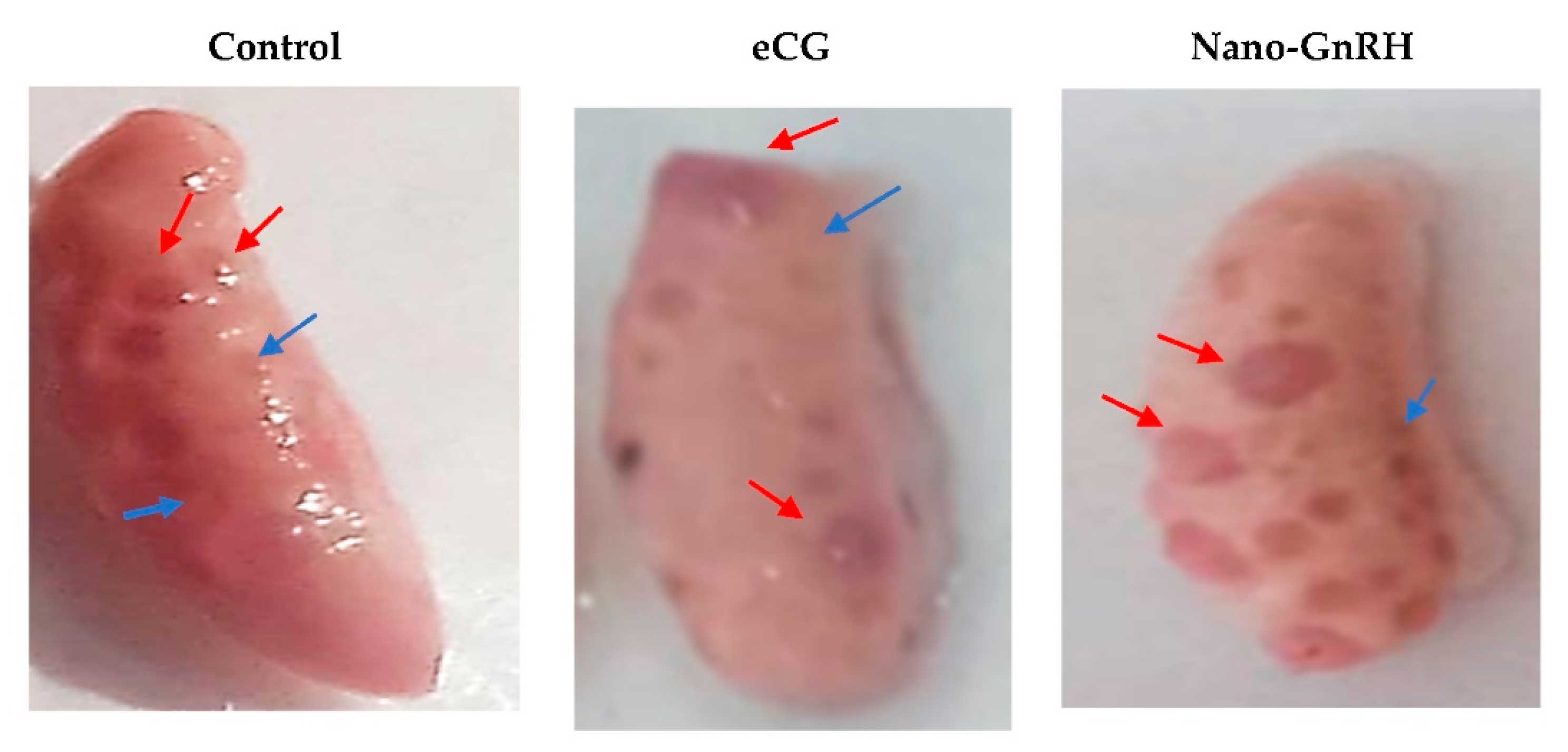
| Figure | Technique | Particle Characteristics | Expected Advances |
|---|---|---|---|
| GnRH-chitosan-TPP NPs [7] | Ionic-gelation | Size = 212 nm, PdI = 0.295, Zp = 8.0 mV, EE = 90% |
|
| GnRH-chitosan-TPP NPs [53] | Size = 93.91 nm, PdI = 0.302, Zp = 11.6mV, EE = 91.2% | ||
| GnRH-chitosan-dextran sulfate NPs [51] | Ionic-gelation | EE = 40−50% | |
| hCG-chitosan-TPP NPs [35] | Ionic-gelation | - | |
| P4-chitosan-TPP-Tween 80 [61] | Spray-drying | Size = 1 and 7 μm, EE = 69–75% |
|
| P4-polymethyl-methacrylate-nanospheres [57] | Miniemulsion polymerization | size = 150–200 nm, EE > 69% | |
| P4-polymethyl-methacrylate-nanocapsules [57] | size = 240–300 nm, EE > 90% | ||
| P4-polylactic acid NPs [55] | Solution blow spinning | Size = 289–441 nm | |
| Melatonin-loaded lipid-core Nps [58] | Interfacial deposition of polymer | size = 168 nm PdI = 0.08 |
|
| Melatonin loaded-lipid (olive oil) NPs [60] | Hot homogenization-ultrasonication | Size = 119nm, PdI = 0.09, EE = 94% |
|
| Formula | Technique | Particle Characteristics | Expected Advances |
|---|---|---|---|
| Zinc oxide NPs [89] | Commercial product | Size = 30.92 nm Zp = 32.16 mV |
|
| Selenium oxide NPs [89] | Commercial product | Size = 78.47 nm Zp =−20.36 mV | |
| Selenium oxide NPs [86] | Chemical reduction method using ascorbic acid and acacia gum | Size = 45.00 nm |
|
| Fish oil or soy oil -in-water NPs Soy oil-fish oil or rapeseed-fish oil-in-water NPs [81] | Nanoemulsion | - |
|
| Solid lipid-lysine NPs [90] | Ultrasonic processor | Size = 200–500 nm Zp = < −30 mV EE = 40−90% |
|
| Alginate-chitosan-glycerol NPs [85] | Ionic-gelation | Size = 3 mm EE = 78.1% |
|
| Type of Drug | Formula | Technique | Particle Characteristics | Drug Activity | Usage |
|---|---|---|---|---|---|
| Antibiotic [99] | Enrofloxacin- poly lactic-co-glycolic acid NPs | - | Size = 102 nm PdI = 0.095 Zp = −32 mV | Antimicrobial agent against Staphylococcus aureus, Escherichia coli | Endometritis and mastitis treatment |
| Antibiotic [102] | Tilmicosin-loaded hydrogenated castor oil NPs | Hot homogenization and ultrasonication | Size = 343 nm PdI = 0.33 Zp = 7.9 mV EE = 60.4% | Antimicrobial agent against Staphylococcus aureus | Mastitis treatment |
| Antibiotic [100] | Triclosan-loaded liposome NPs | Dehydration-rehydration | Size = 53.3 nm EE = 90% | Antimicrobial agent against Toxoplasma gondii | Toxoplasmosis treatment |
| Antibiotic [101] | Atovaquone-poloxamer 188 - sodium dodecyl sulfate | - | - | Antimicrobial agent against Toxoplasma gondii | Toxoplasmosis treatment |
| Nitric oxide (NO) [110] | NO-alginate-chitosan NO-chitosan-TPP | - | Size= 270–375 nm PdI=0.27–0.31 Zp = 16−17 mV | Antimicrobial agent against Staphylococcus aureus, Escherichia coli | Mastitis treatment |
| Metal [91] | Silver NPs | Biosynthesis by apigenin | Size = 10 nm | Antimicrobial agent against Prevotella melaninogenica and Arcanobacterium pyogenes | Antibiotic alternative for endometritis treatment |
| Metal [108] | Silver NPs | Biosynthesis by quercetin | Size = 20 nm Zp= 37.7mV | Antimicrobial agent against Staphylococcus aureus and Pseudomonas aeruginosa | Antibiotic alternative for mastitis treatment |
| Chitosan [106] | Chitosan-TPP Nps | Ionotropic gelation | Size = 19.1 nm PdI = 0.41 Zp = 49.9 mV Yield particle = 92.8% | Antimicrobial agent against Pseudomona sp. | Antibiotic alternative for mastitis treatment |
| Antibiotic + polyphenol [105] | Poly(lactic-co-glycolic) acid-epigallocatechin gallate- doxycycline Nps Singh et al., 2015 | Modified double emulsion solvent evaporation/extraction technique | Size = 176 to 211 nm PdI = 0.124 to 0.466 EE= 78.5 to 86.3% | Anti-inflammatory agent | Assisted-endometritis therapy |
| Essential oil 1 | Oregano oil Nps | - | - | Antimicrobial agent against Staphylococcus aureus, Escherichia coli, Streptococcus spp. | Antibiotic alternative for endometritis treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, N.M.; Gonzalez-Bulnes, A. Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances. Animals 2021, 11, 1932. https://doi.org/10.3390/ani11071932
Hashem NM, Gonzalez-Bulnes A. Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances. Animals. 2021; 11(7):1932. https://doi.org/10.3390/ani11071932
Chicago/Turabian StyleHashem, Nesrein M., and Antonio Gonzalez-Bulnes. 2021. "Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances" Animals 11, no. 7: 1932. https://doi.org/10.3390/ani11071932
APA StyleHashem, N. M., & Gonzalez-Bulnes, A. (2021). Nanotechnology and Reproductive Management of Farm Animals: Challenges and Advances. Animals, 11(7), 1932. https://doi.org/10.3390/ani11071932







