A Serological Survey of Paratuberculosis in the Polish European Bison (Bison bonasus) Population in 2018–2021
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
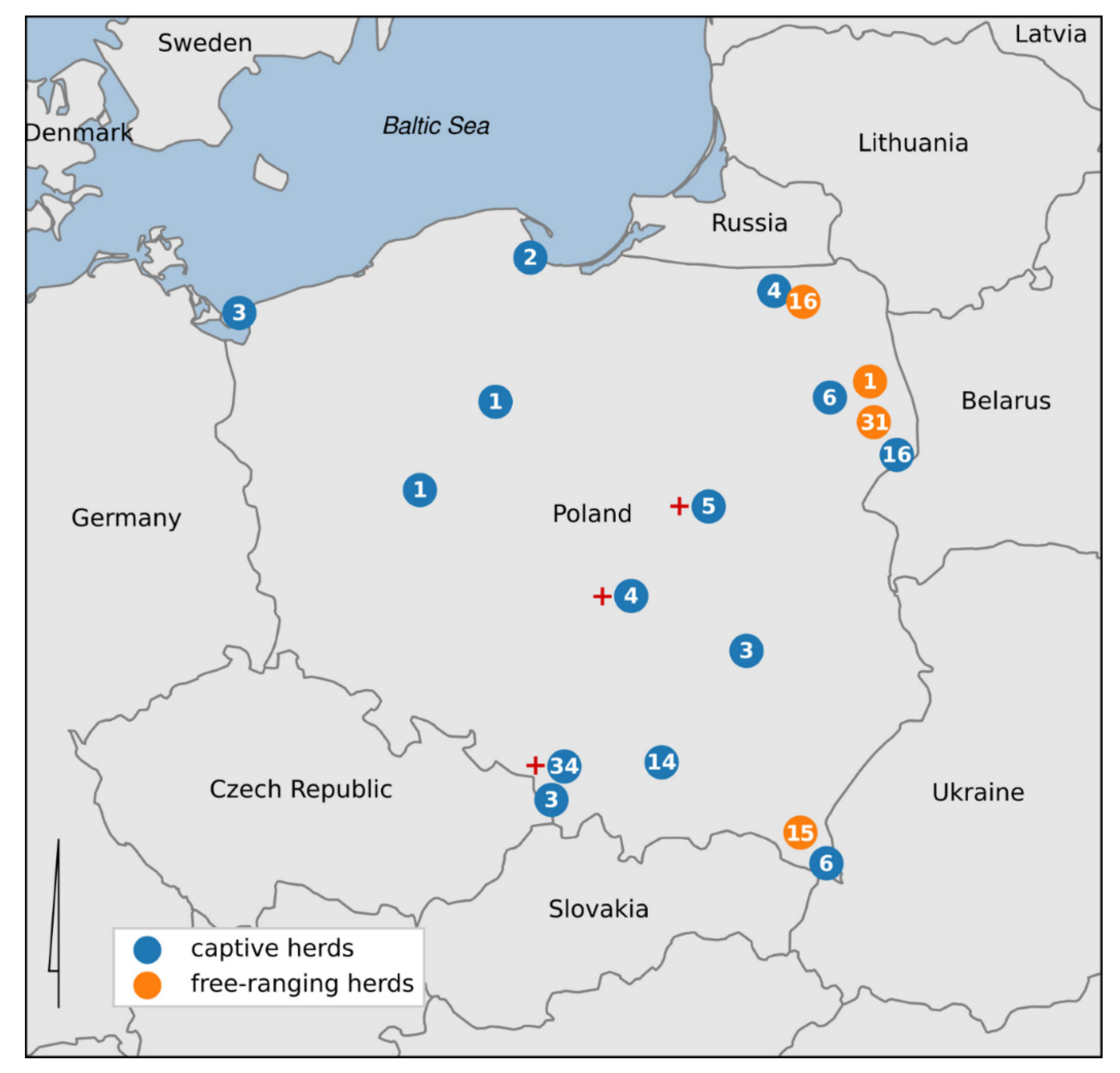
2.1. Animal Sampling
2.2. Diagnostic Analaysis
3. Results
Paratuberculosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Klich, D.; Olech, W.; Łopucki, R.; Danik, K. Community attitudes to the European bison Bison bonasus in areas where its reintroduction is planned and in areas with existing populations in northeastern Poland. Eur. J. Wildl. Res. 2018, 64, 61. [Google Scholar] [CrossRef]
- Klich, D.; Łopucki, R.; Perlińska-Teresiak, M.; Lenkiewicz-Bardzińska, A.; Olech, W. Human–Wildlife Conflict: The Human Dimension of European Bison Conservation in the Bieszczady Mountains (Poland). Animals 2021, 11, 503. [Google Scholar] [CrossRef]
- Pyziel, A.M.; Demiaszkiewicz, A.W. Coccidia (Apicomplexa: Eimeriidae) of the lowland European bison (Bison bonasus bonasus L.) in Poland. Vet. Parasitol. 2014, 202, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Klich, D.; Kitowski, I.; Łopucki, R.; Wiącek, D.; Olech, W. Essential differences in the mineral status of free-ranging European bison Bison bonasus populations in Poland: The effect of the anthroposphere and lithosphere. Sci. Total Environ. 2021, 757, 143926. [Google Scholar] [CrossRef]
- Kwiecień, E.; Stefańska, I.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Moroz, A.; Olech, W.; Spinu, M.; Binek, M.; Rzewuska, M. Trueperella pyogenes Isolates from Livestock and European Bison (Bison bonasus) as a Reservoir of Tetracycline Resistance Determinants. Antibiotics 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Olech, W.; Klich, D.; Perzanowski, K. Development of a new Action Plan for the European bison. Oryx 2019, 53, 214. [Google Scholar] [CrossRef]
- Didkowska, A.; Orłowska, B.; Krajewska-Wędzina, M.; Augustynowicz-Kopeć, E.; Brzezińska, S.; Żygowska, M.; Anusz, K. Microbiological and molecular monitoring for bovine tuberculosis in the Polish population of European bison (Bison bonasus). Ann. Agric. Environ. Med. 2020. [Google Scholar] [CrossRef]
- Koets, A.P.; Eda, S.; Sreevatsan, S. The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: Where time and place matter. Vet. Res. 2015, 46, 61. [Google Scholar] [CrossRef]
- Okuni, J.B.; Hansen, S.; Eltom, K.H.; Eltayeb, E.; Amanzada, A.; Omega, J.A.; Czerny, C.P.; Abd El Wahed, A.; Ojok, L. Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa. Microorganisms 2020, 8, 1007. [Google Scholar] [CrossRef]
- Čurlík, J.; Lazár, P.; Iglódyová, A.; Barbušinová, E.; Šmiga, Ľ.; Novotný, J.; Mojžišová, J.; Ondrejková, A.; Hromada, R.; Konjević, D.; et al. Detection of Mycobacterium avium subsp. paratuberculosis in Slovakian wildlife. Pol. J. Vet. Sci. 2020, 23, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.J.; Smith, L.A.; Stevenson, K.; Davidson, R.S.; Marion, G.; Hutchings, M.R. Infection of non-ruminant wildlife by Mycobacterium avium subsp. paratuberculosis. In Paratuberculosis: Organism, Disease, Control, 2nd ed.; CABI: Wallingford, UK, 2020; pp. 200–212. [Google Scholar] [CrossRef]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Raffo, E.; Steuer, P.; Monti, G.; Salgado, M. Effect of Mycobacterium avium subsp. paratuberculosis (MAP) infection on the diagnostic accuracy for Mycobacterium bovis (M. bovis) infection under field conditions in cattle belonging to low M. bovis prevalence herds. Trop. Anim. Health Prod. 2017, 49, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Krzysiak, M.K.; Jabłoński, A.; Iwaniak, W.; Krajewska, M.; Kęsik-Maliszewska, J.; Larska, M. Seroprevalence and risk factors for selected respiratory and reproductive tract pathogen exposure in European bison (Bison bonasus) in Poland. Vet. Microbiol. 2018, 215, 57–65. [Google Scholar] [CrossRef]
- Didkowska, A.; Krajewska-Wędzina, M.; Bielecki, W.; Brzezińska, S.; Augustynowicz-Kopeć, E.; Olech, W.; Anusz, K.; Sridhara, A.A.; Johnathan-Lee, A.; Elahi, R.; et al. Antibody responses in European bison (Bison bonasus) naturally infected with Mycobacterium caprae. Vet. Microbiol. 2021, 253, 108952. [Google Scholar] [CrossRef] [PubMed]
- Krzysiak, M.K.; Dudek, K.; Krajewska, M.; Bednarek, D.; Szulowski, K. Serological studies to determine the occurrence of Johne’s disease and mycoplasma infection in the Northern-East Polish population of European bison (Bison bonasus). Pol. J. Vet. Sci. 2014, 17, 721–723. [Google Scholar] [CrossRef]
- Münster, P.; Fechner, K.; Völkel, I.; von Buchholz, A.; Czerny, C.P. Distribution of Mycobacterium avium subsp. paratuberculosis in a German zoological garden determined by IS900 semi-nested and quantitative real-time PCR. Vet. Microbiol. 2013, 163, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Roller, M.; Hansen, S.; Knauf-Witzens, T.; Oelemann, W.M.R.; Czerny, C.P.; Abd El Wahed, A.; Goethe, R. Mycobacterium avium subspecies paratuberculosis infection in Zoo animals: A review of susceptibility and disease process. Front. Vet. Sci. 2020, 7, 572724. [Google Scholar] [CrossRef]
- Carta, T.; Álvarez, J.; Pérez de la Lastra, J.M.; Gortázar, C. Wildlife and paratuberculosis: A review. Res. Vet. Sci. 2013, 94, 191–197. [Google Scholar] [CrossRef]
- Ellingson, J.L.; Stabel, J.R.; Radcliff, R.P.; Whitlock, R.H.; Miller, J.M. Detection of Mycobacterium avium subspecies paratuberculosis in free-ranging bison (Bison bison) by PCR. Mol. Cell. Probes. 2005, 19, 219–225. [Google Scholar] [CrossRef]
- Forde, T.; De Buck, J.; Elkin, B.; Kutz, S.; van der Meer, F.; Orsel, K. Contrasting results of culture-dependent and molecular analyses of Mycobacterium avium subsp. paratuberculosis from wood bison. Appl. Environ. Microbiol. 2013, 79, 4448–4454. [Google Scholar] [CrossRef]
- Whittington, R.J.; Marsh, I.B.; Whitlock, R.H. Typing of IS 1311 polymorphisms confirms that bison (Bison bison) with paratuberculosis in Montana are infected with a strain of Mycobacterium avium subsp. paratuberculosis distinct from that occurring in cattle and other domesticated livestock. Mol. Cell. Probes 2001, 15, 139–145. [Google Scholar] [CrossRef]
- Girling, S.; Pizzi, R.; Harley, J.; Richardson, D.; Philbey, A. Diagnosis and management of an outbreak of Mycobacterium avium subspecies paratuberculosis in a wildlife park in Scot-land. In Proceedings of the International Conference on Diseases of Zoo and Wild Animals/Annual Conference of the European Association of Zoo and Wildlife Veterinarians, Lisbon, Portugal, 1–4 June 2011. [Google Scholar]
- Meng, Q.F.; Li, Y.; Yang, F.; Yao, G.Z.; Qian, A.D.; Wang, W.L.; Cong, W. Seroprevalence and risk factors of Mycobacterium avium subspecies paratuberculosis infection in domestic sika deer in China. Trop. Anim. Health Prod. 2015, 47, 999–1003. [Google Scholar] [CrossRef]
- Reyes-García, R.; Pérez-de-la-Lastra, J.M.; Vicente, J.; Ruiz-Fons, F.; Garrido, J.M.; Gortázar, C. Large-scale ELISA testing of Spanish red deer for paratuberculosis. Vet. Immunol. Immunopathol. 2008, 124, 75–81. [Google Scholar] [CrossRef]
- Tryland, M.; Olsen, I.; Vikøren, T.; Handeland, K.; Arnemo, J.M.; Tharaldsen, J.; Djønne, B.; Josefsen, T.D.; Reitan, L.J. Serologic survey for antibodies against Mycobacterium avium subsp. paratuberculosis in free-ranging cervids from Norway. J. Wildl. Dis. 2004, 40, 32–41. [Google Scholar] [CrossRef][Green Version]
- Szteyn, J.; Wiszniewska-Łaszczych, A. Seroprevalence of Mycobacterium avium subsp. paratuberculosis infection in dairy herds in Zuławy, Poland. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 397–400. [Google Scholar] [PubMed]
- Szteyn, J.; Liedtke, K.; Wiszniewska-Łaszczych, A.; Wysok, B.; Wojtacka, J. Isolation and molecular typing of Mycobacterium avium subsp. paratuberculosis from faeces of dairy cows. Pol. J. Vet. Sci. 2020, 23, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Krasińska, M.; Krasiński, Z. Żubr Monografia Przyrodnicza, 2nd ed.; Drukarnia im. A. Półtawskiego: Kielce, Poland, 2017; pp. 186–187. [Google Scholar]
- Pruvot, M.; Forde, T.L.; Steele, J.; Kutz, S.J.; De Buck, J.; van der Meer, F.; Orsel, K. The modification and evaluation of an ELISA test for the surveillance of Mycobacterium avium subsp. paratuberculosis infection in wild ruminants. BMC Vet. Res. 2013, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, A.; De Juan, L.; Bezos, J.; Alvarez, J.; Romero, B.; Lozano, F.; Paramio, J.L.; Lopez-Sanchez, J.; Mateos, A.; Dominguez, L. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 2006, 37, 593–606. [Google Scholar] [CrossRef][Green Version]
| Population | Age | Sex | ||
|---|---|---|---|---|
| Female | Male | Unknown | ||
| Captive herds | ≤1 year | 13 | 14 | - |
| 2–5 years | 22 | 23 | - | |
| ≥6 years | 10 | 10 | - | |
| Unknown | 6 | 4 | - | |
| Total | 51 | 51 | - | |
| Free-range herds | ≤1 year | - | 2 | - |
| 2–5 years | 9 | 5 | - | |
| ≥6 years | 23 | 20 | - | |
| Unknown | 2 | 1 | 1 | |
| Total | 34 | 28 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didkowska, A.; Ptak, M.; Opałczyńska, K.; Wojciechowska, M.; Olech, W.; Anusz, K. A Serological Survey of Paratuberculosis in the Polish European Bison (Bison bonasus) Population in 2018–2021. Animals 2021, 11, 2094. https://doi.org/10.3390/ani11072094
Didkowska A, Ptak M, Opałczyńska K, Wojciechowska M, Olech W, Anusz K. A Serological Survey of Paratuberculosis in the Polish European Bison (Bison bonasus) Population in 2018–2021. Animals. 2021; 11(7):2094. https://doi.org/10.3390/ani11072094
Chicago/Turabian StyleDidkowska, Anna, Marcin Ptak, Katarzyna Opałczyńska, Marlena Wojciechowska, Wanda Olech, and Krzysztof Anusz. 2021. "A Serological Survey of Paratuberculosis in the Polish European Bison (Bison bonasus) Population in 2018–2021" Animals 11, no. 7: 2094. https://doi.org/10.3390/ani11072094
APA StyleDidkowska, A., Ptak, M., Opałczyńska, K., Wojciechowska, M., Olech, W., & Anusz, K. (2021). A Serological Survey of Paratuberculosis in the Polish European Bison (Bison bonasus) Population in 2018–2021. Animals, 11(7), 2094. https://doi.org/10.3390/ani11072094







