Growth Performance, Biochemical Blood Indices, and Large Intestine Physiology of Rats Fed Diets with Alfalfa Protein-Xanthophyll Concentrate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Nutrient Analyses
2.3. Blood Biochemistry
2.4. Measurement of Digesta pH and SCFA Analysis
2.5. β-Glucuronidase Activity Assay
2.6. HPLC Analysis of Amines
2.7. Large Intestine Histology
2.8. Measurement of Mucus Layer Thickness
2.9. Total RNA Isolation
2.10. Analysis of Mucin Gene Expression
2.11. Statistical Analyses
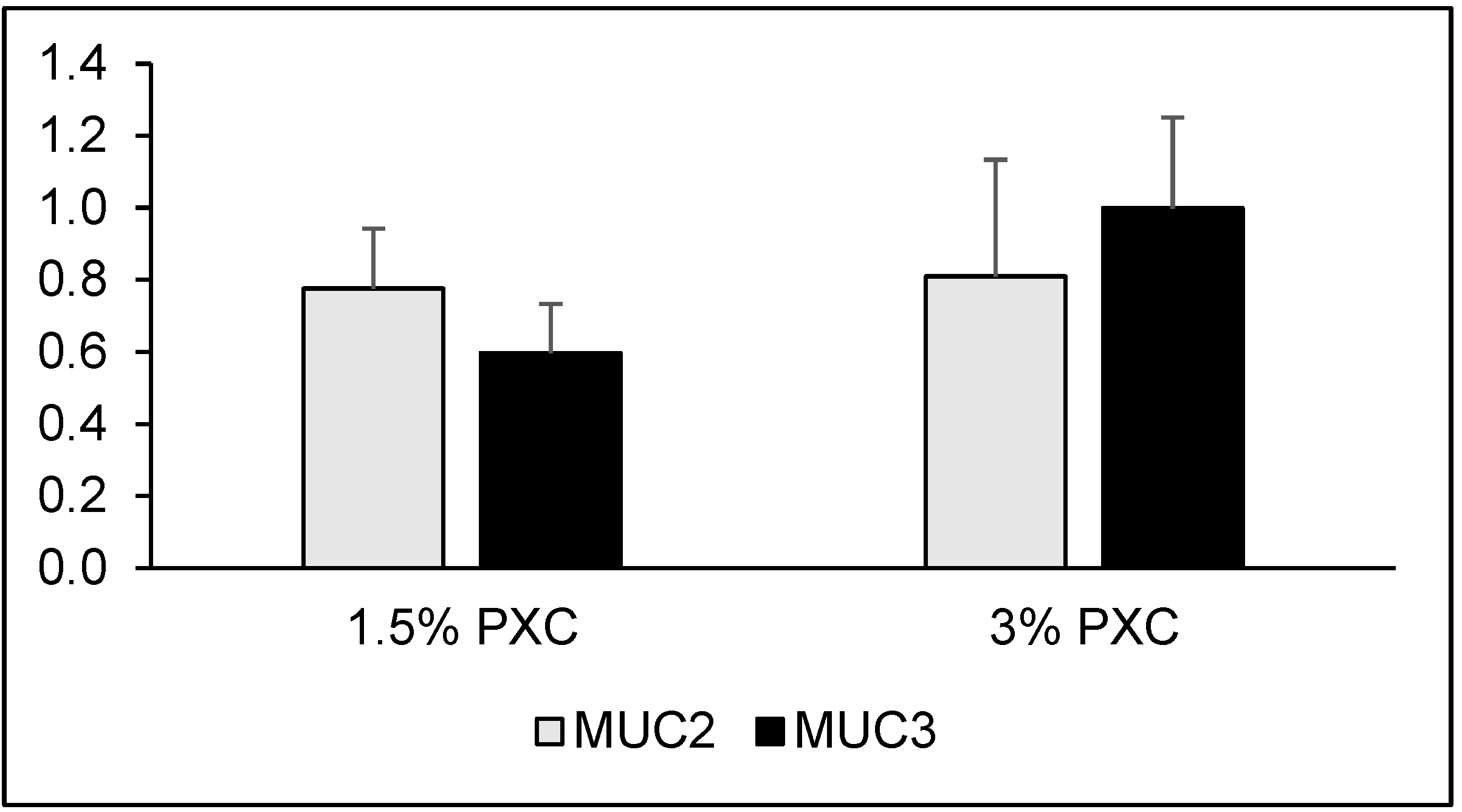
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zanin, V. A New Nutritional Idea for Man: Lucerne Leaf Concentrate; APEF (Association pour la Promotion des Extraits Foliaires en Nutrition): Paris, France, 1998; pp. 1–35. [Google Scholar]
- Gaweł, E.; Grzelak, M. The effect of a protein-xanthophyll concentrate from alfalfa (phytobiotic) on animal production—A current review. Ann. Anim. Sci. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Myer, R.O.; Cheeke, P.R. Utilization of alfalfa meal and alfalfa protein concentrate by rats. J. Anim. Sci. 1975, 40, 500–508. [Google Scholar] [CrossRef]
- Saunders, R.M.; Connor, M.A.; Booth, A.N.; Bickoff, E.M.; Kohler, G.O. Measurement of digestibility of alfalfa protein concentrates by in vivo and in vitro methods. J. Nutr. 1973, 103, 530–535. [Google Scholar] [CrossRef]
- Myer, R.O.; Cheeke, P.R.; Kennick, W.H. Utilization of alfalfa protein concentrate by swine. J. Anim. Sci. 1975, 40, 885–891. [Google Scholar] [CrossRef]
- Ognik, K.; Patkowski, L.; Grela, E.R. Effect of a protein-xanthophyll concentrate from alfalfa and of genotype and sex of lambs on their blood redox profile. Bull. Vet. Inst. Pulawy 2012, 56, 165–169. [Google Scholar] [CrossRef]
- Pietrzak, E.; Grela, E.R. The effects of adding lucerne protein concentrate to diets on the reproductive traits and blood metabolic profiles of sows and piglets. J. Anim. Feed Sci. 2015, 24, 216–225. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Danek-Majewska, A.; Kwiatkowska, K.; Krusiński, R. Effects of dietary alfalfa protein concentrate on lipid metabolism and antioxidative status and dietetic value of muscles in broilers. Poult. Sci. 2021, 100, 100974. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Ognik, K.; Czech, A.; Matras, J. Quality assessment of eggs from laying hens fed a mixture with lucerne protein concentrate. J. Anim. Feed Sci. 2014, 23, 236–243. [Google Scholar] [CrossRef][Green Version]
- Grela, E.R.; Knaga, S.; Winiarska-Mieczan, A.; Zięba, G. Effects of dietary alfalfa protein concentrate supplementation on performance, egg quality, and fatty acid composition of raw, freeze-dried, and hard-boiled eggs from Polbar laying hens. Poult. Sci. 2020, 99, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Collin, S.M.; Bertin, E.; Davys, G.J.; Mathur, B. Leaf concentrate as an alternative to iron and folic acid supplements for anaemic adolescent girls: A randomised controlled trial in India. Public Health Nutr. 2010, 13, 418–423. [Google Scholar] [CrossRef]
- Hadidi, M.; Khaksar, F.B.; Pagan, J.; Ibarz, A. Application of ultrasound-ultrafiltration-assisted alkaline isoelectric precipitation (UUAAIP) technique for producing alfalfa protein isolate for human consumption: Optimization, comparison, physicochemical, and functional properties. Food Res. Int. 2020, 130, 108907. [Google Scholar] [CrossRef]
- Nicholson, J.K. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Barszcz, M.; Taciak, M.; Skomiał, J. A dose-response effects of tannic acid and protein on growth performance, caecal fermentation, colon morphology, and β-glucuronidase activity of rats. J. Anim. Feed Sci. 2011, 20, 613–625. [Google Scholar] [CrossRef]
- Taciak, M.; Barszcz, M.; Tuśnio, A.; Bachanek, I.; Pastuszewska, B.; Skomiał, J. The effects of type of protein and fibre fermented in vitro with different pig inocula on short-chain fatty acids and amines concentration. J. Anim. Feed Sci. 2015, 24, 235–243. [Google Scholar] [CrossRef]
- Smirnov, A.; Sklan, D.; Uni, Z. Mucin dynamics in the chick small intestine are altered by starvation. J. Nutr. 2004, 134, 736–742. [Google Scholar] [CrossRef]
- Amit-Romach, E.; Uni, Z.; Cheled, S.; Berkovich, Z.; Reifen, R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J. Nutr. Biochem. 2009, 20, 70–77. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Anugwa, F.O.I.; Varel, V.H.; Dickson, J.S.; Pond, W.G.; Krook, L.P. Effects of dietary fiber and protein concentration on growth, feed efficiency, visceral organ weights and large intestine microbial populations of swine. J. Nutr. 1989, 119, 879–886. [Google Scholar] [CrossRef]
- Pond, W.G.; Varel, V.H.; Dickson, J.S.; Haschek, W.M. Comparative response of swine and rats to high-fiber or high-protein diets. J. Anim. Sci. 1989, 67, 716–723. [Google Scholar] [CrossRef]
- Woodman, D.D. Assessment of hepatotoxicity. In Animal Clinical Chemistry: A Primer for Toxicologists; Evans, G.O., Ed.; Taylor and Francis Ltd.: London, UK, 1996; pp. 66–82. [Google Scholar]
- Yuan, J.-P.; Peng, J.; Yin, K.; Wang, J.-H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 33, 7119–7131. [Google Scholar]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef]
- Giovannucci, E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. J. Nutr. 2001, 131, 3109S–3120S. [Google Scholar] [CrossRef]
- Sjögren, K.; Liu, J.-L.; Blad, K.; Skrtic, S.; Vidal, O.; Wallenius, V.; LeRoith, D.; Törnell, J.; Isaksson, O.G.P.; Jansson, J.-O.; et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 7088–7092. [Google Scholar] [CrossRef]
- Roberton, A.M.; Lee, S.P.; Lindop, R.; Stanley, R.A.; Thomse, L.; Tasman-Jones, C. Biliary control of β-glucuronidase activity in the luminal contents of the rat ileum, cecum, and rectum. Cancer Res. 1982, 42, 5165–5166. [Google Scholar]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Zaripheh, S.; Erdman, J.W., Jr. Factors that influence the bioavailability of xanthophylls. J. Nutr. 2002, 132, 531S–534S. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Willing, B.; Keeney, K.M.; Menendez, A.; Bergstrom, K.S.; Gill, N.; Russell, S.L.; Vallance, B.A.; Finlay, B.B. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011, 79, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M.; Arvidsson, I.; Karpman, D. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PLoS ONE 2012, 7, e46476. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut 1993, 34, 437–439. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Meguro, S.; Hase, T.; Tokimitsu, I.; Sakata, T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 525–531. [Google Scholar] [CrossRef]
- Hedemann, M.S.; Theil, P.K.; Bach Knudsen, K.E. The thickness of the intestinal mucous layer in the colon of rats fed various sources of non-digestible carbohydrates is positively correlated with the pool of SCFA but negatively with the proportion of butyric acid in digesta. Br. J. Nutr. 2009, 102, 117–125. [Google Scholar] [CrossRef]

| PXC (%) | |||
|---|---|---|---|
| 0 | 1.5 | 3 | |
| Components (g/kg, as-fed basis) | |||
| Protein-xanthophyll concentrate | 15.0 | 30.0 | |
| Soybean meal | 250.0 | 235.0 | 220.0 |
| Wheat | 385.2 | 385.2 | 385.2 |
| Maize | 200.0 | 200.0 | 200.0 |
| Flax meal | 30.0 | 30.0 | 30.0 |
| Dried whey | 30.0 | 30.0 | 30.0 |
| Dried brewer’s yeast | 60.0 | 60.0 | 60.0 |
| Monocalcium phosphate | 10.0 | 10.0 | 10.0 |
| Calcium carbonate | 20.0 | 20.0 | 20.0 |
| Sodium chloride | 3.4 | 3.4 | 3.4 |
| Mineral-vitamin premix 1 | 10.0 | 10.0 | 10.0 |
| L-lysine | 1.0 | 1.0 | 1.0 |
| DL-methionine | 0.4 | 0.4 | 0.4 |
| Nutrient content (% dry matter) | |||
| Dry matter | 90.3 | 90.1 | 90.4 |
| Crude protein | 25.5 | 25.6 | 25.9 |
| Crude ash | 6.5 | 6.6 | 6.7 |
| Ether extract | 4.2 | 4.4 | 4.6 |
| Crude fibre | 4.7 | 4.9 | 5.0 |
| ADF 2 | 6.9 | 6.5 | 6.9 |
| NDF 3 | 24.1 | 24.1 | 27.9 |
| ADL 4 | 2.1 | 1.9 | 2.0 |
| Gross energy (MJ/kg) | 17.1 | 17.1 | 17.0 |
| Gene | Primer | Forward | Product Length (Base Pairs) | Reference |
|---|---|---|---|---|
| MUC2 | Forward | CAGAGTGCATCAGTGGCTGT | 242 | [19] |
| Reverse | CCCGTCGAAGGTGATGTAGT | |||
| MUC3 | Forward | AACTGCGACTGGGGCACCCAGAAA | 366 | [19] |
| Reverse | AAAACCGTTTTTGTGTTAGTAT | |||
| β-actin | Forward | AACTGGGACGATATGGAGAAGATTT | 252 | [19] |
| Reverse | TGGGCACAGTGTGGG TGA |
| PXC (%) | SEM | p Value | Contrasts | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1.5 | 3 | Linear | Quadratic | |||
| Feed intake (g) | 526 | 527 | 547 | 6.10 | 0.308 | ||
| Body weight gain (g) | 154 | 160 | 165 | 2.70 | 0.264 | ||
| Feed efficiency (g/g) | 3.4 | 3.3 | 3.3 | 0.04 | 0.281 | ||
| Relative organ weight (g/100 g body weight) | |||||||
| Heart | 0.30 | 0.31 | 0.30 | 0.003 | 0.800 | ||
| Liver | 5.03 | 4.91 | 4.78 | 0.045 | 0.078 | 0.022 | 0.078 |
| Pancreas | 0.41 | 0.41 | 0.41 | 0.013 | 0.991 | ||
| Spleen | 0.24 | 0.25 | 0.25 | 0.005 | 0.763 | ||
| Kidneys | 0.94 | 0.94 | 0.94 | 0.010 | 0.997 | ||
| Stomach | 0.57 | 0.56 | 0.56 | 0.010 | 0.971 | ||
| Small intestine | 3.12 | 3.13 | 3.02 | 0.039 | 0.486 | ||
| Caecum | 0.52 | 0.47 | 0.47 | 0.013 | 0.198 | ||
| PXC (%) | SEM | p Value | Contrasts | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1.5 | 3 | Linear | Quadratic | |||
| Total protein (g/L) | 66 | 65 | 64 | 0.8 | 0.552 | ||
| Albumin (g/L) | 35 | 33 | 35 | 0.4 | 0.137 | ||
| Urea (mmol/L) | 6.6 | 6.4 | 6.6 | 0.18 | 0.845 | ||
| Cholesterol (mmol/L) | 1.0 | 1.5 | 1.8 | 0.18 | 0.129 | ||
| Triglycerides (mmol/L) | 1.5 | 1.5 | 1.6 | 0.10 | 0.951 | ||
| HDL 1, mmol/L | 0.8 | 0.8 | 0.8 | 0.03 | 0.574 | ||
| Glucose (mmol/L) | 13.6 | 13.7 | 13.9 | 0.29 | 0.951 | ||
| Creatinine (µmol/L) | 36.4 | 36.9 | 37.7 | 0.96 | 0.879 | ||
| Uric acid (µmol/L) | 140 a | 139 a | 242 b | 19.7 | 0.040 | 0.031 | 0.040 |
| Total bilirubin (µmol/L) | 11.4 b | 5.3 a | 8.5 a,b | 1.01 | 0.039 | 0.245 | 0.039 |
| AST 2, U/L | 87 | 91 | 97 | 4.7 | 0.705 | ||
| ALT 3, U/L | 55 | 49 | 52 | 1.4 | 0.289 | ||
| ALP 4, U/L | 762 | 736 | 740 | 27.8 | 0.927 | ||
| ACP 5, U/L | 33 | 31 | 33 | 1.0 | 0.806 | ||
| Cholinesterase (U/L) | 313 | 294 | 332 | 9.7 | 0.289 | ||
| LDH 6, U/L | 409 | 279 | 324 | 25.6 | 0.105 | ||
| CK 7, U/L | 476 | 472 | 530 | 33.8 | 0.750 | ||
| Amylase (U/L) | 635 | 623 | 631 | 15.3 | 0.951 | ||
| Chloride (mmol/L) | 90 | 98 | 100 | 4.7 | 0.632 | ||
| Phosphorus (mmol/L) | 3.3 | 3.2 | 3.1 | 0.06 | 0.373 | ||
| Iron (µmol/L) | 43.7 | 39.6 | 46.8 | 1.40 | 0.104 | ||
| Calcium (mmol/L) | 3.0 | 3.3 | 3.2 | 0.07 | 0.286 | ||
| Magnesium (mmol/L) | 1.0 | 1.0 | 0.9 | 0.02 | 0.843 | ||
| PXC (%) | SEM | p Value | Contrasts | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1.5 | 3 | Linear | Quadratic | |||
| Caecal digesta (g/100 g body weight) | 2.82 | 2.73 | 2.90 | 0.055 | 0.477 | ||
| Digesta pH | 6.47 | 6.51 | 6.61 | 0.038 | 0.315 | ||
| β-glucuronidase (U/g) | 51.9 | 86.6 | 77.9 | 12.98 | 0.534 | ||
| Short-chain fatty acids (µmol/g) | |||||||
| Acetic | 31.5 | 31.4 | 28.3 | 0.83 | 0.204 | ||
| Propionic | 5.45 | 5.75 | 5.12 | 0.147 | 0.220 | ||
| Isobutyric | 0.43 | 0.46 | 0.58 | 0.013 | 0.364 | ||
| Butyric | 19.9 | 17.5 | 18.3 | 0.77 | 0.466 | ||
| Isovaleric | 0.12 | 0.16 | 0.21 | 0.016 | 0.057 | 0.017 | 0.057 |
| Valeric | 0.43 | 0.44 | 0.45 | 0.011 | 0.829 | ||
| Total SCFA | 57.8 | 55.7 | 55.8 | 1.11 | 0.188 | ||
| Amines (µmol/g) | |||||||
| Methylamine | 0.016 | 0.018 | 0.016 | 0.0009 | 0.481 | ||
| Putrescine | 0.011 | 0.010 | 0.011 | 0.0005 | 0.725 | ||
| Cadaverine | 0.009 | 0.010 | 0.011 | 0.0022 | 0.785 | ||
| Tyramine | 0.017 | 0.018 | 0.018 | 0.0012 | 0.670 | ||
| Spermidine | 0.009 | 0.010 | 0.009 | 0.0004 | 0.705 | ||
| PXC (%) | SEM | p Value | Contrasts | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1.5 | 3 | Linear | Quadratic | |||
| Caecum | |||||||
| Crypts depth (µm) | 197 | 209 | 190 | 5.7 | 0.413 | ||
| Myenteron thickness (µm) | 288 | 289 | 275 | 11.5 | 0.873 | ||
| Colon | |||||||
| Crypts depth (µm) | 305 | 310 | 348 | 8.1 | 0.059 | 0.031 | 0.059 |
| Myenteron thickness (µm) | 197 | 205 | 232 | 13.0 | 0.568 | ||
| Mucus layer thickness (µg dye/cm2) | 87 b | 59 a | 63 a | 3.8 | 0.001 | 0.007 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barszcz, M.; Tuśnio, A.; Bachanek-Matusiewicz, I.; Gawin, K.; Skomiał, J.; Taciak, M. Growth Performance, Biochemical Blood Indices, and Large Intestine Physiology of Rats Fed Diets with Alfalfa Protein-Xanthophyll Concentrate. Animals 2021, 11, 2069. https://doi.org/10.3390/ani11072069
Barszcz M, Tuśnio A, Bachanek-Matusiewicz I, Gawin K, Skomiał J, Taciak M. Growth Performance, Biochemical Blood Indices, and Large Intestine Physiology of Rats Fed Diets with Alfalfa Protein-Xanthophyll Concentrate. Animals. 2021; 11(7):2069. https://doi.org/10.3390/ani11072069
Chicago/Turabian StyleBarszcz, Marcin, Anna Tuśnio, Ilona Bachanek-Matusiewicz, Kamil Gawin, Jacek Skomiał, and Marcin Taciak. 2021. "Growth Performance, Biochemical Blood Indices, and Large Intestine Physiology of Rats Fed Diets with Alfalfa Protein-Xanthophyll Concentrate" Animals 11, no. 7: 2069. https://doi.org/10.3390/ani11072069
APA StyleBarszcz, M., Tuśnio, A., Bachanek-Matusiewicz, I., Gawin, K., Skomiał, J., & Taciak, M. (2021). Growth Performance, Biochemical Blood Indices, and Large Intestine Physiology of Rats Fed Diets with Alfalfa Protein-Xanthophyll Concentrate. Animals, 11(7), 2069. https://doi.org/10.3390/ani11072069







