Enhanced Hippocampus-Nidopallium Caudolaterale Connectivity during Route Formation in Goal-Directed Spatial Learning of Pigeons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects, Surgery, and Electrode Implantation
2.2. Goal-Directed Spatial Learning Experiment
2.3. Behavioral and Neural Data Recording and Analysis
2.4. Functional Network Connectivity Analysis
2.5. Statistical Analysis
3. Results
3.1. Behavioral Performanceduring Route Formation
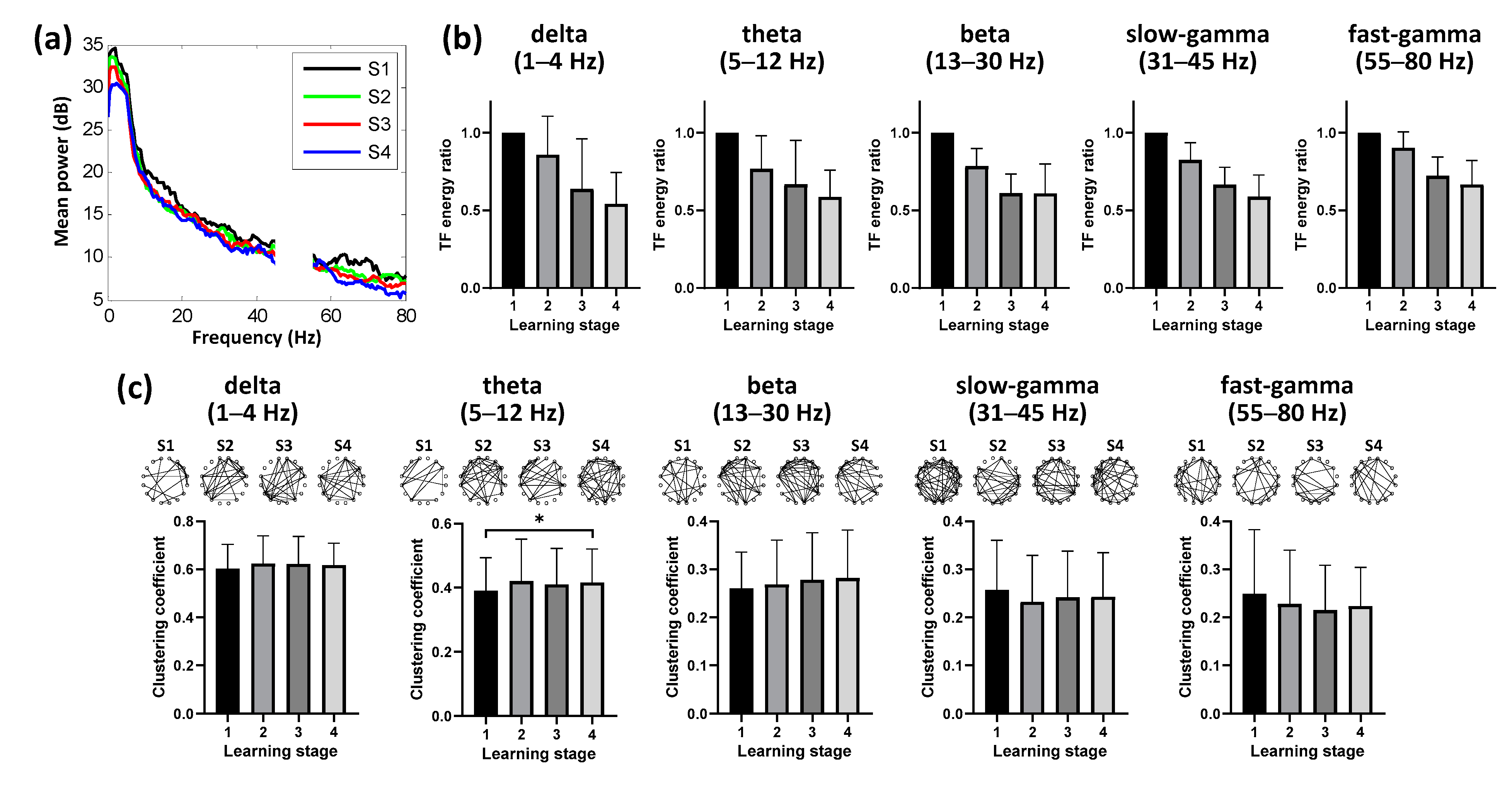
3.2. Dynamics of Hippocampal Activity during Learning
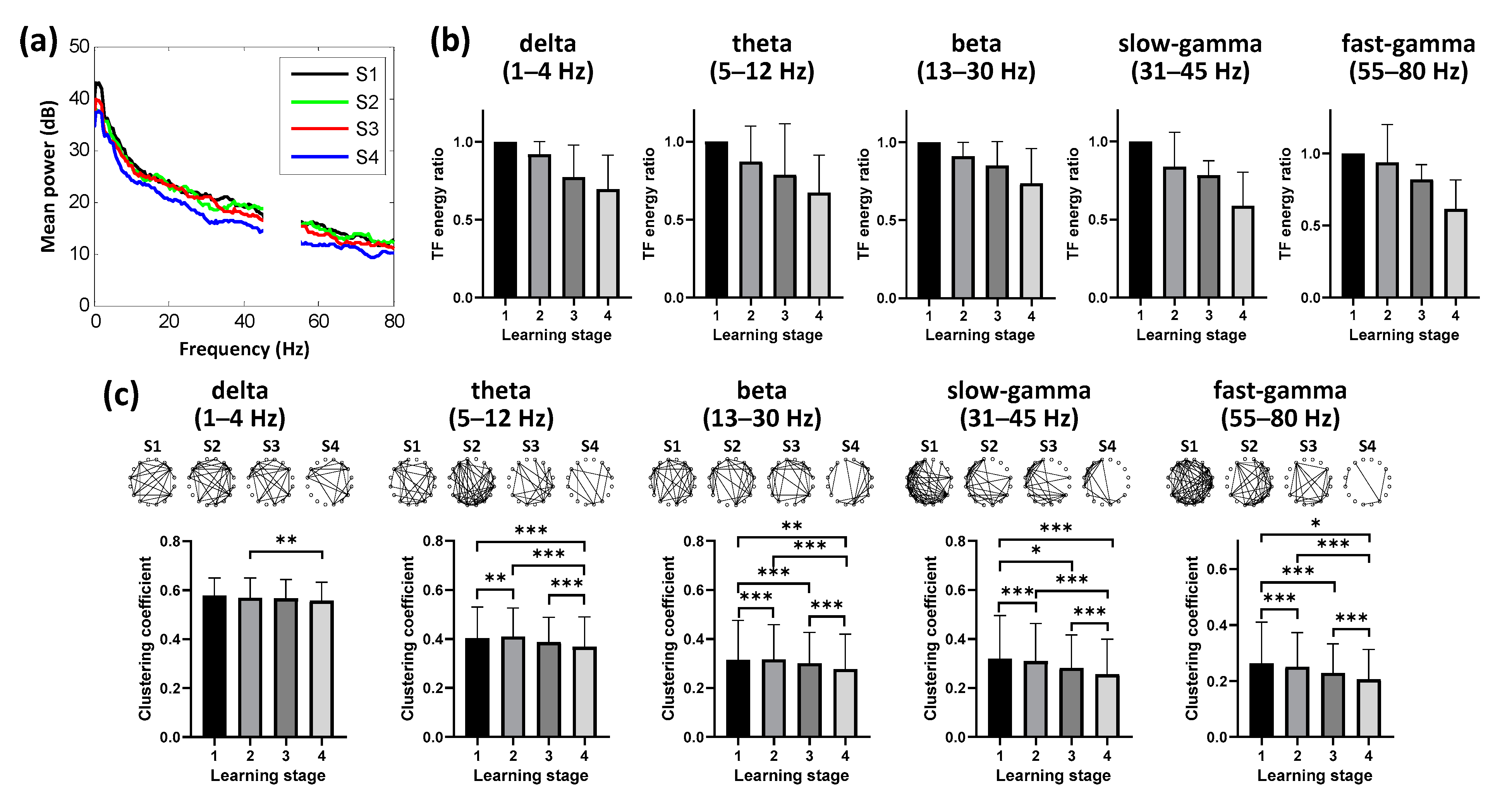
3.3. Dynamic Neural Patterns in NCL during Learning
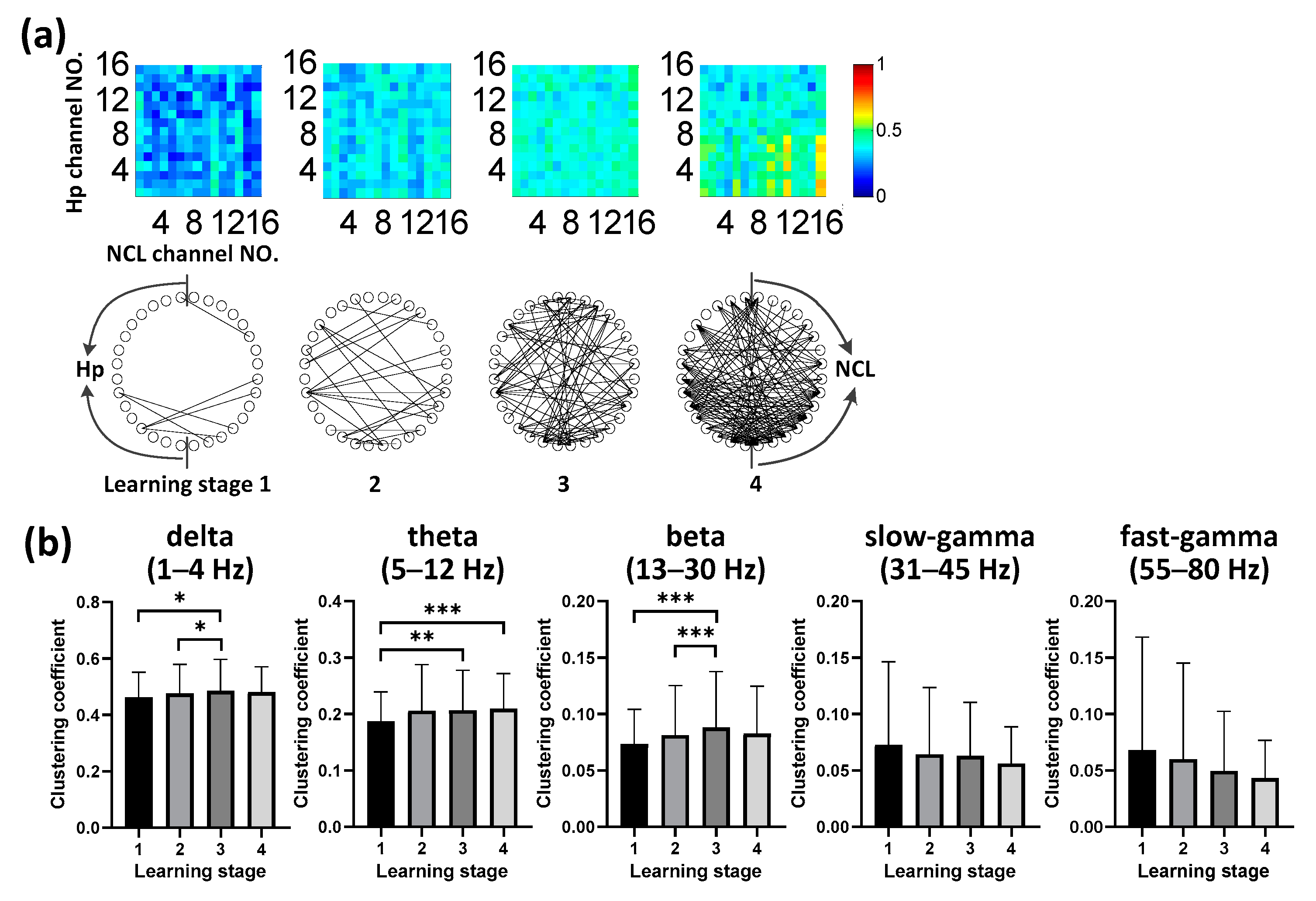
3.4. Evolution of Hp-NCL Connectivity during Learning
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buschman, T.J.; Miller, E.K. Goal-direction and top-down control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130471. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.E.; Foster, D.J. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 2013, 497, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Verschure, P.F.; Pennartz, C.M.; Pezzulo, G. The why, what, where, when and how of goal-directed choice: Neuronal and computational principles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130483. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Brain Res. 1971, 34, 171–175. [Google Scholar] [PubMed]
- Taube, J.S.; Muller, R.U.; Ranck, J.J. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990, 10, 420–435. [Google Scholar] [CrossRef]
- Solstad, T.; Boccara, C.N.; Kropff, E.; Moser, M.; Moser, E.I. Representation of geometric borders in the entorhinal cortex. Science 2008, 322, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Recce, M.L. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 1993, 3, 317–330. [Google Scholar] [CrossRef]
- Stachenfeld, K.L.; Botvinick, M.M.; Gershman, S.J. The hippocampus as a predictive map. Nat. Neurosci. 2017, 20, 1643–1653. [Google Scholar] [CrossRef]
- Ma, L.; Hyman, J.M.; Durstewitz, D.; Phillips, A.G.; Seamans, J.K. A quantitative analysis of context-dependent remapping of medial frontal cortex neurons and ensembles. J. Neurosci. 2016, 36, 8258–8272. [Google Scholar] [CrossRef]
- Negrón-Oyarzo, I.; Espinosa, N.; Aguilar-Rivera, M.; Fuenzalida, M.; Aboitiz, F.; Fuentealba, P. Coordinated prefrontal-hippocampal activity and navigation strategy-related prefrontal firing during spatial memory formation. Proc. Natl. Acad. Sci. USA 2018, 115, 7123–7128. [Google Scholar] [CrossRef]
- Zielinski, M.C.; Shin, J.D.; Jadhav, S.P. Coherent coding of spatial position mediated by theta oscillations in the hippocampus and prefrontal cortex. J. Neurosci. 2019, 39, 4550–4565. [Google Scholar] [CrossRef]
- Stacho, M.; Herold, C.; Rook, N.; Wagner, H.; Axer, M.; Amunts, K.; Güntürkün, O. A cortex-like canonical circuit in the avian forebrain. Science 2020, 369, eabc5534. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.; Wagener, L.; Rinnert, P. A neural correlate of sensory consciousness in a corvid bird. Science 2020, 369, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Schloemer, P.; Mafoppa-Fomat, I.; Mehlhorn, J.; Amunts, K.; Axer, M. The hippocampus of birds in a view of evolutionary connectomics. Cortex 2019, 118, 165–187. [Google Scholar] [CrossRef]
- Herold, C.; Bingman, V.P.; Ströckens, F.; Letzner, S.; Sauvage, M.; Palomero-Gallagher, N.; Zilles, K.; Güntürkün, O. Distribution of neurotransmitter receptors and zinc in the pigeon (Columba livia) hippocampal formation: A basis for further comparison with the mammalian hippocampus. J. Comp. Neurol. 2014, 522, 2553–2575. [Google Scholar] [CrossRef]
- Herold, C.; Coppola, V.J.; Bingman, V.P. The maturation of research into the avian hippocampal formation: Recent discoveries from one of the nature’s foremost navigators. Hippocampus 2015, 25, 1193–1211. [Google Scholar] [CrossRef] [PubMed]
- Sherry, D.F.; Grella, S.L.; Guigueno, M.F.; White, D.J.; Marrone, D.F. Are there place cells in the avian hippocampus? Brain Behav. Evolut. 2017, 90, 73–80. [Google Scholar] [CrossRef]
- Bingman, V.P.; Hough, G.E.; Kahn, M.C.; Siegel, J.J. The homing pigeon hippocampus and space: In search of adaptive specialization. Brain Behav. Evolut. 2003, 62, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hough, G.E.; Bingman, V.P. Spatial response properties of homing pigeon hippocampal neurons: Correlations with goal locations, movement between goals, and environmental context in a radial-arm arena. J. Comp. Physiol. A 2004, 190, 1047–1062. [Google Scholar] [CrossRef]
- Kahn, M.C.; Siegel, J.J.; Jechura, T.J.; Bingman, V.P. Response properties of avian hippocampal formation cells in an environment with unstable goal locations. Behav. Brain Res. 2008, 191, 153–163. [Google Scholar] [CrossRef]
- Mouritsen, H.; Heyers, D.; Güntürkün, O. The neural basis of long-distance navigation in birds. Annu. Rev. Physiol. 2016, 78, 133–154. [Google Scholar] [CrossRef]
- Mogensen, J.; Divac, I. The prefrontal ‘cortex’ in the pigeon. Behavioral evidence. Brain Behav. Evol. 1982, 21, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O. Cognitive impairments after lesions of the neostriatum caudolaterale and its thalamic afferent in pigeons: Functional similarities to the mammalian prefrontal system? J. Hirnforsch. 1997, 38, 133–143. [Google Scholar] [PubMed]
- Güntürkün, O. The avian ‘prefrontal cortex’ and cognition. Curr. Opin. Neurobiol. 2005, 15, 686–693. [Google Scholar] [CrossRef]
- Liu, X.; Wan, H.; Li, S.; Shang, Z.; Shi, L. The role of nidopallium caudolaterale in the goal-directed behavior of pigeons. Behav. Brain Res. 2017, 326, 112–120. [Google Scholar] [CrossRef]
- Li, M.; Shang, Z.; Zhao, K.; Cheng, S.; Wan, H. The role of Hp-NCL network in goal-directed routing information encoding of bird: A review. Brain Sci. 2020, 10, 617. [Google Scholar] [CrossRef]
- Shanahan, M.; Bingman, V.P.; Shimizu, T.; Wild, M.; Güntürkün, O. Large-scale network organization in the avian forebrain: A connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 2013, 7, 89. [Google Scholar] [CrossRef]
- Zhao, K.; Nie, J.; Yang, L.; Liu, X.; Shang, Z.; Wan, H. Hippocampus-nidopallium caudolaterale interactions exist in the goal-directed behavior of pigeon. Brain Res. Bull. 2019, 153, 257–265. [Google Scholar] [CrossRef]
- Hennig, J.A.; Oby, E.R.; Golub, M.D.; Bahureksa, L.A.; Sadtler, P.T.; Quick, K.M.; Ryu, S.I.; Tyler-Kabara, E.C.; Batista, A.P.; Chase, S.M.; et al. Learning is shaped by abrupt changes in neural engagement. Nat. Neurosci. 2021, 24, 727–736. [Google Scholar] [CrossRef]
- Alemany-Gonzalez, M.; Gener, T.; Nebot, P.; Vilademunt, M.; Dierssen, M.; Puig, V.M. Prefrontal-hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability. Proc. Natl. Acad. Sci. USA 2020, 117, 11788–11798. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Yang, L.; Wang, H.; Wan, H.; Shang, Z. Functional connectivity changes in the intra- and inter-brain during the construction of the multi-brain network of pigeons. Brain Res. Bull. 2020, 161, 147–157. [Google Scholar] [CrossRef]
- Karten, H.; Hodos, W. A Stereotaxic Atlas of the Brain of the Pigeon (Columba livia); Johns Hopkins Press: Baltimore, MD, USA, 1967. [Google Scholar]
- Bastos, A.M.; Schoffelen, J. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Li, S.; Wan, H. Decoding pigeon behavior outcomes using functional connections among local field potentials. Comput. Intell. Neurosci. 2018, 2018, 3505371. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and functions of theta rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef]
- Nieder, A. Inside the corvid brain—Probing the physiology of cognition in crows. Curr. Opin. Behav. Sci. 2017, 16, 8–14. [Google Scholar] [CrossRef]
- Meij, J.; Rattenborg, N.C.; Beckers, G.J.L. Divergent neuronal activity patterns in the avian hippocampus and nidopallium. Eur. J. Neurosci. 2020, 52, 3124–3139. [Google Scholar] [CrossRef]
- Hallock, H.L.; Wang, A.; Griffin, A.L. Ventral midline thalamus is critical for hippocampal-prefrontal synchrony and spatial working memory. J. Neurosci. 2016, 36, 8372–8389. [Google Scholar] [CrossRef]
- Jones, M.W.; Wilson, M.A. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005, 3, e402. [Google Scholar] [CrossRef]
- Liu, T.; Bai, W.; Xia, M.; Tian, X. Directional hippocampal-prefrontal interactions during working memory. Behav. Brain Res. 2018, 338, 1–8. [Google Scholar] [CrossRef]
- Sigurdsson, T.; Stark, K.L.; Karayiorgou, M.; Gogos, J.A.; Gordon, J.A. Impaired hippocampal–prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 2010, 464, 763–767. [Google Scholar] [CrossRef]
- Hyafil, A.; Giraud, A.; Fontolan, L.; Gutkin, B. Neural cross-frequency coupling: Connecting architectures, mechanisms, and functions. Trends Neurosci. 2015, 38, 725–740. [Google Scholar] [CrossRef]
- Jiang, H.; Bahramisharif, A.; Gerven, M.A.J.; Jensen, O. Distinct directional couplings between slow and fast gamma power to the phase of theta oscillations in the rat hippocampus. Eur. J. Neurosci. 2020, 51, 2070–2081. [Google Scholar] [CrossRef]
- Goodman, J. Place vs. response learning: History, controversy, and neurobiology. Front. Behav. Neurosci. 2021, 14, 598570. [Google Scholar] [CrossRef]
- Packard, M.G.; McGaugh, J.L. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 1996, 65, 65–72. [Google Scholar] [CrossRef]
- de Bruin, J.P.C.; Moita, M.P.; de Brabander, H.M.; Joosten, R.N.J.M. Place and response learning of rats in a Morris water maze: Differential effects of fimbria fornix and medial prefrontal cortex lesions. Neurobiol. Learn. Mem. 2001, 75, 164–178. [Google Scholar] [CrossRef][Green Version]
- Geerts, J.P.; Chersi, F.; Stachenfeld, K.L.; Burgess, N. A general model of hippocampal and dorsal striatal learning and decision making. Proc. Natl. Acad. Sci. USA 2020, 117, 31427–31437. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.-M.; Fan, J.-T.; Cheng, S.-G.; Yang, L.-F.; Yang, L.; Wang, L.-F.; Shang, Z.-G.; Wan, H. Enhanced Hippocampus-Nidopallium Caudolaterale Connectivity during Route Formation in Goal-Directed Spatial Learning of Pigeons. Animals 2021, 11, 2003. https://doi.org/10.3390/ani11072003
Li M-M, Fan J-T, Cheng S-G, Yang L-F, Yang L, Wang L-F, Shang Z-G, Wan H. Enhanced Hippocampus-Nidopallium Caudolaterale Connectivity during Route Formation in Goal-Directed Spatial Learning of Pigeons. Animals. 2021; 11(7):2003. https://doi.org/10.3390/ani11072003
Chicago/Turabian StyleLi, Meng-Meng, Jian-Tao Fan, Shu-Guan Cheng, Li-Fang Yang, Long Yang, Liao-Feng Wang, Zhi-Gang Shang, and Hong Wan. 2021. "Enhanced Hippocampus-Nidopallium Caudolaterale Connectivity during Route Formation in Goal-Directed Spatial Learning of Pigeons" Animals 11, no. 7: 2003. https://doi.org/10.3390/ani11072003
APA StyleLi, M.-M., Fan, J.-T., Cheng, S.-G., Yang, L.-F., Yang, L., Wang, L.-F., Shang, Z.-G., & Wan, H. (2021). Enhanced Hippocampus-Nidopallium Caudolaterale Connectivity during Route Formation in Goal-Directed Spatial Learning of Pigeons. Animals, 11(7), 2003. https://doi.org/10.3390/ani11072003





