Use of Hydrolyzed Chinese Gallnut Tannic Acid in Weaned Piglets as an Alternative to Zinc Oxide: Overview on the Gut Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets for Piglets
2.2. Animals and Management
2.3. Sampling and Collection
2.4. Analysis of Intestinal Morphology and Intestinal Flora
2.5. Statistical Analysis
3. Results
3.1. Production Performance
3.2. Diarrhea Rate
3.3. Antioxidant Capacity
3.4. Intestinal Tissue Morphology and Intestinal Barrier
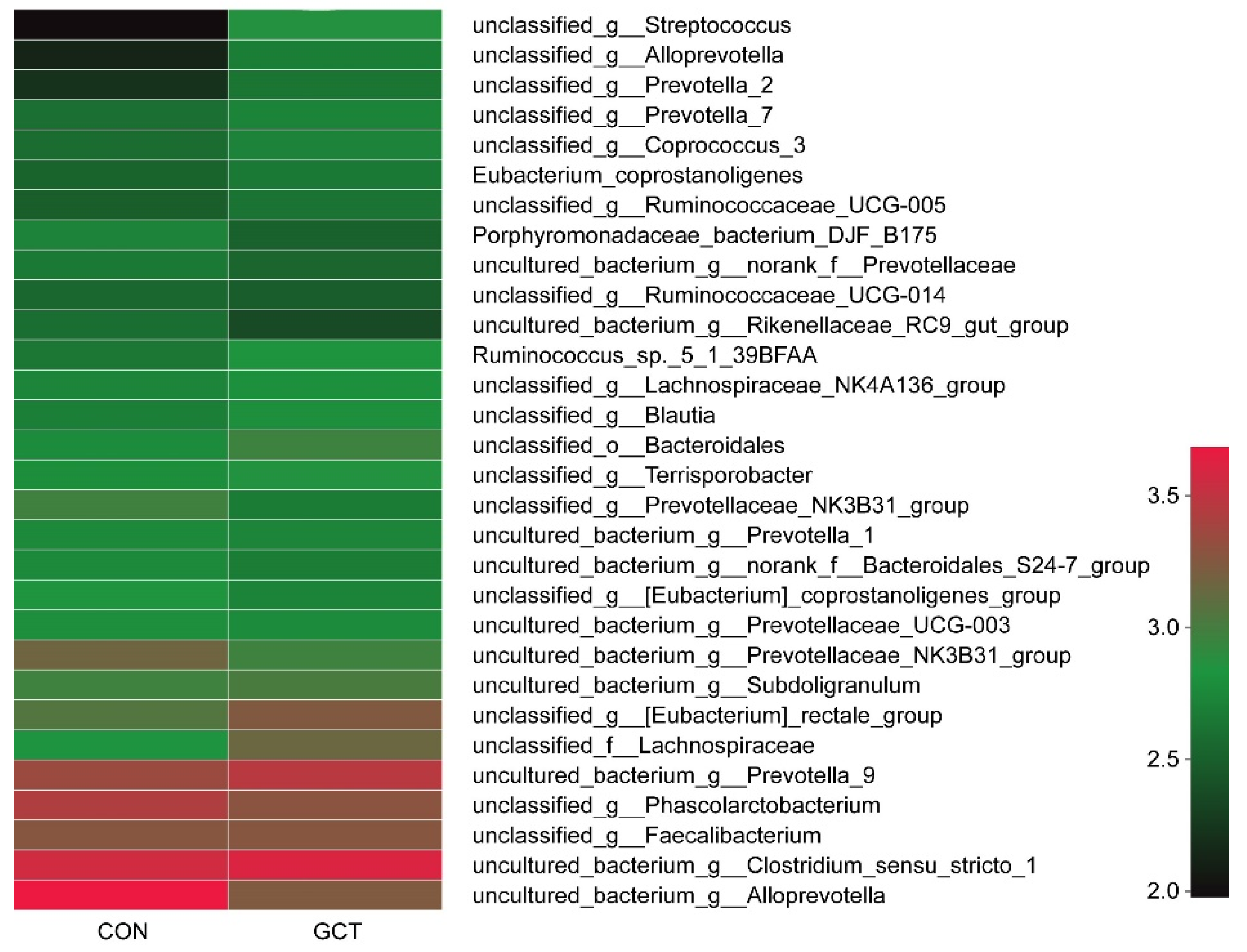
3.5. Changes of Intestinal Flora
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs1. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Kloubert, V.; Blaabjerg, K.; Dalgaard, T.S.; Poulsen, H.D.; Rink, L.; Wessels, I. Influence of zinc supplementation on immune parameters in weaned pigs. J. Trace Elements Med. Biol. 2018, 49, 231–240. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Jondreville, C.; Revy, P.; Dourmad, J. Dietary means to better control the environmental impact of copper and zinc by pigs from weaning to slaughter. Livest. Prod. Sci. 2003, 84, 147–156. [Google Scholar] [CrossRef]
- Tosi, G.; Massi, P.; Antongiovanni, M.; Buccioni, A.; Minieri, S.; Marenchino, L.; Mele, M. Efficacy Test of a Hydrolysable Tannin Extract Against Necrotic Enteritis in Challenged Broiler Chickens. Ital. J. Anim. Sci. 2013, 12, e62. [Google Scholar] [CrossRef]
- Rezar, V.; Salobir, J.; Levart, A.; Tomažin, U.; Škrlep, M.; Lukač, N.B.; Čandek-Potokar, M. Supplementing entire male pig diet with hydrolysable tannins: Effect on carcass traits, meat quality and oxidative stability. Meat Sci. 2017, 133, 95–102. [Google Scholar] [CrossRef]
- Girard, M.; Thanner, S.; Pradervand, N.; Hu, D.; Ollagnier, C.; Bee, G. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: Efficacy on an experimental ETEC F4 model. PLoS ONE 2018, 13, e0197878. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.K.; Kannan, A.; Singh, B.; Sharma, O.P. Value Addition of Feed and Fodder by Alleviating the Antinutritional Effects of Tannins. Agric. Res. 2013, 2, 189–206. [Google Scholar] [CrossRef] [Green Version]
- Ozkose, E.; Kulolu, R.; Comlekcioglu, U.; Kar, B.; Ekinci, S. Effects of tannic acid on the fibrolytic enzyme activity and survival of some ruminal bacteria. Int. J. Agric. Biol. 2011, 13, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Sathe, S.; Sze-Tao, K. Effects of sodium chloride, phytate and tannin on in vitro proteolysis of phaseolin. Food Chem. 1997, 59, 253–259. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Yan, H.; et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Bee, G.; Silacci, P.; Ampuero-Kragten, S.; Čandek-Potokar, M.; Wealleans, A.; Litten-Brown, J.; Salminen, J.-P.; Mueller-Harvey, I. Hydrolysable tannin-based diet rich in gallotannins has a minimal impact on pig performance but significantly reduces salivary and bulbourethral gland size. Anim. An Int. J. Anim. Biosci. 2017, 11, 1617–1625. [Google Scholar] [CrossRef] [Green Version]
- Kotrotsios, N.V.; Christaki, E.V.; Bonos, E.M.; Florou-Paneri, P.C. The effect of dietary carob pods on nutrient digestibility in weaning, growing and fattening periods of pigs. J. Food Agric. Environ. 2010, 8, 779–782. [Google Scholar]
- Biagia, G.; Cipollini, I.; Paulicks, B.R.; Roth, F.X. Effect of tannins on growth performance and intestinal ecosystem in weaned piglets. Arch. Anim. Nutr. 2010, 64, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.P.; Pająk, J.J.; Skomiał, J.; Miltko, R.; Kowalik, B. The effect of lingonberry leaves and oak cortex addition to sheep diets on pancreatic enzymes activity. J. Anim. Feed. Sci. 2017, 26, 354–358. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res. Int. 2014, 905215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madec, F.; Bridoux, N.; Bounaix, S.; Cariolet, R.; Duval-Iflah, Y.; Hampson, D.J.; Jestin, A. Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Veter Microbiol. 2000, 72, 295–310. [Google Scholar] [CrossRef]
- Trckova, M.; Lorencova, A.; Babak, V.; Neca, J.; Ciganek, M. The effect of leonardite and lignite on the health of weaned piglets. Res. Veter Sci. 2018, 119, 134–142. [Google Scholar] [CrossRef]
- Ye, M.-H.; Nan, Y.-L.; Ding, M.-M.; Hu, J.-B.; Liu, Q.; Wei, W.-H.; Yang, S.-M. Effects of dietary tannic acid on the growth, hepatic gene expression, and antioxidant enzyme activity in Brandt’s voles (Microtus brandti). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 196–197, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.A.S.; Grahn, M.F.; Hutton, M.; Williams, N.S. Butyrate metabolism in the terminal ileal mucosa of patients with ulcerative colitis. BJS 2005, 82, 36–38. [Google Scholar] [CrossRef]
- Riskin, A.; Agostoni, C.; Shamir, R. Physiology of the Gastrointestinal Tract; Springer: Milan, Italy, 2012; pp. 263–280. [Google Scholar]
- Van Ampting, M.T.J.; Schonewille, A.J.; Vink, C.; Brummer, R.J.M.; Van Der Meer, R.; Bovee-Oudenhoven, I.M.J. Damage to the Intestinal Epithelial Barrier by Antibiotic Pretreatment of Salmonella-Infected Rats Is Lessened by Dietary Calcium or Tannic Acid. J. Nutr. 2010, 140, 2167–2172. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.J.; Zheng, M.L.; Ren, A.; Zhou, C.S.; Yan, Q.X.; Tan, Z.L.; Zhang, P.H.; Yi, K.L. Effects of high rice diet on growth performance, nutrients apparent digestibility, nitrogen metabolism, blood parameters and rumen fermentation in growing goats. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 749–755. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Li, J.; Pang, G.; Ren, F.; Fang, B. Effects of Diethyl Phosphate, a Non-Specific Metabolite of Organophosphorus Pesticides, on Serum Lipid, Hormones, Inflammation, and Gut Microbiota. Molecules 2019, 24, 2003. [Google Scholar] [CrossRef] [Green Version]
- Biddle, A.; Stewart, L.; Blanchard, J.L.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Kameyama, K.; Fujii, T.; Nishikawa, S.; Kajiura, T.; Nakamura, E. Isolation of a novel indigenous Lachnospiraceae bacterium which induces the development of obesity and diabetes in gnotobiotic mice. Diabetes 2014, 63, A74. [Google Scholar]
- Surana, N.; Kasper, D. Lachnospiraceae protect from colitis by regulating colonic group 3 innate lymphoid cells. Eur. J. Immunol. 2016, 46, 346. [Google Scholar]
- Rodríguez-Sanoja, R.; Ruiz, B.; Guyot, J.P.; Sanchez, S. Starch-Binding Domain Affects Catalysis in Two Lactobacillus α-Amylases. Appl. Environ. Microbiol. 2005, 71, 297–302. [Google Scholar] [CrossRef] [Green Version]



| Composition (%) | Content | Nutrient Levels of Basal Diets | Content |
|---|---|---|---|
| Corn | 56.5 | Crude protein | 18.2 |
| Soybean meal | 10 | Crude fat | 6.2 |
| Puffed soybean | 8 | Crude fiber | 2.5 |
| Fermented soybean meal | 5 | Crude ash | 5 |
| Flour | 5 | Lysine | 1.35 |
| Fish meal | 4 | Chloride | 0.6 |
| Soybean oil | 3.5 | P | 0.55 |
| Glucose | 2.5 | Ca | 0.8 |
| Whey powder | 2.5 | Digestive energy (MJ/kg) | 14.23 |
| Premix A | 3 |
| Item | CON | GCT | SEM | p Value |
|---|---|---|---|---|
| Initial weight (kg) | 10.10 | 10.05 | 0.047 | 0.615 |
| Final weight (kg) | 18.83 | 18.95 | 0.075 | 0.469 |
| ADG (g/day) | 418.86 | 420.22 | 2.50 | 0.731 |
| ADFI (g/day) | 748.90 | 752.96 | 5.52 | 0.799 |
| FCR | 1.81 | 1.79 | 0.008 | 0.411 |
| Item | CON | GCT | SEM | p Value |
|---|---|---|---|---|
| 0–7 days | 3.17% | 3.97% | 0.0095 | 0.383 |
| 7–14 days | 2.78% | 1.98% | 0.0059 | 0.263 |
| 14–21 days | 1.19% | 0 | 0.0031 | 0.049 |
| Item | CON | GCT | SEM | p Value |
|---|---|---|---|---|
| GSH (mg/mL) | 5.57 | 6.43 | 0.19 | 0.012 |
| MDA (nmol/mL) | 4.15 | 3.58 | 0.14 | 0.032 |
| SOD (U/mL) | 97.16 | 105.47 | 2.06 | 0.036 |
| Item | CON | GCT | SEM | p Value |
|---|---|---|---|---|
| Villus height (um) | ||||
| Duodenum | 454.25 | 401.02 | 15.63 | 0.088 |
| Jejunum | 422.44 | 410.70 | 16.99 | 0.747 |
| Ileum | 384.67 | 399.66 | 14.95 | 0.639 |
| Crypt depth (um) | ||||
| Duodenum | 142.70 | 148.20 | 2.90 | 0.367 |
| Jejunum | 159.04 | 150.06 | 4.02 | 0.284 |
| Ileum | 144.69 | 128.25 | 4.08 | 0.036 |
| D-lac | 1288 | 1106 | 36.29 | 0.004 |
| Item | CON | GCT | SEM | p Value |
|---|---|---|---|---|
| Community richness | ||||
| Sobs | 394.83 | 436.33 | 13.04 | 0.114 |
| Ace | 435.11 | 474.88 | 13.13 | 0.136 |
| Chao | 448.93 | 482.20 | 13.54 | 0.236 |
| Community evenness | ||||
| Heip | 0.15 | 0.165 | 0.0087 | 0.294 |
| Simpsoneven | 0.058 | 0.07 | 0.0057 | 0.358 |
| Shannoneven | 0.68 | 0.7 | 0.0098 | 0.251 |
| Community diversity | ||||
| Shannon | 4.05 | 4.26 | 0.072 | 0.152 |
| Simpson | 0.047 | 0.036 | 0.0046 | 0.236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, K.; Xu, B.; Peng, X.; Chai, B.; Nong, S.; Li, Z.; Shen, S.; Si, H. Use of Hydrolyzed Chinese Gallnut Tannic Acid in Weaned Piglets as an Alternative to Zinc Oxide: Overview on the Gut Microbiota. Animals 2021, 11, 2000. https://doi.org/10.3390/ani11072000
Sun J, Wang K, Xu B, Peng X, Chai B, Nong S, Li Z, Shen S, Si H. Use of Hydrolyzed Chinese Gallnut Tannic Acid in Weaned Piglets as an Alternative to Zinc Oxide: Overview on the Gut Microbiota. Animals. 2021; 11(7):2000. https://doi.org/10.3390/ani11072000
Chicago/Turabian StyleSun, Junying, Kaijun Wang, Baichang Xu, Xiaomin Peng, Beibei Chai, Siwei Nong, Zheng Li, Shuibao Shen, and Hongbin Si. 2021. "Use of Hydrolyzed Chinese Gallnut Tannic Acid in Weaned Piglets as an Alternative to Zinc Oxide: Overview on the Gut Microbiota" Animals 11, no. 7: 2000. https://doi.org/10.3390/ani11072000
APA StyleSun, J., Wang, K., Xu, B., Peng, X., Chai, B., Nong, S., Li, Z., Shen, S., & Si, H. (2021). Use of Hydrolyzed Chinese Gallnut Tannic Acid in Weaned Piglets as an Alternative to Zinc Oxide: Overview on the Gut Microbiota. Animals, 11(7), 2000. https://doi.org/10.3390/ani11072000







