Influence of Infectious Pancreatic Necrosis Virus and Yersinia ruckeri Co-Infection on a Non-Specific Immune System in Rainbow Trout (Oncorhynchus mykiss)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Experimental Infection
- group C—uninfected control;
- group Yer—infected with Y. ruckeri;
- group IPN—infected with IPNV;
- group Yer+IPN—co-infected with Y. ruckeri and IPNV.
2.2.1. Experiment I
2.2.2. Experiment II
2.2.3. Sampling
2.3. Immunological Tests
2.3.1. Tests Performed on Blood Serum
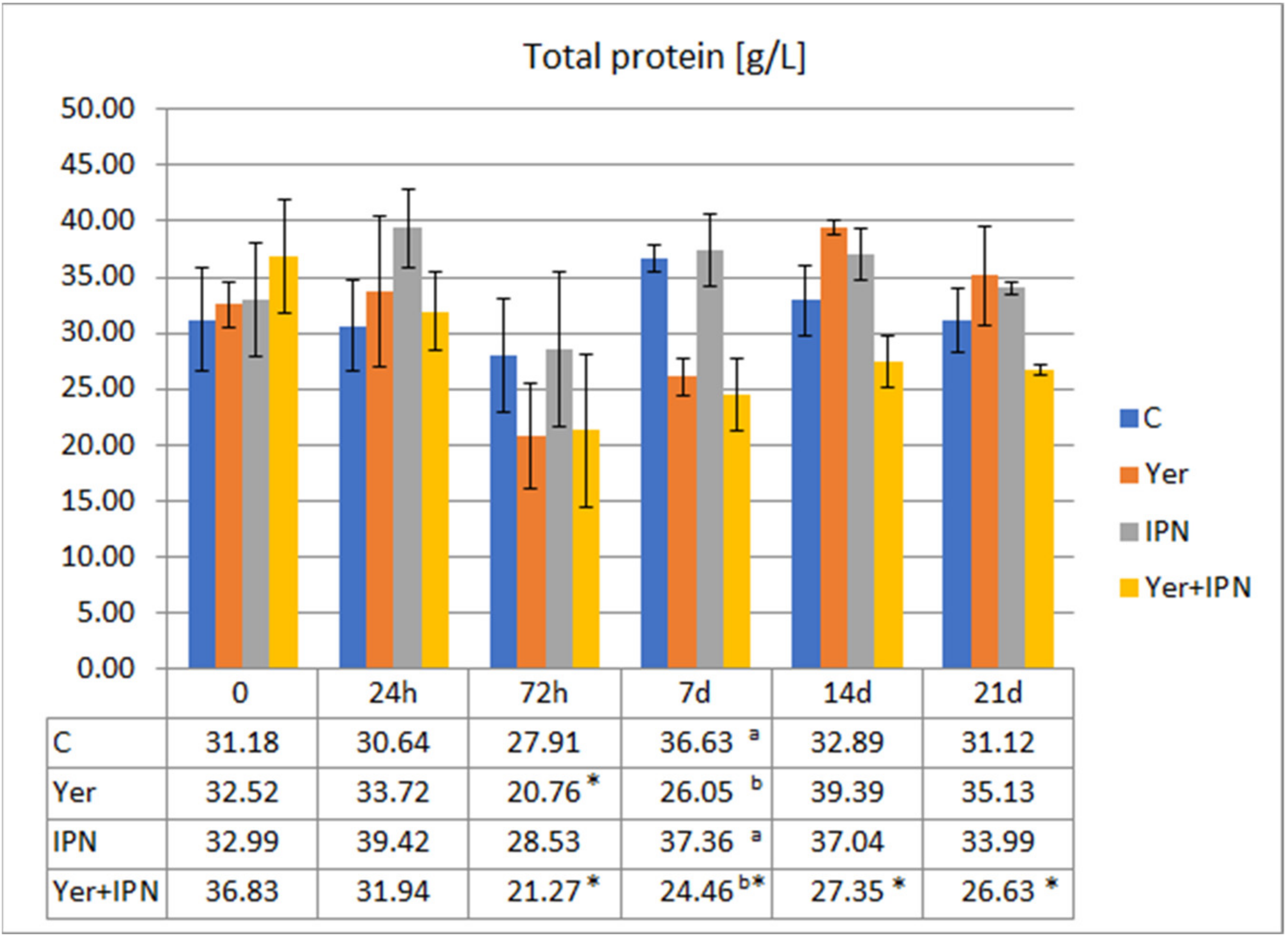
Total Protein
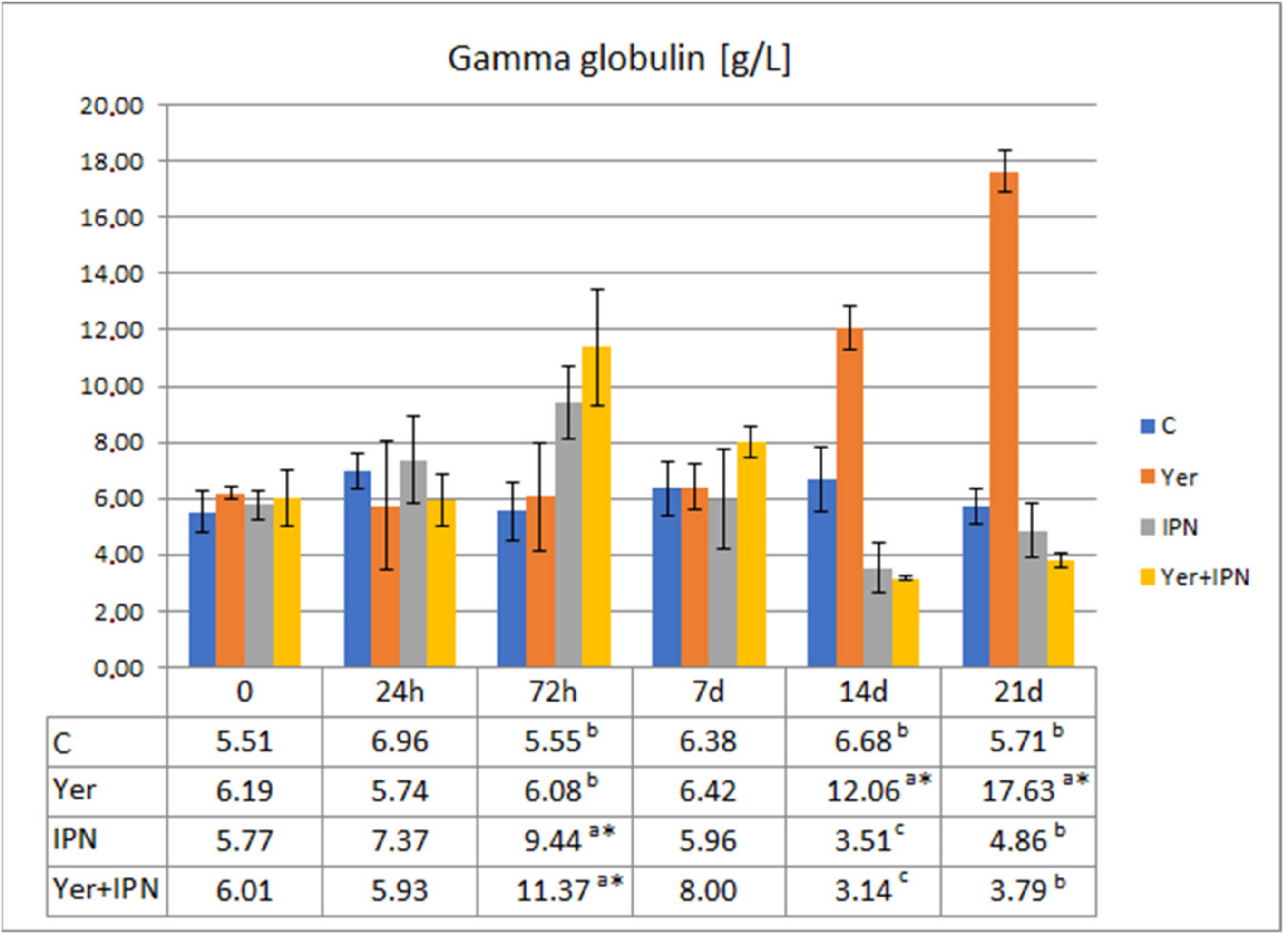
Gamma Globulin Level
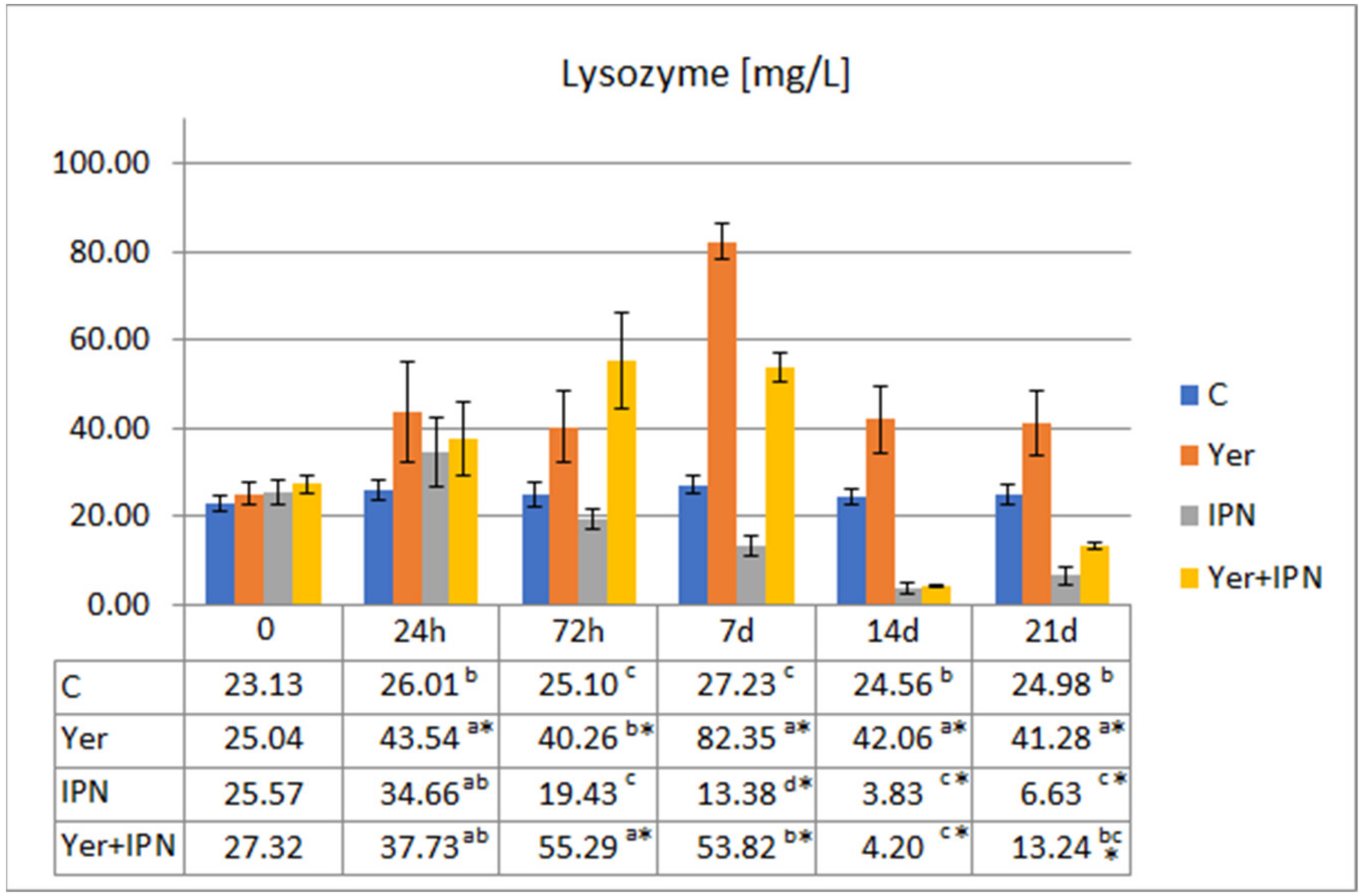
Lysozyme Activity
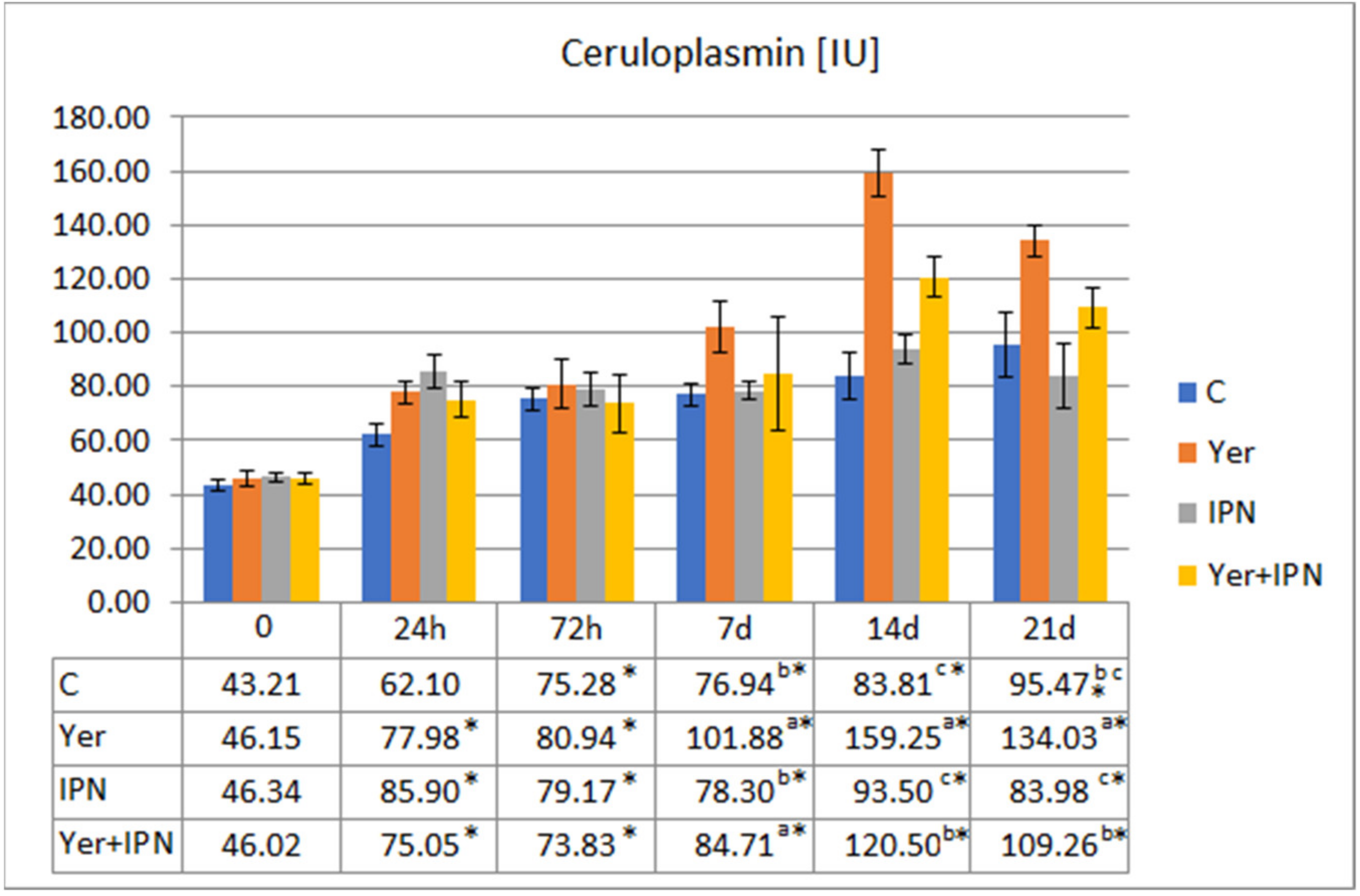
Ceruloplasmin Activity
- acetate buffer (pH 5.2 containing crystalline acetic acid, sodium acetate trihydrate, and 15 mg EDTA);
- buffered substrate solution (0.2% p-phenylenediamine (PPD) in acetic buffer);
- sodium azide solution (0.02% sodium azide solution in deionized water).
2.3.2. Tests Performed on Tissue
- nitro blue tetrazolium (NBT)—0.1% NBT (Sigma-Aldrich) to the first two rows (two replicates);
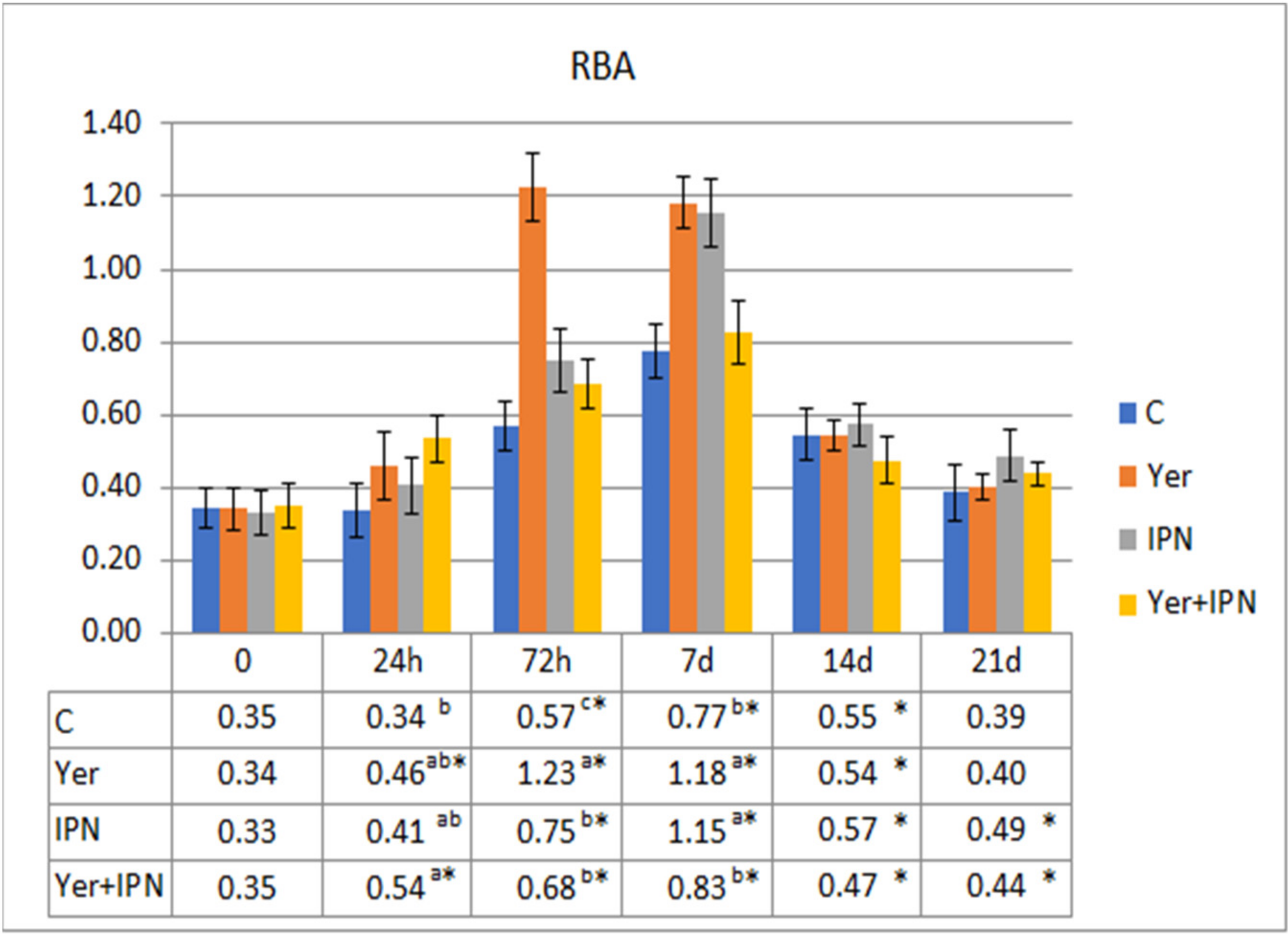
- RBA—PMA solution with 0.1% NBT to the next three rows (three replicates);
- PKA—bacterial suspension and 0.1% NBT to the last three rows (triplicate).
2.4. Statistical Analysis
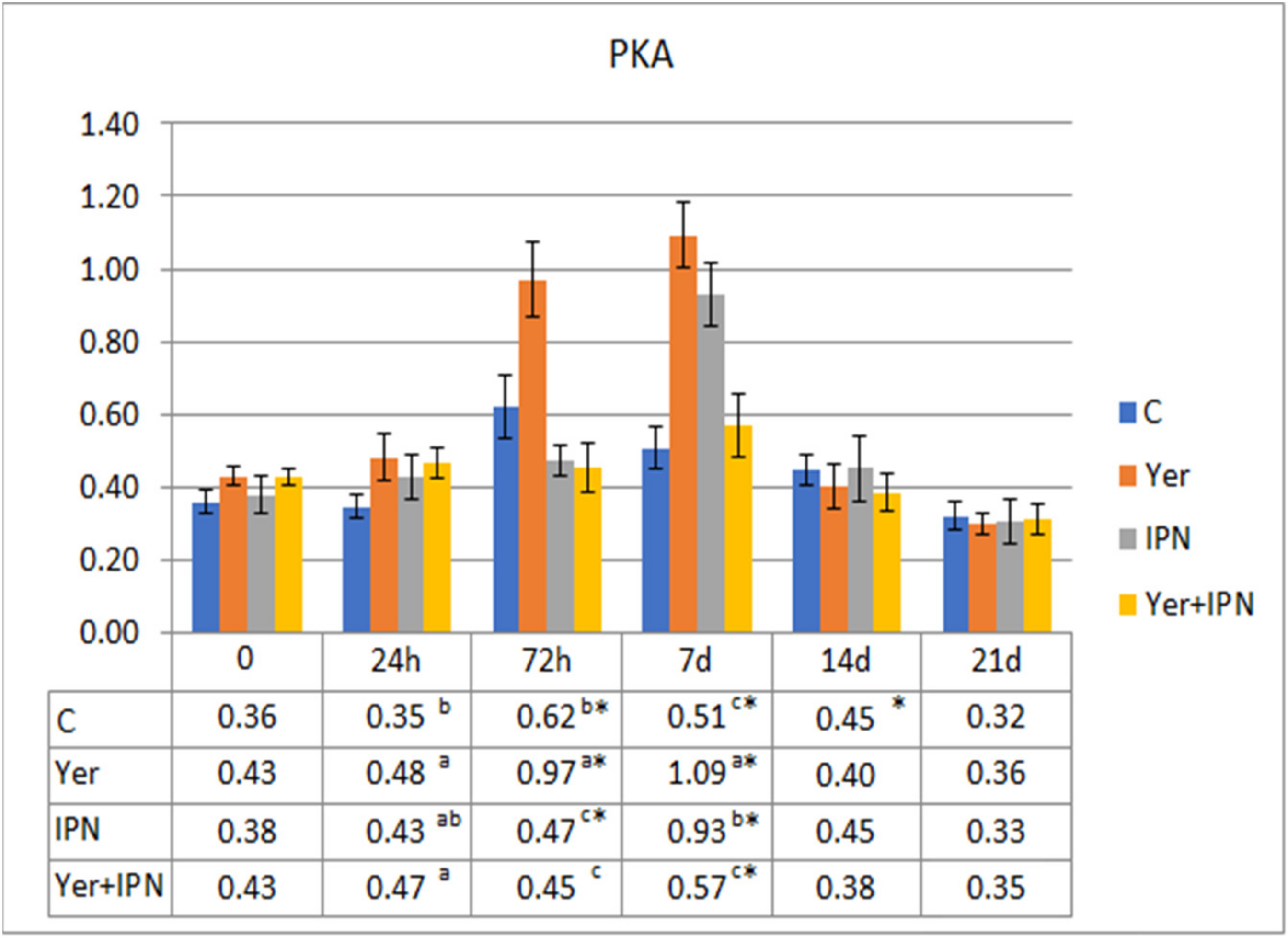
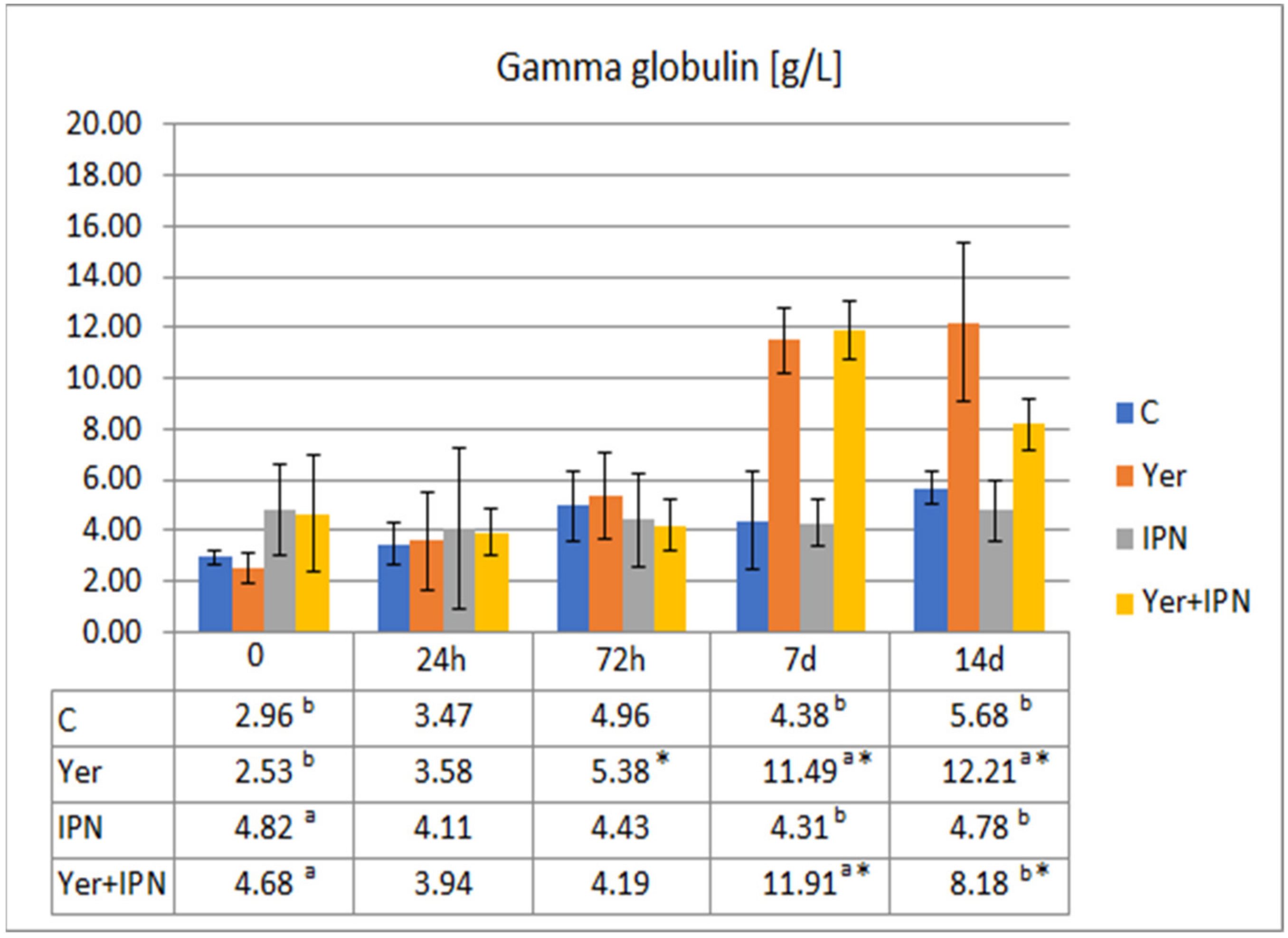
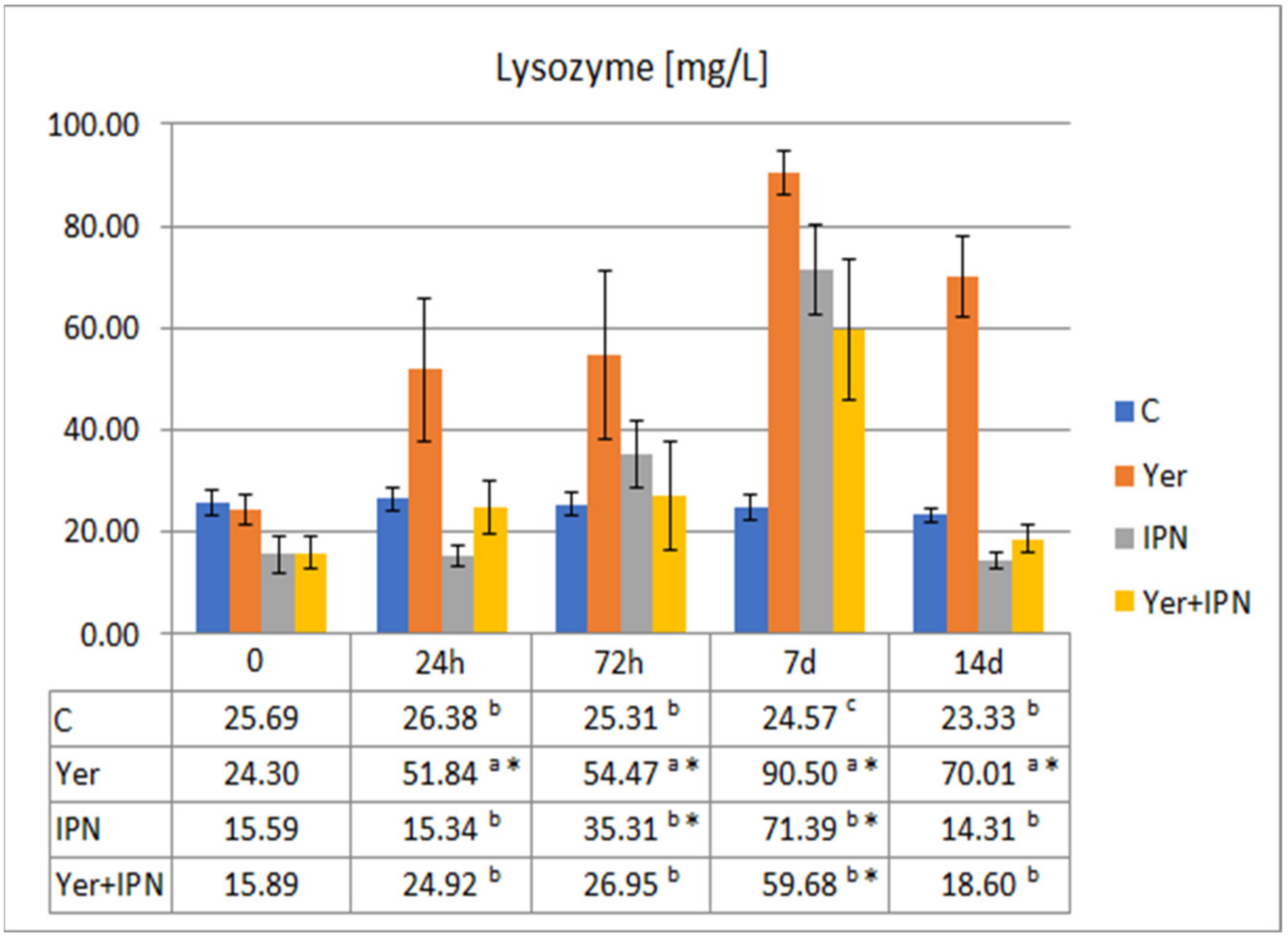
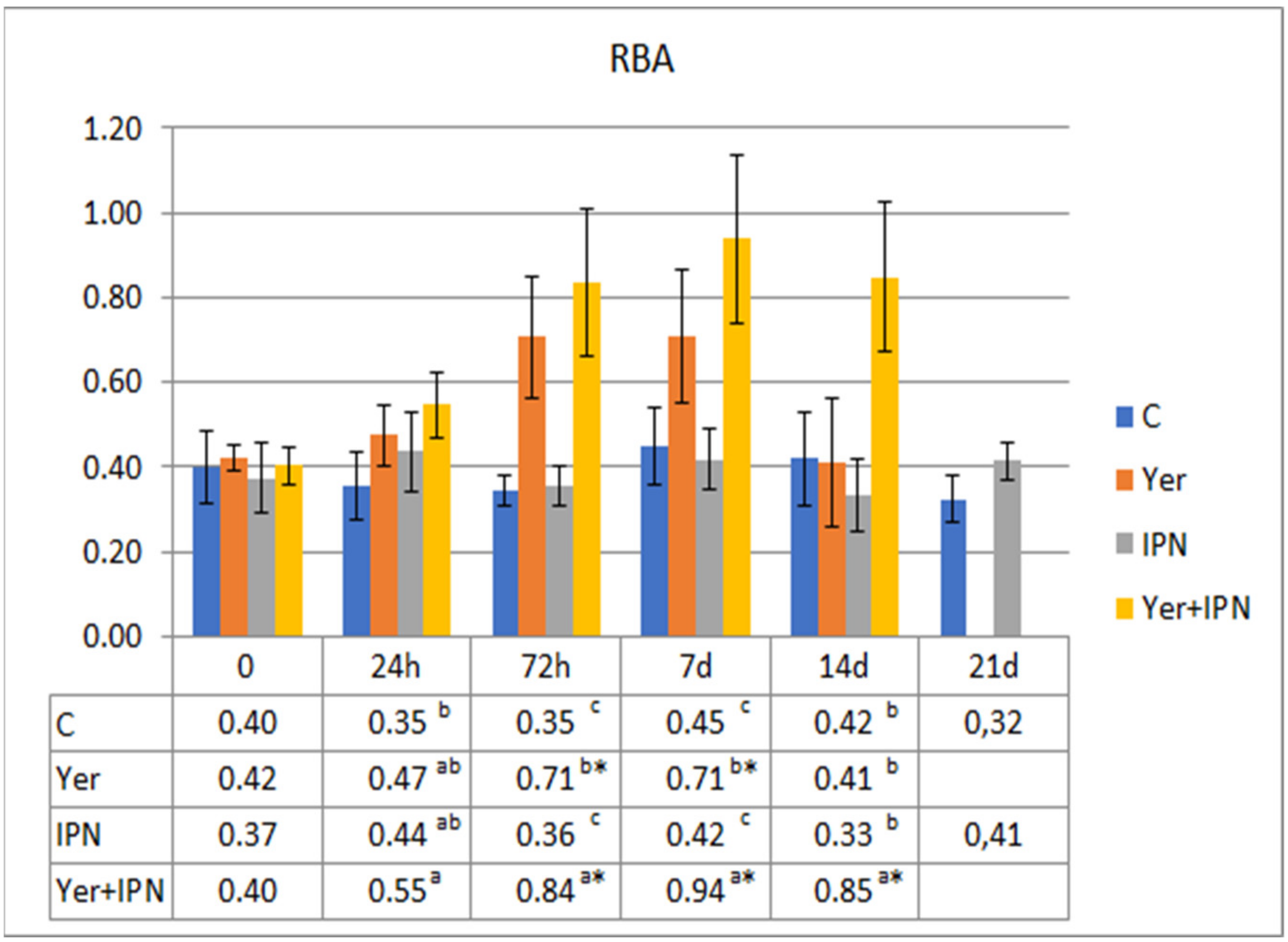
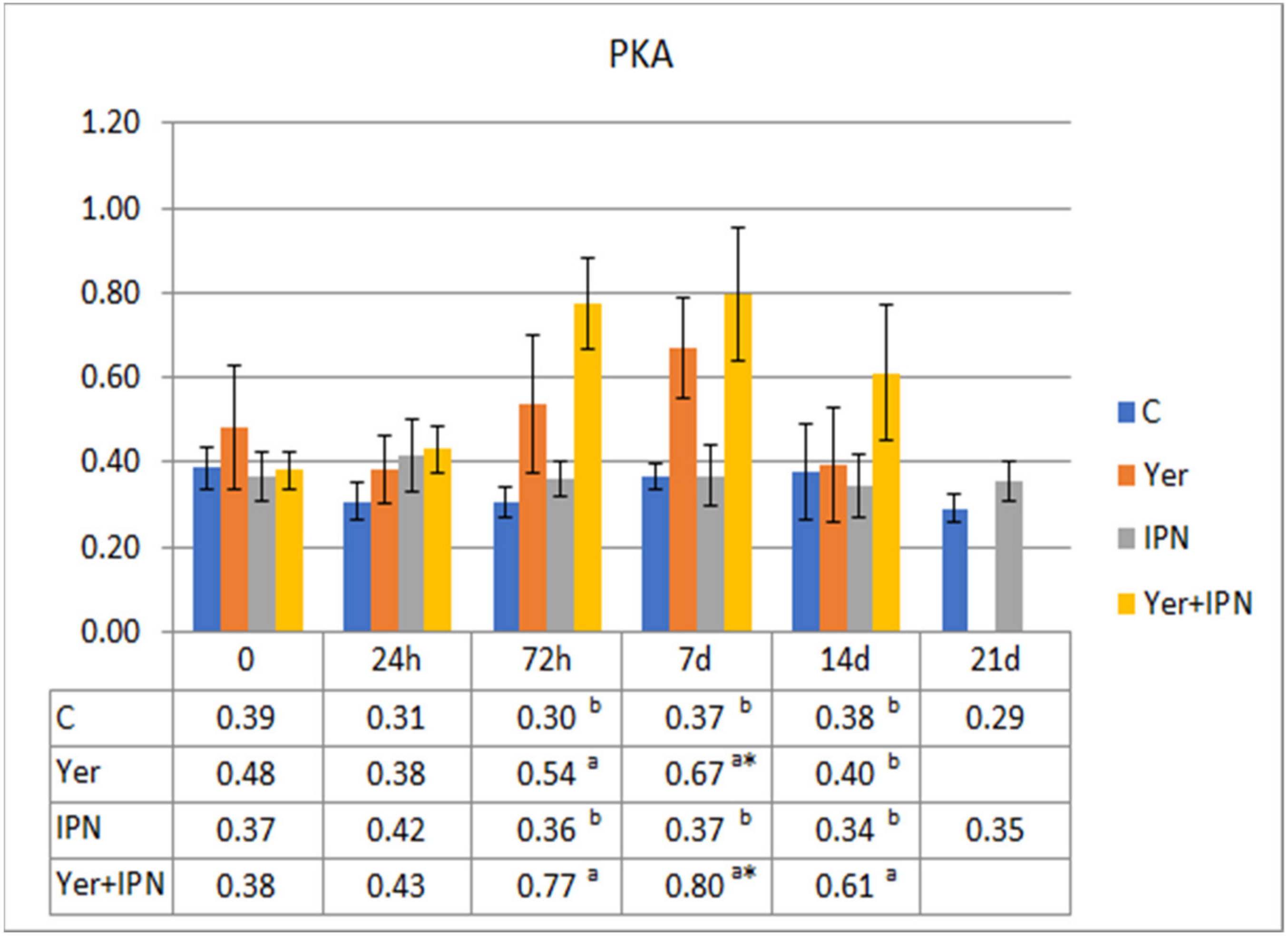
3. Results
3.1. Experiment I
3.2. Experiment II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018; United Nations: Rome, Italy, 2018; pp. 2–75. [Google Scholar] [CrossRef] [Green Version]
- FAO. FAO Fisheries & Aquaculture-Cultured Aquatic Species Information Programme (CASIP). Available online: http://www.fao.org/fishery/collection/cultured-species/en (accessed on 20 January 2021).
- Bruno, D.W.; Noguera, P.A.; Poppe, T.T. Viral Diseases. In A Colour Atlas of Salmonid Diseases, 2nd ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 52–72. [Google Scholar]
- Dhar, A.K.; LaPatra, S.; Orry, A.; Allnutt, F.C.T. Infectious Pancreatic Necrosis Virus. In Fish Viruses and Bacteria: Pathobiology and Protection; Woo, P.T.K., Cipriano, R.C., Eds.; Cabi Publishing: Wallingford, UK, 2017; pp. 1–12. [Google Scholar]
- Dopazo, C.P. The Infectious Pancreatic Necrosis Virus (IPNV) and its virulence determinants: What is known and what should be known. Pathogens 2020, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Menanteau-Ledouble, S.; Saleh, M.; El-Matbouli, M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet. Res. 2015, 46, 103. [Google Scholar] [CrossRef] [Green Version]
- Pajdak-Czaus, J.; Platt-Samoraj, A.; Szweda, W.; Siwicki, A.K.; Terech-Majewska, E. Yersinia ruckeri—A threat not only to rainbow trout. Aquac. Res. 2019, 50, 3083–3096. [Google Scholar] [CrossRef]
- Romalde, J.L.; Barja, J.L.; Magarinos, B.; Toranzo, A.E. Starvation-survival processes of the bacterial fish pathogen Yersinia ruckeri. Syst. Appl. Microbiol. 1994, 17, 161–168. [Google Scholar] [CrossRef]
- Busch, R.A.; Lingg, A.J. Establishment of an asymptomatic carrier state infection of Enteric Redmouth Disease in Rainbow trout (Salmo gairdneri). J. Fish. Res. Board. Can. 1975, 32, 2429–2432. [Google Scholar] [CrossRef]
- Coquet, L.; Cosette, P.; Quillet, L.; Petit, F.; Junter, G.A.; Jouenne, T. Occurrence and phenotypic characterization of Yersinia ruckeri strains with biofilm-forming capacity in a rainbow trout farm. Appl. Environ. Microbiol. 2002, 68, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willumsen, B. Birds and wild fish as potential vectors of Yersinia ruckeri. J. Fish Dis. 1989, 12, 275–277. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish, 6th ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 366–379. [Google Scholar] [CrossRef]
- Ohtani, M.; Villumsen, K.R.; Strom, H.K.; Raida, M.K. 3D visualization of the initial Yersinia ruckeri infection route in rainbow trout (Oncorhynchus mykiss) by optical projection tomography. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormsby, M.; Davies, R. Yersinia ruckeri. In Fish Viruses and Bacteria: Pathobiology and Protection; Woo, P.T.K., Cipriano, R.C., Eds.; Cabi Publishing: Wallingford, UK, 2017; pp. 339–351. [Google Scholar]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The impact of co-infections on fish: A review. Vet. Res. 2016, 47, 98. [Google Scholar] [CrossRef] [Green Version]
- Baba, E.; Acar, U.; Yilmaz, S.; Zemheri, F.; Ergun, S. Dietary olive leaf (Olea europea L.) extract alters some immune gene expression levels and disease resistance to Yersinia ruckeri infection in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 2018, 79, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Yang, T.I.; Liu, P.C.; Wu, J.L.; Hsu, Y.L. Dual challenges of Infectious Pancreatic Necrosis Virus and Vibrio carchariae in the grouper, Epinephelus sp. Virus Res. 1999, 63, 131–134. [Google Scholar] [CrossRef]
- Pajdak, J.; Terech-Majewska, E.; Platt-Samoraj, A.; Schulz, P.; Siwicki, A.K.; Szweda, W. Characterization of pathogenic Yersinia ruckeri strains and their importance in rainbow trout immunoprophylaxis. Med. Weter. 2017, 73, 579–584. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D. Immunostimulation in fish: Measuring the effects of stimulants by serological and immunological methods. US Fish. Wildl. Serv. IFI 1993, 1, 17. [Google Scholar]
- Siwicki, A.K.; Cossarini-Dunier, M. Effect of levamisole on the lymphocyte and macrophage activity in carp (Cyprinus carpio). Ann. Rech. Vet. 1990, 21, 95–100. [Google Scholar]
- Monte, M.M.; Urquhart, K.; Secombes, C.J.; Collet, B. Individual monitoring of immune responses in rainbow trout after cohabitation and intraperitoneal injection challenge with Yersinia ruckeri. Fish Shellfish Immunol. 2016, 55, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordmo, R. Strengths and weaknesses of different challenge methods. Dev. Biol. Stand. 1997, 90, 303–309. [Google Scholar] [PubMed]
- Johansen, L.H.; Sommer, A.I. Infectious pancreatic necrosis virus infection in Atlantic salmon Salmo salar post-smolts affects the outcome of secondary infections with infectious salmon anaemia virus or Vibrio salmonicida. Dis. Aquat. Organ. 2001, 47, 109–117. [Google Scholar] [CrossRef]
- Wedemeyer, G.; Chatterton, K. Some blood chemistry values for rainbow trout (Salmo gairdneri). J. Fish. Res. Board. Can. 1970, 27, 1162–1164. [Google Scholar] [CrossRef]
- McCarthy, D.H.; Stevenson, J.P.; Roberts, M.S. Some blood parameters of rainbow trout (Salmo gairdneri Richardson) I. The Kamloops variety. J. Fish. Biol. 1973, 5, 1–8. [Google Scholar] [CrossRef]
- McCarthy, D.H.; Stevenson, J.P.; Roberts, M.S. Some blood parameters of rainbow trout (Salmo gairdneri Richardson) II. The Shasta variety. J. Fish. Biol. 1975, 7, 215–219. [Google Scholar] [CrossRef]
- Rehulka, J.; Minarik, B.; Adamec, V.; Rehulkova, E. Investigations of physiological and pathological levels of total plasma protein in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 2005, 36, 22–32. [Google Scholar] [CrossRef]
- Altun, S.; Diler, O. Some haematological parameters of the rainbow trouts experimentally infected with Yersinia ruckeri. Turk. J. Vet. Anim. Sci. 1999, 23, 301–309. [Google Scholar]
- Wobeser, G. Outbreak of redmouth disease in rainbow trout (Salmo gairdneri) in Saskatchewan. J. Fish. Res. Board. Can. 1973, 30, 571–575. [Google Scholar] [CrossRef]
- Bruno, D.W.; Munro, A.L.S. Immunity in Atlantic salmon, Salmo salar L., fry following vaccination against Yersinia ruckeri, and the influence of body-weight and Infectious Pancreatic Necrosis Virus (IPNV) on the detection of carriers. Aquaculture 1989, 81, 205–211. [Google Scholar] [CrossRef]
- Johansen, L.-H.; Eggset, G.; Sommer, A.-I. Experimental IPN virus infection of Atlantic salmon parr; recurrence of IPN and effects on secondary bacterial infections in post-smolts. Aquaculture 2009, 290, 9–14. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Moyner, K.; Roed, K.H.; Sevatdal, S.; Heum, M. Changes in nonspecific immune parameters in Atlantic salmon, Salmo salar L., induced by Aeromonas salmonicida infection. Fish Shellfish Immunol. 1993, 3, 253–265. [Google Scholar] [CrossRef]
- Siwicki, A.; Studnicka, M. The phagocytic ability of neutrophils and serum lysozyme activity in experimentally infected carp, Cyprinus carpio L. J. Fish. Biol. 1987, 31, 57–60. [Google Scholar] [CrossRef]
- Studnicka, M.; Siwicki, A.; Ryka, B. Lysozyme level in carp (Cyprinus carpio L.). Bamidgeh 1986, 38, 22–25. [Google Scholar]
- Liu, H.; Peatman, E.; Wang, W.Q.; Abernathy, J.; Liu, S.K.; Kucuktas, H.; Terhune, J.; Xu, D.H.; Klesius, P.; Liu, Z.J. Molecular responses of ceruloplasmin to Edwardsiella ictaluri infection and iron overload in channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2011, 30, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Das, S.; Das Mahapatra, K.; Saha, J.N.; Baranski, M.; Odegard, J.; Robinson, N. Characterization of the ceruloplasmin gene and its potential role as an indirect marker for selection to Aeromonas hydrophila resistance in rohu, Labeo rohita. Fish Shellfish Immunol. 2013, 34, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Collet, B. Innate immune responses of salmonid fish to viral infections. Dev. Comp. Immunol. 2014, 43, 160–173. [Google Scholar] [CrossRef]
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.H.; Sommer, A.I. In vitro studies of Infectious Pancreatic Necrosis Virus-infections in leukocytes isolated from Atlantic salmon (Salmo salar L.). Aquaculture 1995, 132, 91–95. [Google Scholar] [CrossRef]
- Dopazo, C.P.; Moreno, P.; Olveira, J.G.; Borrego, J.J. The theoretical reliability of PCR-based fish viral diagnostic methods is critically affected when they are applied to fish populations with low prevalence and virus loads. J. Appl. Microbiol. 2018, 124, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Storset, A.; Evensen, O.; Midtlyng, P.J. A user’s inter-laboratory comparison of broodfish screening for infectious pancreatic necrosis virus using molecular and conventional diagnostic methods. Dev. Biol. 2006, 126, 101–105. [Google Scholar]
- Maj-Paluch, J.; Reichert, M. Role of infectious pancreatic necrosis virus (IPNV) infection in co-infections with other viruses. Med. Weter. 2018, 74, 243–246. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajdak-Czaus, J.; Schulz, P.; Terech-Majewska, E.; Szweda, W.; Siwicki, A.K.; Platt-Samoraj, A. Influence of Infectious Pancreatic Necrosis Virus and Yersinia ruckeri Co-Infection on a Non-Specific Immune System in Rainbow Trout (Oncorhynchus mykiss). Animals 2021, 11, 1974. https://doi.org/10.3390/ani11071974
Pajdak-Czaus J, Schulz P, Terech-Majewska E, Szweda W, Siwicki AK, Platt-Samoraj A. Influence of Infectious Pancreatic Necrosis Virus and Yersinia ruckeri Co-Infection on a Non-Specific Immune System in Rainbow Trout (Oncorhynchus mykiss). Animals. 2021; 11(7):1974. https://doi.org/10.3390/ani11071974
Chicago/Turabian StylePajdak-Czaus, Joanna, Patrycja Schulz, Elżbieta Terech-Majewska, Wojciech Szweda, Andrzej Krzysztof Siwicki, and Aleksandra Platt-Samoraj. 2021. "Influence of Infectious Pancreatic Necrosis Virus and Yersinia ruckeri Co-Infection on a Non-Specific Immune System in Rainbow Trout (Oncorhynchus mykiss)" Animals 11, no. 7: 1974. https://doi.org/10.3390/ani11071974
APA StylePajdak-Czaus, J., Schulz, P., Terech-Majewska, E., Szweda, W., Siwicki, A. K., & Platt-Samoraj, A. (2021). Influence of Infectious Pancreatic Necrosis Virus and Yersinia ruckeri Co-Infection on a Non-Specific Immune System in Rainbow Trout (Oncorhynchus mykiss). Animals, 11(7), 1974. https://doi.org/10.3390/ani11071974





