Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals
Abstract
Simple Summary
Abstract
1. Introduction
Strategies of Maternal Care
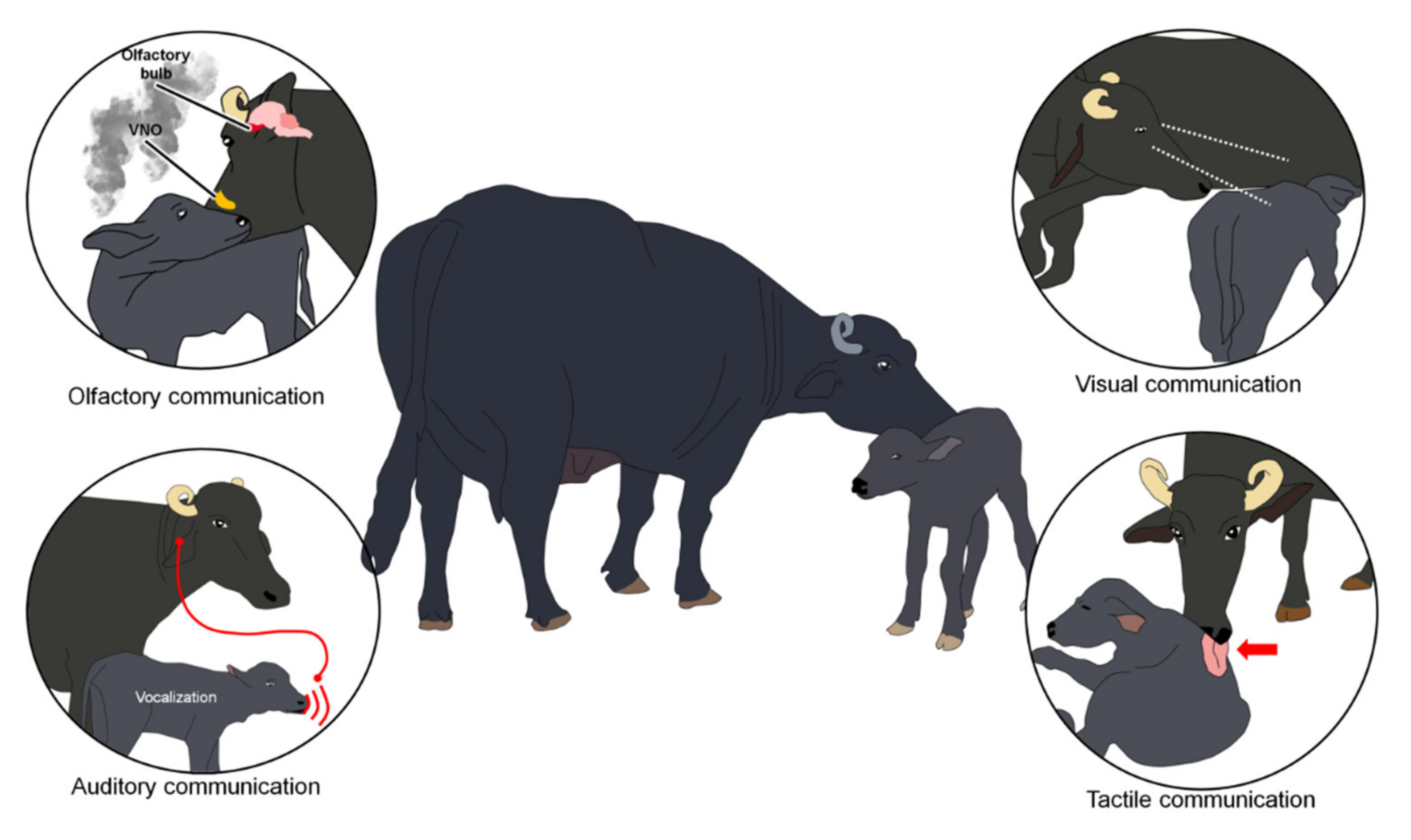
2. Sensory Stimulation and Communication during the Imprinting Process
2.1. Tactile Communication
2.2. Auditory Communication
2.3. Olfactory Communication
2.4. Visual Communication
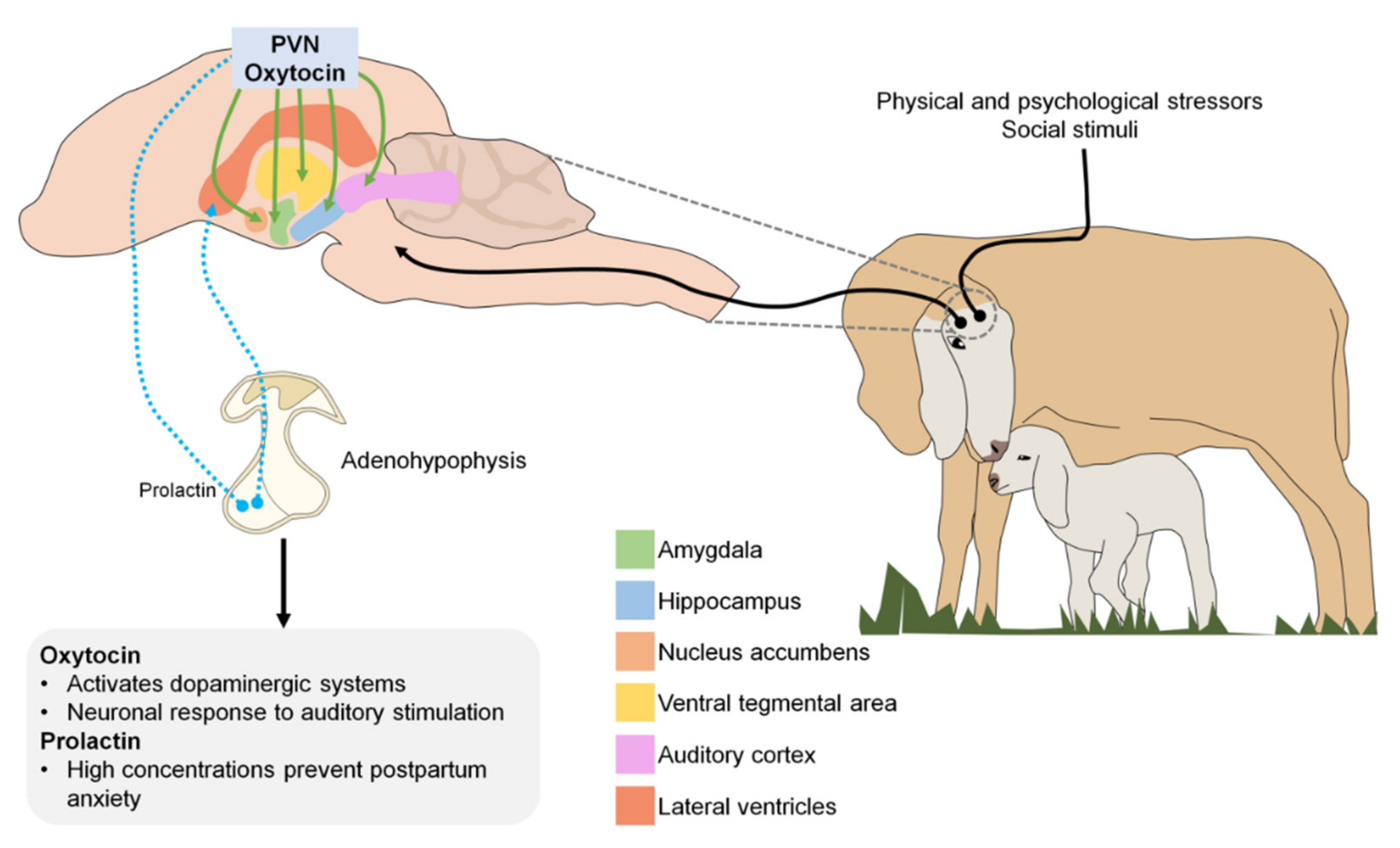
3. Neurobiological Mechanism in the Sensitive Period
4. Neurotransmitters Involved in Imprinting
5. Factors That Interfere with Imprinting during the Sensitive Period
5.1. Factors Inherent to Animals
5.2. Factors Related to the Environment
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zilkha, N.; Sofer, Y.; Beny, Y.; Kimchi, T. From classic ethology to modern neuroethology: Overcoming the three biases in social behavior research. Curr. Opin. Neurobiol. 2016, 38, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Mandujano-Camacho, H. Ecología y sociobiología de la impronta: Perspectivas para su estudio en los Crocodylia. Ciencia y Mar 2010, 14, 49–54. [Google Scholar]
- Mora-Medina, P.; Orihuela, A.; Arch-Tirado, E.; Vázquez, C.; Mota-Rojas, D. Metabolic changes during brief periods of ewe–lamb separation at different ages. Anim. Prod. Sci. 2018, 58, 1297–1306. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Aoki, N.; Kitajima, T.; Iikubo, E.; Katagiri, S.; Matsushima, T.; Homma, K.J. Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat. Commun. 2012, 3, 1081. [Google Scholar] [CrossRef]
- Fraser, A.F. Comportamiento de los Animales de Granja; Acribia: Zaragoza, España, 1980; p. 292. [Google Scholar]
- Maier, R. La evolución del aprendizaje. In Comportamiento Animal. Un Enfoque Evolutivo y Ecológico; Mc Graw Hill: Madrid, España, 2001; p. 541. [Google Scholar]
- Solano, J.; Orihuela, A.; Galina, C.S.; Aguirre, V. A note on behavioral responses to brief cow-calf separation and reunion in cattle (Bos indicus). J. Vet. Behav. 2007, 2, 10–14. [Google Scholar] [CrossRef]
- Sirovnik, J.; Varth, K.; decOliveira, D.; Ferneborg, S.; Haskell, M.J.; Hillmann, E.; Jensen, M.B.; Mejdell, C.M.; Napolitano, F.; Vaarst, M.; et al. Methodological terminology and definitions for research and discussion of cow-calf contact systems. J. Dairy Res. 2020, 87, S1. [Google Scholar] [CrossRef] [PubMed]
- Madigan, S.; Bakermans-Kranenburg, M.J.; Van Ljzendoorn, M.H.; Moran, G.; Pederson, D.R.; Benoit, D. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: A review and meta-analysis of a transmission gap. Attach. Hum. Dev. 2006, 8, 89–111. [Google Scholar] [CrossRef]
- Fillion, T.J.; Blass, E.M. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science 1986, 231, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Oxley, G.; Lovic, V.; Fleming, A. Effects of preweaning exposure to novel maternal odors on maternal responsiveness and selectivity in adulthood. Dev. Psychobiol. 2002, 41, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, K.M.; Hinton, M.R.; Atkins, K. Mothers determine sexual preferences. Nature 1998, 395, 229–230. [Google Scholar] [CrossRef]
- Galef, B.G.; Laland, K.N. Social learning in animals: Empirical studies and theoretical models. AIBS Bull. 2005, 55, 489–499. [Google Scholar] [CrossRef]
- Price, E.O.; Wallach, S.J.R. Physical isolation of hand-reared Hereford bulls increases their aggressiveness toward humans. Appl. Anim. Behav. Sci. 1999, 27, 263–267. [Google Scholar] [CrossRef]
- Laland, K.N. On the evolutionary consequences of sexual imprinting. Evolution 1994, 48, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Langmore, N.E. Functions of duet and solo songs of female birds. Trends Ecol. Evol. 1998, 13, 136–140. [Google Scholar] [CrossRef]
- Damián, J.P.; Hötzel, M.J.; Banchero, G.; Ungerfeld, R. Growing without a mother during rearing affects the response to stressors in rams. Appl. Anim. Behav. Sci. 2018, 209, 36–40. [Google Scholar] [CrossRef]
- Damián, J.P.; Beracochea, F.; Machado, S.; Hötzel, M.J.; Banchero, G.; Ungerfeld, R. Growing without a mother results in poorer sexual behaviour in adult rams. Animal 2018, 12, 98–105. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Nowak, R. Socio-spatial criteria are important for the establishment of maternal preference in lambs. Appl. Anim. Behav. Sci. 2006, 96, 269–280. [Google Scholar] [CrossRef]
- Murphey, R.M.; Paranhos da Costa, M.J.R.; de Souza Lima, L.O.; de Moura Duarte, F.A. Communal suckling in water buffalo (Bubalus bubalis). Appl. Anim. Behav. Sci. 1991, 28, 341–352. [Google Scholar] [CrossRef][Green Version]
- Murphey, R.M.; Paranhos da Costa, M.J.R.; Gomes da Silva, R.; de Souza, R. Allonursing in river buffalo, Bubalus bubalis: Nepotism, incompetence, or thievery? Anim. Behav. 1995, 49, 1611–1616. [Google Scholar] [CrossRef]
- Lanzoni, L.; Chincarini, M.; Giammarco, M.; Fusaro, I.; Gloria, A.; Contri, A.; Ferri, N.; Vignola, G. Materal and neonatal behaviour in Italian Mediterranean Buffaloes. Animals 2021, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Poindron, P. Mechanisms of activation of maternal behaviour in mammals. Reprod. Nutr. Dev. 2005, 45, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Mora-Medina, P.; Orihuela, A.; Arch-Tirado, E.; Roldan-Santiago, P.; Terrazas, A.; Mota-Rojas, D. Sensory factors involved in mother-young bonding in sheep: A review. Vet. Med-Czech. 2016, 61, 595e611. [Google Scholar] [CrossRef]
- Napolitano, F.; Mota-Rojas, D.; Guerrero Legarreta, I.; Orihuela, A. The Latin American River Buffalo, Recent Findings, 3rd ed.; BM Editores Press: Mexico City, Mexico, 2020; pp. 1–1505. (In Spanish) [Google Scholar]
- Nowak, R.; Porter, R.H.; Levy, F.; Orgeur, P.; Schaal, B. Role of mother-young interactions in the survival of offspring in domestic mammals. Rev. Reprod. 2000, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Mariti, C.; Mota-Rojas, D.; Martínez-Burnes, J.; Barrios-García, H.; Gazzano, A. Maternal behaviour in domestic dogs. Intl. J. Vet. Sci. Med. 2019, 7, 20–30. [Google Scholar] [CrossRef]
- Rutberg, A.T. Birth synchrony in American Bison (Bison bison): Response to predation or season? J. Mammal. 1984, 65, 418–423. [Google Scholar] [CrossRef]
- Dwyer, C.M. Behavioural development in the neonatal lamb: Effect of maternal and birth-related factors. Theriogenology 2003, 59, 1027–1050. [Google Scholar] [CrossRef]
- Keverne, E.B.; Kendrick, K.M. Oxytocin facilitation of maternal behavior in sheep. Ann. N.Y. Acad. Sci. 1992, 652, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kamboj, M.L.; Chandra, S.; Kumar, R. Effect of calf suckling dummy calf used and weaning on milk ejection stimuli and milk yield of Murrah buffaloes (Bubalus bubalis). J. Pharmacogn. Phytochem. 2017, SP1, 1012–1015. [Google Scholar]
- Dubey, P.; Singh, R.R.; Choudhary, S.S.; Verma, K.K.; Kumar, A.; Gamit, P.M.; Dubey, S.; Prajapati, K. Post parturient neonatal behaviour and their relationship with maternal behaviour score, parity and sex in Surti buffaloes. J. Appl. Anim. Res. 2018, 46, 360–364. [Google Scholar] [CrossRef]
- Vince, M.A. Newborn lambs and their dams: The interaction that leads to sucking. Adv. Stud. Behav. 1993, 22, 239–268. [Google Scholar]
- Nowak, R.; Keller, M.; Val-Laillet, D.; Levy, F. Perinatal visceral events and brain mechanisms involved in the development of mother-young bonding in sheep. Horm. Behav. 2007, 52, 92–98. [Google Scholar] [CrossRef]
- González-Mariscal, G.; Poindron, P. Parental care in mammals: Immediate internal and sensory factors of control. In Hormones, Brain and Behavior, 1st ed.; Pfaff, D.W., Arnold, A.P., Etgen, A.M., Fahrfbach, S.E., Rubin, R.T., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 215–298. [Google Scholar]
- Numan, M.; Fleming, A.S.; Lévy, F. Maternal Behavior. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, J.D., Ed.; Elsevier: London, UK, 2006; pp. 1921–1993. [Google Scholar]
- Mora-Medina, P.; Napolitano, F.; Mota-Rojas, D.; Berdugo-Gutiérrez, J.; Ruiz-Buitrago, J.; Guerrero-Legarreta, I. Imprinting, Sucking and Allosucking Behaviors in Buffalo Calves. J. Buffalo Sci. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- De Rosa, G.; Grasso, F.; Braghieri, A.; Bilancione, A.; Di Francia, A.; Napolitano, F. Behavior and milk production of buffalo cows as affected by housing system. J. Dairy Sci. 2009, 92, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.M.; Quirino, C.R.; Bastos, R. Effect of nursing behaviour, sex of the calf, and parity order on milk production of buffaloes. Rev. Colomb. Cienc. Pec. 2017, 30, 30–38. [Google Scholar] [CrossRef][Green Version]
- Vichová, J.; Bartos, L. Allosucking in cattle: Gain or compensation? Appl. Anim. Behav. Sci. 2005, 94, 223–235. [Google Scholar] [CrossRef]
- Das, S.M.; Redbo, I.; Wiktorsson, H. Effects of age of calf on suckling behaviour and other behavioural activities of Zebu and crossbred calves during restricted suckling periods. Appl. Anim. Behav. Sci. 2000, 67, 47–57. [Google Scholar] [CrossRef]
- Roulin, A.; Geeb, P. The immunological function of allosuckling. Ecol. Lett. 1999, 2, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Paranhos da Costa, M.J.R.; Andriolo, A.; de Oliveira, J.F.S.; Schmidek, W.R. Suckling and allosuckling in river buffalo calves and its relation with weight gain. Appl. Anim. Behav. Sci. 2000, 66, 1–10. [Google Scholar] [CrossRef]
- Roulin, A. Why do lactating females nurse alien offspring? A review of hypotheses and empirical evidence. Anim. Behav. 2002, 63, 201–208. [Google Scholar] [CrossRef]
- Wierucka, K.; Pitcher, B.J.; Harcourt, R.; Charrier, I. Multimodal mother–offspring recognition: The relative importance of sensory cues in a colonial mammal. Anim. Behav. 2018, 146, 135–142. [Google Scholar] [CrossRef]
- Horn, G. Neural mechanisms of learning: An analysis of imprinting in the domestic chick. Proc. R. Soc. Lond. Series B 1981, 213, 101–137. [Google Scholar]
- Knudsen, E.I. Sensitive Periods in the Development of the Brain and Behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef]
- Keller, M.; Meurisse, M.; Poindron, P.; Nowak, R.; Ferreira, G.; Shayit, M.; Levy, F. Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Dev. Psychobiol. 2003, 43, 167–176. [Google Scholar] [CrossRef]
- Griffith, M.K.; Williams, G.L. Contribution of maternal vision and olfaction to suckling-mediated inhibition of LH secretion, the expression of maternal selectivity, and lactation in beef cows. Biol. Reprod. 1996, 54, 761–768. [Google Scholar] [CrossRef]
- Hudson, S.J.; Mullord, M.M. Investigations of maternal bonding in dairy cattle. Appl. Anim. Ethol. 1977, 3, 271–276. [Google Scholar] [CrossRef]
- Johnsen, J.F.; Zipp, K.A.; Kälber, T.; de Passillé, A.M.; Knierim, U.; Barth, K.; Mejdell, C.M. Is rearing calves with the dam a feasible option for dairy farms? Current and future research. Appl. Anim. Behav. Sci. 2016, 181, 1–11. [Google Scholar] [CrossRef]
- Lévy, F.; Keller, M.; Poindron, P. Olfactory regulation of maternal behavior in mammals. Hom. Behav. 2004, 46, 284–302. [Google Scholar] [CrossRef]
- Corona, R.; Meurisse, M.; Cornilleau, F.; Moussu, C.; Keller, M.; Lévy, F. Disruption of adult olfactory neurogenesis induces deficits in maternal behavior in sheep. Behav. Brain Res. 2018, 347, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Arenas, A. Aprendizaje olfativo temprano en la abeja (Apis mellifera) y su rol en la toma de Decisiones Relacionadas con la obtención de recursos. Ph.D. Thesis, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina, 2009. [Google Scholar]
- Rosillon-Warnier, A.; Paquay, R. Development and consequences of teat-order in piglets. Appl. Anim. Behav. Sci. 1984, 13, 47–58. [Google Scholar] [CrossRef]
- Matamala, F.; Strappini, A.; Sepúlveda-Varas, P. Dairy cow behaviour around calving: Its relationship with management practices and environmental conditions. Austral J. Vet. Sci. 2021, 53, 9–22. [Google Scholar] [CrossRef]
- Bordi, F.; LeDoux, J.E. Response properties of single unit I areas of rat auditory thalamus that project to the amygdala. Exp. Brain Res. 1994, 98, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Pramanik, P.S.; Kashyap, S.S. Dam-calf interactions in Murrah buffaloes up to six hours post-parturition. Indian J. Anim. Prod. Manag. 2009, 25, 78–80. [Google Scholar]
- Nowak, R.; Boivin, X. Filial attachment in sheep: Similarities and differences between ewe-lamb and human-lamb relationships. Appl. Anim. Behav. Sci. 2015, 164, 12–28. [Google Scholar] [CrossRef]
- Sato, S.; Sako, S.; Maeda, A. Social licking patterns in cattle (Bos taurus): Influence of environmental and social factors. Appl. Anim. Behav. Sci. 1991, 32, 3–12. [Google Scholar] [CrossRef]
- Mills, M.; Melhuish, E. Recognition of mother’s voice in early infancy. Nature 1974, 252, 123–124. [Google Scholar] [CrossRef]
- Balcombe, P. Vocal recognition of pups by mother Mexican free-tailed bats, Tadarida brasiliensis mexicana. Anim. Behav. 1990, 39, 60–66. [Google Scholar] [CrossRef]
- Knörnschild, M.; Von Helversen, O. Nonmutual vocal mother–pup recognition in the greater sac-winged bat. Anim. Behav. 2008, 76, 1001–1009. [Google Scholar] [CrossRef]
- Kober, M.; Trillmich, F.; Naguib, M. Vocal mother-offspring communication in guinea pigs: Females adjust maternal responsiveness to litter size. Front. Zool. 2008, 5, 13. [Google Scholar] [CrossRef]
- Torriani, M.V.G.; Vannoni, E.; McElliogot, A.G. Mother-Young recognition in an Ungulate Hider Species: An unidirectional process. Am. Naturalist 2006, 168, 412–420. [Google Scholar] [CrossRef]
- Charrier, I.; Burlet, A.; Aubin, T. Social vocal communication in captive Pacific walruses Odobenus rosmarus divergens. Mamm. Biol. 2010, 76, 622–627. [Google Scholar] [CrossRef]
- Briefer, E.; McElligott, A.G. Mutual mother-offspring vocal recognition in an ungulate hiders species (Capra hircus). Anim. Cong. 2011, 14, 585–598. [Google Scholar] [CrossRef]
- De la Torre, M.P.; Briefer, E.F.; Ochocki, B.M.; McElligott, A.G.; Reader, T. Mother–offspring recognition via contact calls in cattle, Bos taurus. Anim. Behav. 2016, 114, 147–154. [Google Scholar] [CrossRef]
- Sèbe, F.; Nowak, R.; Poindron, P.; Aubin, T. Establishment of vocal communication and discrimination between ewes and their lamb in the first two days after parturition. Dev. Psychobiol. 2007, 49, 375–386. [Google Scholar] [CrossRef]
- Pickup, H.; Dwyer, C. Breed differences in the expression of maternal care at parturition persist throughout the lactation period in sheep. Appl. Anim. Behav. Sci. 2011, 132, 33–41. [Google Scholar] [CrossRef]
- Sèbe, F.; Duboscq, J.; Aubin, T.; Ligout, S.; Poindron, P. Early vocal recognition of mother by lambs: Contribution of low- and high- frequency vocalizations. Anim. Behav. 2010, 79, 1055–1066. [Google Scholar] [CrossRef]
- Morgan, P.D.; Boundy, C.A.P.; Arnold, G.W.; Lindsay, D.R. The roles played by the senses of the ewe in the location and recognition of lambs. Reprod. Dev. Behav. Sheep 1985, 181–192. [Google Scholar] [CrossRef]
- Corona, R.; Frédéric, L. Chemical olfactory signals and parenthood in mammals. Horm. Behav. 2015, 68, 77–90. [Google Scholar] [CrossRef]
- Sánchez-Andrade, G.; James, B.M.; Kendrick, K.M. Neural encoding of olfactory recognition memory. J. Reprod. Dev. 2005, 51, 547–558. [Google Scholar] [CrossRef]
- Poindron, P.; Otal, J.; Ferreira, G.; Keller, M.; Guesdon, V.; Nowak, R.; Lévy, F. Amniotic fluid is important for the maintenance of maternal responsiveness and the establishment of maternal selectivity in sheep. Animal 2010, 4, 2057–2064. [Google Scholar] [CrossRef]
- Špinka, M.; Maletínská, J.; Víchová, J.; Stěhulová, I. Individual recognition of piglets by sows in the early post-partum period. Behaviour 2002, 139, 975–991. [Google Scholar] [CrossRef]
- Poindron, P.; Levy, F.; Keller, M. Maternal responsiveness and maternal selectivity in domestic sheep and goat: The two facets of maternal attachments. Dev. Psychol. 2007, 49, 54–70. [Google Scholar] [CrossRef]
- Hernández, H.; Terrazas, A.; Poindron, P.; Ramírez-Vera, S.; Flores, J.A.; Delgadillo, J.A.; Vielma, J.; Duarte, G.; Fernández, I.G.; Fitz-Rodríguez, G.; et al. Sensorial and physiological control of maternal behavior in small ruminants: Sheep and goats. Trop. Subtrop. Agroecosys. 2012, 15, s91–s102. [Google Scholar]
- Ramírez, M.; Soto, R.; Poindron, P.; Alvarez, L.; Valencia, J.J.; González, F.; Terrazas, A. Maternal behaviour around birth and mother-young recognition in Pelibuey sheep. Vet. Mex. 2011, 42, 27–46. [Google Scholar]
- Booth, K.K.; Katz, L.S. Role of the vomeronasal organ in neonatal offspring recognition in sheep. Biol. Reprod. 2000, 63, 953–958. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Orihuela, A.; Napolitano, F.; Mora-Medina, P.; Alonso-Spilsbury, M. Olfaction in animal behaviour and welfare. CAB Rev. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Alexander, G. Odour, and the recognition of lambs by Merino ewes. Appl. Anim. Ethol. 1978, 4, 153–158. [Google Scholar] [CrossRef]
- Poindron, P.; Gilling, G.; Hernandez, H.; Serafin, N.; Terrazas, A. Early recognition of newborn goat kids by their mother: I. Nonolfactory discrimination. Dev. Psychobiol. 2003, 43, 82–89. [Google Scholar] [CrossRef]
- Brennan, P.A.; Kendrick, K.M. Mammalian social odours: Attraction and individual recognition. Philos. T. R. Soc. B. 2006, 361, 2061–2078. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A. The cognitive neuroscience of creativity. Psychon. B. Rev. 2004, 11, 1011–1026. [Google Scholar] [CrossRef]
- Horn, G.; McCabe, B.J.; Bateson, P.P.G. An autoradiographic study of the chick brain after imprinting. Brain Res. 1979, 168, 361–373. [Google Scholar] [CrossRef]
- Bradley, P. Development of two regions of the chick telencephalon. Dev. Brain Res. 1985, 20, 83–88. [Google Scholar] [CrossRef]
- Bale, T.L.; Picetti, R.; Contarino, A.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002, 22, 193–199. [Google Scholar] [CrossRef]
- Bale, T.L.; Vale, W.W. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. 2004, 44, 525–557. [Google Scholar] [CrossRef]
- Bustos, M. Núcleo accumbens y el sistema motivacional a cargo del apego. Rev. Chil. Neuro. Psiquiat. 2008, 46, 207–215. [Google Scholar] [CrossRef]
- Insel, T.R.; Young, L.J. The neurobiology of attachment. Neuroscience 2001, 2, 129–136. [Google Scholar] [CrossRef]
- Ikemoto, S.; Panksepp, J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 1999, 31, 6–41. [Google Scholar] [CrossRef]
- Guardini, G.; Bowen, J.; Mariti, C.; Fatjó, J.; Sighieri, C.; Gazzano, A. Influence of maternal care on behavioural development of domestic dogs (Canis familiaris) living in a home environment. Animals 2017, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sierra, E.; Chico, P.L.F.; Gordillo, D.L.F.; Portugal, R.A. Neurotransmisores del sistema límbico. Hipocampo, GABA y memoria. Primera parte. Salud Mental. 2007, 30, 7–15. [Google Scholar]
- Love, T.M. Oxytocin, motivation and the role of Dopamine. Pharmacol. Biochem. Behav. 2014, 119, 49–60. [Google Scholar] [CrossRef]
- Acevedo-Rodríguez, A.; Mani, S.K.; Handa, R.J. Oxytocin and estrogen receptor β in the brain: An overview. Front. Endocrinol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Le Neindre, P.; Poindron, P.; Delouis, C. Hormonal induction of maternal behavior in non-pregnant ewes. Physiol. Behav. 1979, 22, 731–734. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Caldwell, J.D.; Walker, C.; Ayers, G.; Mason, G.A. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav. Neurosci. 1994, 108, 1163–1171. [Google Scholar] [CrossRef]
- Boccia, M.L.; Goursaud, A.P.S.; Bachevalier, J.; Anderson, K.D.; Pedersen, C.A. Peripherally administered non-peptide oxytocin antagonist, L368,899®, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Horm. Behav. 2007, 52, 344–351. [Google Scholar] [CrossRef]
- Kim, S.; Soken, T.A.; Cromer, S.J.; Martínez, S.R.; Hardy, L.R.; Strathearn, L. Oxytocin and postpartum depression: Delivering on what´s known and what´s not. Brain Res. 2014, 11, 219–232. [Google Scholar] [CrossRef]
- Rich, M.E.; de Cárdenas, E.J.; Lee, H.J.; Caldwell, H.K. Impairments in the Initiation of Maternal Behavior in Oxytocin Receptor Knockout Mice. PLoS ONE 2014, 9, e98839. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.K. Neurobiology of sociability. Adv. Exp. Med. Biol. 2012, 739, 187–205. [Google Scholar]
- Strathearn, L. Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. J. Neuroendocrinol. 2011, 23, 1054–1065. [Google Scholar] [CrossRef]
- Chaiseha, Y.; Ngernsoungner, P.; Sartsoongnoen, N.; Prakobsaeng, N.; El Halawani, M.E. Presence of prolactin mRNA in extra-pituitary brain areas in the domestic turkey. Acta Histochem. 2012, 114, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.S.; Gratta, D.R. Prolactin-induced neurogenesis in the maternal brain. Trends Endocrin. Mets. 2003, 14, 199–201. [Google Scholar] [CrossRef]
- Larsen, C.M.; Grattan, D.R. Prolactin, neurogenesis, and maternal behaviors. Brain Behav. Immun. 2012, 26, 201–209. [Google Scholar] [CrossRef]
- Edwards, S.A.; Broom, D.M. behavioural interactions of dairy cows with their newborn calves and the effects of parity. Anim. Behav. 1982, 30, 525–535. [Google Scholar] [CrossRef]
- Dwyer, C.M. Maternal behaviour and lamb survival: From neuroendocrinology to practical application. Animal 2014, 8, 102–112. [Google Scholar] [CrossRef]
- Dwyer, C.M. Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. J. Anim. Sci. 2008, 86, E259–E270. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.; Smith, L. Parity effects on maternal behaviour are not related to circulating oestradiol concentrations in two breeds of sheep. Physiol. Behav. 2008, 93, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M.; Lawrence, A.B. Maternal behaviour in domestic sheep (Ovis aries): Constancy and change with maternal experience. Behaviour 2000, 137, 1391–1413. [Google Scholar] [CrossRef]
- González-Stagnaro, C. Comportamiento maternal en ovejas West African y mortalidad de corderos. Mundo Pecuario 2012, 8, 33–48. [Google Scholar]
- Meurisse, M.; González, A.; Delsol, G.; Caba, M.; Levy, F.; Poindron, P. Estradiol receptor-α expression in hypothalamic and limbic regions of ewes is influenced by physiological state and maternal experience. Horm. Behav. 2005, 48, 34–43. [Google Scholar] [CrossRef]
- González, E.G.; Cuellar, A.; Hernández, H.; Nandayapa, E.; Álvarez, L.; Tórtora, J.; Terrazas, A. Maternal experience in Romanov sheep impairs mother-lamb recognition during the first 24 hours postpartum. J. Vet. Behav. 2015, 10, 66–72. [Google Scholar] [CrossRef]
- El-Regalaty, H.A.; Aboul-Ela, H.B. Non-genetic factors affecting incidence of abortion, stillbirth and post–natal mortality of Egyptian buffaloes. J. Anim. Poultry Prod. 2014, 5, 313–324. [Google Scholar] [CrossRef]
- Ghavi, H.-Z.N.; Madad, M.; Shadparvar, A.; Kianzad, D. An observational analysis of secondary sex ratio, stillbirth and birth weight in Iranian Buffaloes (Bubalus bubalis). J. Agric. Sci. Tech. 2012, 14, 1477–1484. [Google Scholar]
- Mota-Rojas, D.; De Rosa, G.; Mora-Medina, P.; Braghieri, A.; Guerrero-Legarreta, I.; Napolitano, F. Dairy buffalo behaviour and welfare from calving to milking. CAB Rev. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Lévy, F.; Kendrick, K.M.; Goode, J.A.; Guevara-Guzman, R.; Keverne, E.B. Oxytocin and vasopressin release in the olfactory bulb of parturient ewes: Changes with maternal experience and effects on acetylcholine, γ-aminobutyric acid, glutamate and noradrenaline release. Brain Res. 1995, 669, 197–206. [Google Scholar] [CrossRef]
- Owens, J.L.; Bindon, B.M.; Edey, T.N.; Piper, L.R. Behaviour at parturition and lamb survival of Booroola Merino sheep. Livest. Prod. Sci. 1985, 13, 359–372. [Google Scholar] [CrossRef]
- Castanheira, M.; McManus, C.M.; Neto, P.; da Costa, M.; Méndez, F.D.; Sereno, J.R.; Bertoli, C.D.; Fioravanti, M. Maternal offspring behaviour in Curraleiro Pé Duro naturalized cattle in Brazil. Rev. Bras. Zootecn. 2013, 42, 584–591. [Google Scholar] [CrossRef]
- O’Connor, C.E.; Lawrence, A.B.; Wood-Gush, D.G.M. Influence of litter size and parity on maternal behaviour at parturition on Scottish Blackface sheep. Appl. Anim. Behav. Sci. 1992, 33, 345–355. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martínez-Burnes, J.; Napolitano, F.; Domínguez-Muñoz, M.; Guerrero-Legarreta, I.; Mora-Medina, P.; González-Lozano, M. Dystocia: Factors affecting parturition in domestic animals. CAB Rev. 2020, 15, 1–16. [Google Scholar] [CrossRef]
- Purohit, G.N.; Barolia, Y.; Shekhar, C.; Kumar, P. Maternal dystocia in cows and buffaloes: A review. Open J. Anim. Sci. 2011, 1, 41. [Google Scholar] [CrossRef]
- Darwish, R.A.; Ashmawy, T.A.M. The impact of lambing stress on post-parturient behaviour of sheep with consequences on neonatal homeothermy and survival. Theriogenology 2011, 6, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Amjad Ali, M.; Lodhi, L.A.; Awais, M.M.; Hassan, F.U.; Ahmad, W. Effect of birth weight and sex of the calf on incidence of calving disorders in buffaloes in Punjab province, Pakistan (a case study). Sci. Int. 2016, 28, 3179–3182. [Google Scholar]
- Colmenares, O.; Coss, D.; Vargas, D.; Herrera, P.; Birbe, B. Análisis de sobrevivencia hasta el destete de un rebaño bufalino en condiciones de sabanas bien drenadas. Zoo Trop. 2009, 27, 105–111. [Google Scholar]
- Freitas de Melo, A.; Ungerfeld, R.; Hötzel, M.J.; Orihuela, A.; Pérez-Clariget, R. Low pasture allowance until late gestation in ewes: Behavioral and physiological changes in ewes and lambs from lambing to weaning. Animal 2017, 11, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M.; Lawrence, A.B. A review of the behavioural and physio-logical adaptations of extensively managed breeds of sheep that favour lamb survival. Appl. Anim. Behav. Sci. 2005, 92, 235–260. [Google Scholar] [CrossRef]
- Freitas de Melo, A.; Terrazas, A.; Ungerfeld, R.; Hötzel, M.J.; Orihuela, A.; Pérez-Clariget, R. Influence of low pasture allowance during pregnancy of the attachment between ewes and their lambs at birth and during lactation. Appl. Anim. Behav. Sci. 2018, 199, 9–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orihuela, A.; Mota-Rojas, D.; Strappini, A.; Serrapica, F.; Braghieri, A.; Mora-Medina, P.; Napolitano, F. Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals. Animals 2021, 11, 1968. https://doi.org/10.3390/ani11071968
Orihuela A, Mota-Rojas D, Strappini A, Serrapica F, Braghieri A, Mora-Medina P, Napolitano F. Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals. Animals. 2021; 11(7):1968. https://doi.org/10.3390/ani11071968
Chicago/Turabian StyleOrihuela, Agustín, Daniel Mota-Rojas, Ana Strappini, Francesco Serrapica, Ada Braghieri, Patricia Mora-Medina, and Fabio Napolitano. 2021. "Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals" Animals 11, no. 7: 1968. https://doi.org/10.3390/ani11071968
APA StyleOrihuela, A., Mota-Rojas, D., Strappini, A., Serrapica, F., Braghieri, A., Mora-Medina, P., & Napolitano, F. (2021). Neurophysiological Mechanisms of Cow–Calf Bonding in Buffalo and Other Farm Animals. Animals, 11(7), 1968. https://doi.org/10.3390/ani11071968









