Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Forage, Treatments, and Ensiling
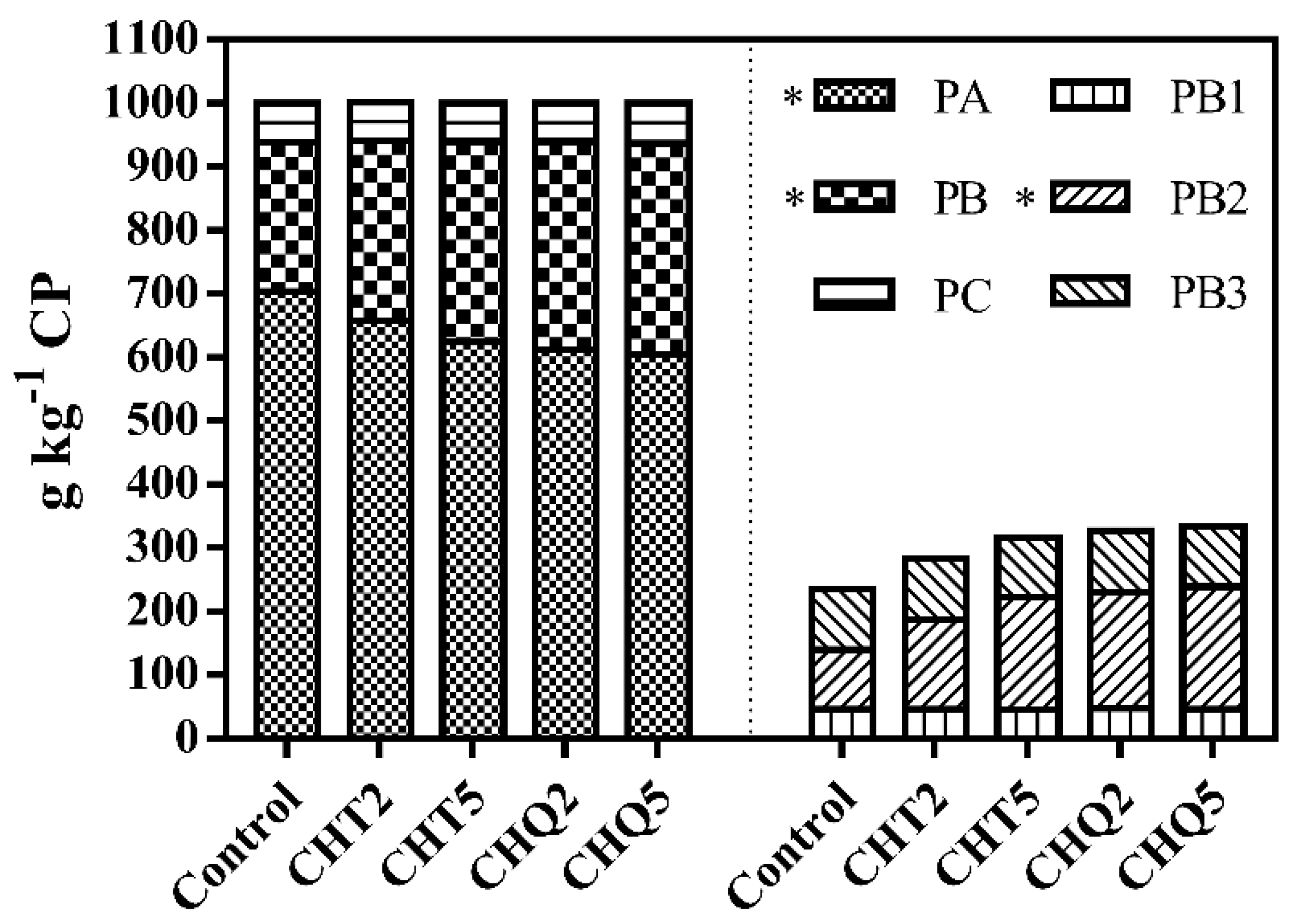
2.2. Chemical and Microbial Analyses
2.3. In Vitro Incubation
2.4. Total DNA Extraction and Real-Time PCR
2.5. Statistical Analyses
3. Results
3.1. Ensiling Characteristics
3.2. Ruminal Gas and CH4 Production
3.3. Ruminal Fermentation Patterns and Nutrient Degradation
3.4. Ruminal Enzyme Activity and Microbes
4. Discussion
4.1. Fermentation Characteristics of Alfalfa Silage
4.2. Gas and CH4 Production and Rumen Microbiota
4.3. Rumen Fermentation and Rumen Microbes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamra, D.N.; Pawar, M.; Singh, B. Effect of Plant Secondary Metabolites on Rumen Methanogens and Methane Emissions by Ruminants; Springer: Dordrecht, The Netherlands, 2012; pp. 351–370. [Google Scholar]
- Eckard, R.J.; Grainger, C.; de Klein, C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livestock Sci. 2011, 130, 47–56. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalzamarinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545, 556–568. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Masahito, O.; Ramon, C.A.; Koenig, K.M.; Iwaasa, A.D.; Ann, B.K. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef]
- Ugbogu, E.A.; Elghandour, M.M.; Ikpeazu, V.O.; Buendia, G.; Molina, O.M.; Arunsi, U.O.; Emmanuel, O.; Salem, A.Z. The potential impacts of dietary plant natural products on the sustainable mitigation of methane emission from livestock farming. J. Clean. Prod. 2019, 213, 915–925. [Google Scholar] [CrossRef]
- Tabacco, E.; Borreani, G.; Crovetto, G.M.; Galassi, G.; Colombo, D.; Cavallarin, L. Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage. J. Dairy Sci. 2006, 89, 4736–4746. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Lv, H.; Chen, N.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Improving fermentation, protein preservation and antioxidant activity of Moringa oleifera leaves silage with gallic acid and tannin acid. Bioresour. Technol. 2020, 297, 122390. [Google Scholar] [CrossRef]
- Niderkorn, V.; Barbier, E.; Macheboeuf, D.; Torrent, A.; Mueller-Harvey, I.; Hoste, H. In vitro rumen fermentation of diets with different types of condensed tannins derived from sainfoin (Onobrychis viciifolia Scop.) pellets and hazelnut (Corylus avellana L.) pericarps. Anim. Feed Sci. Technol. 2020, 259, 114357. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; McAllister, T.A.; Ominski, K.H. Condensed tannin concentrations found in vegetative and mature forage legumes grown in western Canada. Can. J. Plant Sci. 2011, 91, 669–675. [Google Scholar] [CrossRef]
- Cavallarin, L.; Antoniazzi, S.; Borreani, G.; Tabacco, E. Effects of wilting and mechanical conditioning on proteolysis in sainfoin (Onobrychis viciifolia Scop) wilted herbage and silage. J. Sci. Food Agric. 2005, 85, 831–838. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Z.; Li, J.; Shao, T. Ensiling characteristics, in vitro rumen fermentation, microbial communities and aerobic stability of low-dry matter silages produced with sweet sorghum and alfalfa mixtures. J. Sci. Food Agric. 2019, 99, 2140–2151. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and non-starch carbohydrates in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Pang, D.G.; Yang, H.J.; Cao, B.B.; Wu, T.T.; Wang, J.Q. The beneficial effect of Enterococcus faecium on the in vitro ruminal fermentation rate and extent of three typical total mixed rations in northern China. Livestock Sci. 2014, 167, 154–160. [Google Scholar] [CrossRef]
- Fievez, V.; Babayemi, O.J.; Demeyer, D. Estimation of direct and indirect gas production in syringes: A tool to estimate short chain fatty acid production requiring minimal laboratory facilities. Anim. Feed Sci. Technol. 2005, 123, 197–210. [Google Scholar] [CrossRef]
- Mullins, C.R.; Mamedova, L.; Carpenter, A.; Ying, Y.; Allen, M.; Yoon, I.; Bradford, B. Analysis of rumen microbial populations in lactating dairy cattle fed diets varying in carbohydrate profiles and Saccharomyces cerevisiae fermentation product. J. Dairy Sci. 2013, 96, 5872–5881. [Google Scholar] [CrossRef]
- Du, H.S.; Wang, C.; Wu, Z.Z.; Zhang, G.W.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of rumen-protected folic acid and rumen-protected sodium selenite supplementation on lactation performance, nutrient digestion, ruminal fermentation and blood metabolites in dairy cows. J. Sci. Food Agric. 2019, 99, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.J.; Acharya, S.; Wang, S.X.; et al. Condensed tannins affect bacterial and fungal microbiomes and mycotoxin production during ensiling and upon aerobic exposure. Appl. Environ. Microbiol. 2018, 84, e02274-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Govea, F.E.; Muck, R.E.; Mertens, D.R.; Weimer, P.J. Microbial inoculant effects on silage and in vitro ruminal fermentation, and microbial biomass estimation for alfalfa, bmr corn, and corn silages. Anim. Feed Sci. Technol. 2011, 163, 2–10. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Ruminant Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhury, P.K.; Carro, M.D.; Griffith, G.W.; Dagar, S.S.; Puniya, M. New aspects and strategies for methane mitigation from ruminants. Appl. Microbiol. Biotechnol. 2014, 98, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Castleberry, L.; Allen, H.; Parker, D.; Waldrip, H.; Brauer, D.; Willis, W. Associative effects of wet distiller’s grains plus solubles and tannin-rich peanut skin supplementation on in vitro rumen fermentation, greenhouse gas emissions, and microbial changes. J. Anim. Sci. 2019, 97, 4668–4681. [Google Scholar] [CrossRef]
- Lan, W.; Yang, C. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef]
- Belanche, A.; Fuente, G.D.L.; Newbold, C.J. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 2014, 90, 663–667. [Google Scholar] [CrossRef] [Green Version]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef] [Green Version]
- Newbold, C.J.; de la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef] [Green Version]
- Mountfort, D.O.; Asher, R.A.; Bauchop, T. Fermentation of cellulose to methane and carbon dioxide by a rumen anaerobic fungus in a triculture with Methanobrevibacter sp. Strain RAl and Methanosarcina barkeri. Appl. Environ. Microbiol. 1982, 44, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Khiaosa-Ard, R.; Metzler-Zebeli, B.U.; Ahmed, S.; Muro-Reyes, A.; Deckardt, K.; Chizzola, R.; Böhm, J.; Zebeli, Q. Fortification of dried distillers grains plus solubles with grape seed meal in the diet modulates methane mitigation and rumen microbiota in Rusitec. J. Dairy Sci. 2015, 98, 2611–2626. [Google Scholar] [CrossRef] [Green Version]
- Latham, M.J.; Wolin, M.J. Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Appl. Environ. Microbiol. 1977, 34, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.L.; Currenti, E.; Wolin, M.J. Anaerobic bioconversion of cellulose by Ruminococcus albus, Methanobrevibactor smithii, and Methanosarcina barkeri. Appl. Microbiol. Biotechnol. 2000, 54, 494–498. [Google Scholar] [CrossRef]
- Wang, Y.; Alexander, T.W.; Mcallister, T.A. In vitro effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on rumen bacterial populations and fermentation. J. Sci. Food Agric. 2009, 89, 2252–2260. [Google Scholar] [CrossRef]
- Mcallister, T.A.; Martinez, T.; Bae, H.D.; Muir, A.D.; Yanke, L.J.; Jones, G.A. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J. Chem. Ecol. 2005, 31, 2049–2068. [Google Scholar] [CrossRef]
- Mitsumori, M.; Shinkai, T.; Takenaka, A.; Enishi, O.; Higuchi, K.; Kobayashi, Y.; Mcsweeney, C.S. Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. Br. J. Nutr. 2012, 108, 482–491. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Mint, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.; Waldron, M. Nutritional management of transition dairy cows: Strategies to optimize metabolic health. J. Dairy Sci. 2004, 87, E105–E119. [Google Scholar] [CrossRef] [Green Version]
- Grosse Brinkhaus, A.; Bee, G.; Schwarm, A.; Kreuzer, M.; Dohme-Meier, F.; Zeitz, J.O. Rumen microbial protein synthesis and nitrogen efficiency as affected by tanniferous and non-tanniferous forage legumes incubated individually or together in rumen simulation technique. J. Sci. Food Agric. 2018, 98, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, S. A review on environmental impacts of nutritional strategies in ruminants. J. Anim. Sci. 1996, 74, 3112–3124. [Google Scholar] [CrossRef] [PubMed]
- Aguerre, M.J.; Capozzolo, M.C.; Lendoni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Item a | Alfalfa |
|---|---|
| DM (g kg−1) | 235 |
| pH | 6.31 |
| Buffer capacity (mEq kg−1 DM) | 426 |
| CP (g kg−1 DM) | 233 |
| PA (g kg−1 CP) | 275 |
| PB (g kg−1 CP) | 680 |
| PC (g kg−1 CP) | 45 |
| NDF (g kg−1 DM) | 306 |
| ADF (g kg−1 DM) | 259 |
| WSC (g kg−1 DM) | 46.5 |
| LAB (log10 cfu g−1 FM) | 4.86 |
| Yeasts (log10 cfu g−1 FM) | 4.85 |
| Item A | Treatments B | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | CHT2 | CHT5 | CHQ2 | CHQ5 | |||
| Fermentation Characteristics | |||||||
| DML (g kg−1) | 96.2 a | 75.9 b | 74.1 bc | 63.7 d | 65.6 cd | 3.17 | <0.001 |
| pH | 5.32 a | 5.13 b | 5.03 c | 4.92 d | 5.00 cd | 0.04 | <0.001 |
| Lactic acid (g kg−1 DM) | 33.3 a | 31.3 b | 25.0 d | 33.6 a | 27.6 c | 0.91 | <0.001 |
| Acetic acid (g kg−1 DM) | 18.6 a | 17.0 b | 13.7 d | 16.0 c | 14.5 d | 0.46 | <0.001 |
| Butyric acid (g kg−1 DM) | 2.35 a | 0.79 b | 0.07 c | 0.09 c | 0.07 c | 0.24 | <0.001 |
| NH3-N (g kg−1 TN) | 120 a | 88.9 b | 77.8 c | 69.7 d | 60.1 e | 5.56 | <0.001 |
| Microbial counts | |||||||
| LAB (log10 cfu g−1 FM) | 7.34 a | 6.84 bc | 5.88 d | 6.98 b | 6.70 c | 0.23 | <0.001 |
| Yeasts (log10 cfu g−1 FM) | 4.26 | 4.26 | 4.23 | 4.25 | 4.24 | 0.10 | 0.856 |
| Chemical composition | |||||||
| DM (g kg−1 DM) | 215 b | 226 a | 224 a | 229 a | 228 a | 1.52 | 0.004 |
| CP (g kg−1 DM) | 233 | 228 | 230 | 227 | 229 | 1.39 | 0.720 |
| NDF (g kg−1 DM) | 316 c | 325 b | 328 ab | 326 ab | 329 a | 1.23 | <0.001 |
| ADF (g kg−1 DM) | 260 | 258 | 259 | 251 | 258 | 1.24 | 0.233 |
| Item A | Treatments B | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | CHT2 | CHT5 | CHQ2 | CHQ5 | |||
| Fermentation characteristics | |||||||
| pH | 6.75 | 6.79 | 6.75 | 6.79 | 6.78 | 0.01 | 0.766 |
| Total VFA (mM) | 64.6 a | 59.5 b | 57.8 bc | 60.9 b | 55.3 c | 0.87 | <0.001 |
| VFA, mol 100 mol−1 | |||||||
| Acetic acid | 68.9 | 68.8 | 68.4 | 68.3 | 68.7 | 0.09 | 0.143 |
| Propionic acid | 13.8 c | 15.3 b | 15.8 a | 15.9 a | 16.0 a | 0.22 | <0.001 |
| Iso-butyric acid | 1.84 a | 1.53 b | 1.50 bc | 1.51 b | 1.35 c | 0.04 | <0.001 |
| Butyric acid | 9.29 | 9.09 | 9.06 | 9.10 | 9.03 | 0.04 | 0.346 |
| Iso-valeric acid | 3.53 a | 3.09 b | 3.19 b | 3.17 b | 2.85 c | 0.06 | <0.001 |
| Valeric acid | 2.11a | 1.73 b | 1.75 b | 1.76 b | 1.70 b | 0.04 | <0.001 |
| Acetic acid/propionic acid | 4.98 a | 4.46 b | 4.30 c | 4.31 c | 4.28 c | 0.07 | <0.001 |
| NH3-N (mg dL−1) | 27.0 a | 23.7 b | 21.4 c | 22.4 bc | 20.1 d | 0.59 | <0.001 |
| MCP (mg dL−1) | 25.8 c | 27.1 b | 28.5 a | 28.1 a | 28.2 a | 0.27 | <0.001 |
| Nutrient degradation | |||||||
| DM (g kg−1) | 636 a | 617 b | 586 c | 608 b | 568 d | 6.37 | <0.001 |
| CP (g kg−1) | 861 a | 840 b | 832 cd | 838 bc | 829 d | 3.13 | <0.001 |
| NDF (g kg−1) | 466 a | 444 b | 422 c | 435 b | 406 d | 5.75 | <0.001 |
| Item | Treatments A | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | CHT2 | CHT5 | CHQ2 | CHQ5 | |||
| Enzyme activity B | |||||||
| Carboxymethyl-cellulase | 0.624 a | 0.604 abc | 0.578 bc | 0.623 ab | 0.574 c | 0.01 | 0.011 |
| Cellobiase | 1.36 a | 1.22 b | 1.04 d | 1.17 bc | 1.09 cd | 0.03 | <0.001 |
| Xylanase | 3.35 a | 2.49 c | 2.21 d | 2.96 b | 2.94 b | 0.11 | <0.001 |
| Pectinase | 3.29 a | 2.55 c | 2.32 c | 2.96 b | 2.61 c | 0.10 | 0.001 |
| α-amylase | 12.3 a | 9.87 bc | 8.37 c | 11.5 ab | 11.6 ab | 0.41 | <0.001 |
| Protease | 4.93 a | 4.48 b | 3.66 d | 4.60 b | 4.65 b | 0.12 | <0.001 |
| Microbes (copies mL−1) | |||||||
| Total bacteria, ×1011 | 1.30 | 1.37 | 1.27 | 1.35 | 1.39 | 0.04 | 0.841 |
| Total anaerobic fungi, ×107 | 7.74 a | 5.94 c | 4.41 d | 6.63 b | 5.87 c | 0.29 | <0.001 |
| Total protozoa, ×106 | 6.69 a | 6.63 a | 3.55 b | 6.68 a | 3.83 b | 0.39 | <0.001 |
| Total methanogens, ×108 | 4.08 a | 3.31 b | 2.60 c | 3.29 b | 2.86 bc | 0.14 | <0.001 |
| Rumincoccus albus, ×108 | 3.26 a | 2.17 c | 1.38 d | 2.92 b | 2.21 c | 0.18 | <0.001 |
| Rumincoccus flavefaciens, ×109 | 1.81 a | 1.54 b | 1.25 d | 1.64 b | 1.41 c | 0.05 | <0.001 |
| Fibrobacter succinogenes, ×109 | 3.69 c | 4.66 b | 4.44 b | 5.35 a | 5.19 a | 0.16 | <0.001 |
| Butyrivibrio fibrisolvens, ×107 | 4.12 a | 2.87 c | 2.56 c | 3.68 b | 3.56 b | 0.16 | <0.001 |
| Prevotella ruminicola, ×1010 | 5.63 a | 3.67 d | 2.91e | 4.70 c | 5.16 b | 0.27 | <0.001 |
| Ruminobacer amylophilus, ×107 | 2.86 a | 2.44 b | 2.06 c | 2.86 a | 2.98 a | 0.10 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Bao, X.; Guo, G.; Huo, W.; Xu, Q.; Wang, C.; Li, Q.; Liu, Q. Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota. Animals 2021, 11, 1967. https://doi.org/10.3390/ani11071967
Chen L, Bao X, Guo G, Huo W, Xu Q, Wang C, Li Q, Liu Q. Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota. Animals. 2021; 11(7):1967. https://doi.org/10.3390/ani11071967
Chicago/Turabian StyleChen, Lei, Xueyan Bao, Gang Guo, Wenjie Huo, Qingfang Xu, Cong Wang, Qinghong Li, and Qiang Liu. 2021. "Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota" Animals 11, no. 7: 1967. https://doi.org/10.3390/ani11071967
APA StyleChen, L., Bao, X., Guo, G., Huo, W., Xu, Q., Wang, C., Li, Q., & Liu, Q. (2021). Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa Silage Fermentation Characteristics and In Vitro Ruminal Methane Production, Fermentation Patterns, and Microbiota. Animals, 11(7), 1967. https://doi.org/10.3390/ani11071967






