Towards an Integrated Approach for Monitoring Toxoplasmosis in Southern Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
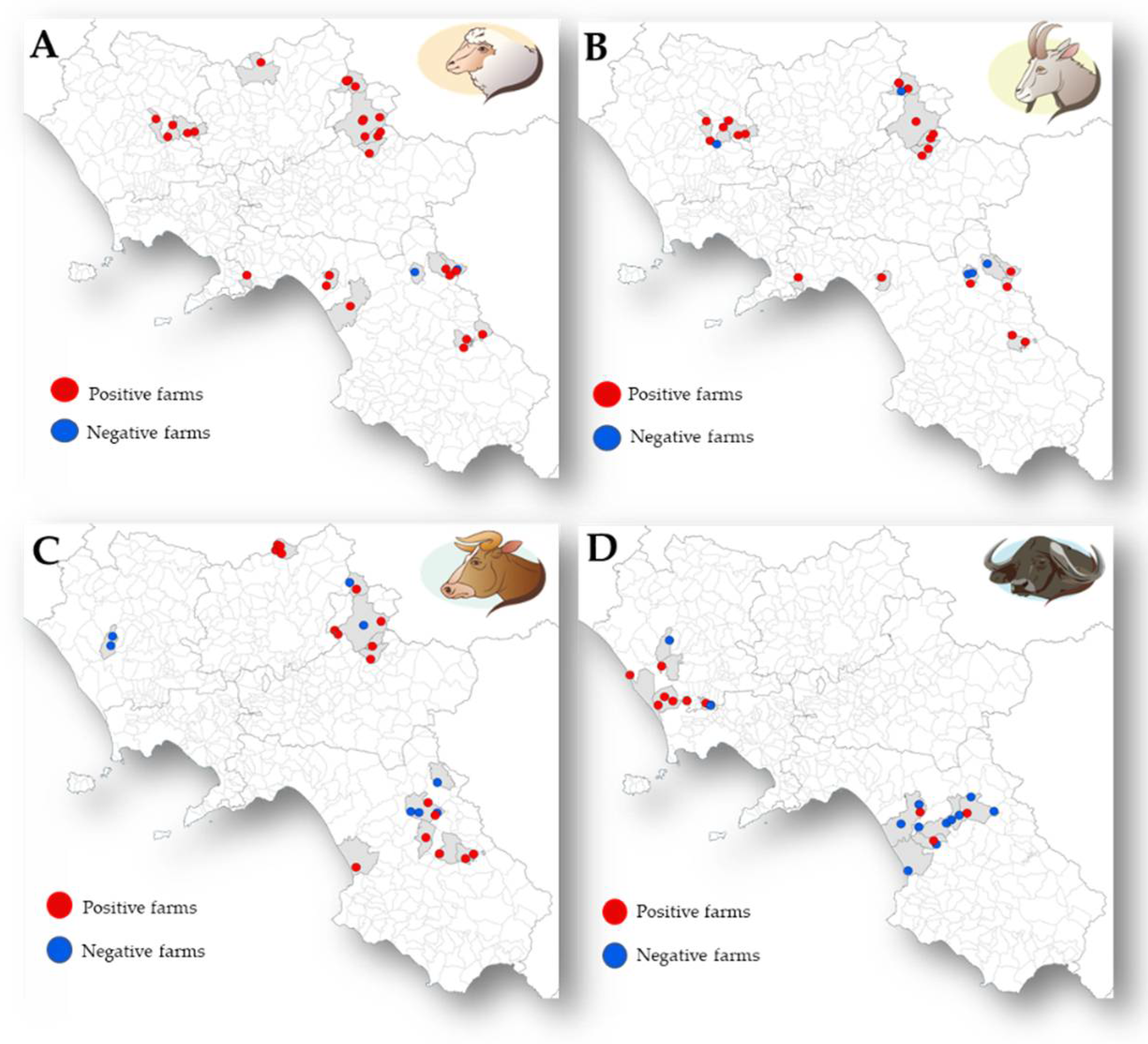
2.2. Task 1. Parasitological Analysis and Risk Factors for T. gondii in Ruminant Livestock Farms
2.2.1. Selection of Livestock Farms
2.2.2. Serological Analysis (Livestock)
2.2.3. Serological and Copromicroscopic Analysis (Cats)
2.2.4. Questionnaire Data Collection
2.2.5. Statistical Analysis
2.3. Task 2. Serological and Molecular Monitoring in Meat-Producing Livestock at slaughterouses
2.3.1. Serological Analysis
2.3.2. K-Agreement
2.3.3. Molecular Analysis
2.4. Task 3. Hospital Discharge Records (HDRs) Analysis
2.5. Task 4. Outreach Activities
3. Results
3.1. Task 1. Parasitological Analysis and Risk Factors for T. gondii in Ruminant Livestock Farms
Statistical Analysis
3.2. Task 2. Serological and Molecular Monitoring of Meat-Producing Livestock at Slaughterouses
K-Agreement
3.3. Task 3. Hospital Discharge Records (HDRs) Analysis
3.4. Task 4. Outreach Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.; Kwok, O.C.; Hill, D.; Yang, Y.R.; Su, C. All about Toxoplasma gondii infections in pigs: 2009–2020. Vet. Parasitol. 2020, 288, 109185. [Google Scholar] [CrossRef]
- Opsteegh, M.; Schares, G.; Blaga, R.; Van der Giessen, J.; on behalf of the consortium. Experimental Studies on Toxoplasma Gondii in the Main Livestock Species (GP/EFSA/BIOHAZ/2013/01); Final Report; EFSA Supporting Publication, EFSA: Parma, Italy, 2016. [Google Scholar]
- FERG. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Peyron, F.; Wallon, M.; Kieffer, F.; Graweg, G. Toxoplasmosis. In Infectious Diseases of the Fetus and Newborn Infant, 8th ed.; Remington, J.S., Klein, J.O., Wilson, C.B., Nizet, V., Maldonado, Y.A., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2016; pp. 949–1042. [Google Scholar]
- Schlüter, D.; Barragan, A. Advances and Challenges in Understanding Cerebral Toxoplasmosis. Front. Immunol. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.; Dubey, J. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.E.; Chirukandoth, S.; Dubey, J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005, 6, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Djurković-Djaković, O.; Dupouy-Camet, J.; Van Der Giessen, J.; Dubey, J.P. Toxoplasmosis: Overview from a One Health perspective. Food Waterborne Parasitol. 2019, 15, e00054. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth 2019, 16, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Dubey, J.P.; Hill, D.; Buchanan, R.L.; Gamble, H.R.; Jones, J.L.; Pradhan, A.K. Prevalence and risk factors for Toxo-plasma gondii infection in meat animals and meat products destined for human consumption. J. Food Prot. 2015, 78, 457–476. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Kelly, P.J.; Bartley, P.M.; Burrells, A.; Porco, A.; Metzler, D.; Crouch, K.; Ketzis, J.K.; Innes, E.A.; Katzer, F. Toxoplasma gondii in livestock in St. Kitts and Nevis, West Indies. Parasites Vectors 2015, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Cenci-Goga, B.T.; Ciampelli, A.; Sechi, P.; Veronesi, F.; Moretta, I.; Cambiotti, V.; Thompson, P.N. Seroprevalence and risk factors for Toxoplasma gondii in sheep in Grosseto district, Tuscany, Italy. BMC Vet. Res. 2013, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Gazzonis, A.L.; Zanzani, S.A.; Villa, L.; Manfredi, M.T. Toxoplasma gondii infection in meat-producing small ruminants: Meat juice serology and genotyping. Parasitol. Int. 2020, 76, 102060. [Google Scholar] [CrossRef] [Green Version]
- Gazzonis, A.L.; Marino, A.M.F.; Garippa, G.; Rossi, L.; Mignone, W.; Dini, V.; Giunta, R.P.; Luini, M.; Villa, L.; Zanzani, S.A.; et al. Toxoplasma gondii seroprevalence in beef cattle raised in Italy: A multicenter study. Parasitol. Res. 2020, 119, 3893–3898. [Google Scholar] [CrossRef]
- Vesco, G.; Buffolano, W.; La Chiusa, S.; Mancuso, G.; Caracappa, S.; Chianca, A.; Villari, S.; Currò, V.; Liga, F.; Petersen, E. Toxoplasma gondii infections in sheep in Sicily, southern Italy. Vet. Parasitol. 2007, 146, 3–8. [Google Scholar] [CrossRef]
- Fusco, G.; Rinaldi, L.; Guarino, A.; Proroga, Y.T.R.; Pesce, A.; Giuseppina, D.M.; Cringoli, G. Toxoplasma gondii in sheep from the Campania region (Italy). Vet. Parasitol. 2007, 149, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Ciuca, L.; Borriello, G.; Bosco, A.; D’Andrea, L.; Cringoli, G.; Ciaramella, P.; Maurelli, M.P.; Di Loria, A.; Rinaldi, L.; Guccione, J. Seroprevalence and Clinical Outcomes of Neospora caninum, Toxoplasma gondii and Besnoitia besnoiti Infections in Water Buffaloes (Bubalus bubalis). Animals 2020, 10, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgroi, G.; Viscardi, M.; Santoro, M.; Borriello, G.; D’Alessio, N.; Boccia, F.; Pacifico, L.; Fioretti, A.; Veneziano, V.; Fusco, G. Genotyping of Toxoplasma gondii in wild boar (Sus scrofa) in southern Italy: Epidemiological survey and associated risk for consumers. Zoonoses Public Health 2020, 67, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Thrusfield, M. Veterinary Epidemiology; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health 2019, 66, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.; Murata, F.; Cerqueira-Cézar, C.; Kwok, O.; Su, C. Economic and public health importance of Toxoplasma gondii infections in sheep: 2009–2020. Vet. Parasitol. 2020, 286, 109195. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.; Cerqueira-Cézar, C.K.; Kwok, O. Public health and economic importance of Toxoplasma gondii infec-tions in goats: The last decade. Res. Vet. Sci. 2020, 132, 292–307. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Yang, Y.R. Public Health Significance of Toxoplasma gondii Infections in Cattle: 2009–2020. J. Parasitol. 2020, 106, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.; Veronesi, F.; Di Cerbo, A.R.; Zanzani, S.; Molineri, G.; Moretta, I.; Moretti, A.; Fioretti, D.P.; Invernizzi, A.; Manfredi, M.T. Toxoplasma gondii in small ruminants in Northern Italy—prevalence and risk factors. Ann. Agric. Environ. Med. 2015, 22, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Opsteegh, M.; Kortbeek, T.M.; Havelaar, A.H.; Van Der Giessen, J.W.B. Intervention Strategies to Reduce Human Toxoplasma gondii Disease Burden. Clin. Infect. Dis. 2014, 60, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.; Cezar, C.C.; Murata, F.H.A.; Kwok, O.; Yang, Y.; Su, C. All about toxoplasmosis in cats: The last decade. Vet. Parasitol. 2020, 283, 109145. [Google Scholar] [CrossRef]
- Veronesi, F.; Santoro, A.; Milardi, G.L.; Diaferia, M.; Morganti, G.; Ranucci, D.; Gabrielli, S. Detection of Toxoplasma gondii in faeces of privately owned cats using two PCR assays targeting the B1 gene and the 529-bp repetitive element. Parasitol. Res. 2017, 116, 1063–1069. [Google Scholar] [CrossRef]
- Spada, E.; Proverbio, D.; della Pepa, A.; Perego, R.; Baggiani, L.; DeGiorgi, G.B.; Domenichini, G.; Ferro, E.; Cremonesi, F. Seroprevalence of feline immunodeficiency virus, feline leukaemia virus and Toxoplasma gondii in stray cat colonies in northern Italy and correlation with clinical and laboratory data. J. Feline Med. Surg. 2012, 14, 369–377. [Google Scholar] [CrossRef]
- Dubey, J. Toxoplasmosis—A waterborne zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef]
- Stelzer, S.; Basso, W.; Benavides Silván, J.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Conraths, F.J.; Schares, G. Tox-oplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef]
- Basso, W.; Sollberger, E.; Schares, G.; Küker, S.; Ardüser, F.; Moore-Jones, G.; Zanolari, P. Toxoplasma gondii and Neospora caninum infections in South American camelids in Switzerland and assessment of serological tests for diagnosis. Parasites Vectors 2020, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Canada, N.; Meireles, C.S.; Rocha, A.; Correia Da Costa, J.M.; Erickson, M.W.; Dubey, J.P. Isolation of viable Toxoplasma gondii from naturally infected aborted bovine fetuses. J. Parasitol. 2002, 88, 1247–1248. [Google Scholar] [CrossRef]
- De Barros, L.D.; Garcia, J.L.; Bresciani, K.D.S.; Cardim, S.T.; Storte, V.S.; Headley, S.A. A Review of Toxoplasmosis and Neosporosis in Water Buffalo (Bubalus bubalis). Front. Vet. Sci. 2020, 7, 455. [Google Scholar] [CrossRef]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii as a Parasite in Food: Analysis and Control. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Ducournau, C.; Moiré, N.; Carpentier, R.; Cantin, P.; Herkt, C.; Lantier, I.; Betbeder, D.; Dimier-Poisson, I. Effective Nano-particle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep. Front. Immunol. 2020, 11, 2183. [Google Scholar] [CrossRef]
- Papini, R.; Di Ciccio, P.; Marangi, M.; Ghidini, S.; Zanardi, E.; Vergara, A.; Giangaspero, A.; Nardoni, S.; Rocchigiani, G.; Mancianti, F.; et al. Occurrence of Toxoplasma gondii in Carcasses of Pigs Reared in Intensive Systems in Northern Italy. J. Food Prot. 2017, 80, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Vismarra, A.; Barilli, E.; Miceli, M.; Mangia, C.; Genchi, M.; Brindani, F.; Kramer, L.; Bacci, C. Toxoplasma gondii in the Cornigliese sheep breed in Italy: Meat juice serology, in vitro isolation and genotyping. Vet. Parasitol. 2017, 243, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.L.; Marangi, M.; Villa, L.; Ragona, M.E.; Olivieri, E.; Zanzani, S.A.; Giangaspero, A.; Manfredi, M.T. Toxoplasma gondii infection and biosecurity levels in fattening pigs and sows: Serological and molecular epidemiology in the intensive pig industry (Lombardy, Northern Italy). Parasitol. Res. 2018, 117, 539–546. [Google Scholar] [CrossRef]
- Condoleo, R.; Rinaldi, L.; Sette, S.; Mezher, Z. Risk Assessment of Human Toxoplasmosis Associated with the Consumption of Pork Meat in Italy. Risk Anal. 2018, 38, 1202–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranucci, D.; Veronesi, F.; Moretti, A.; Branciari, R.; Miraglia, D.; Manfredi, M.T.; Piergili, F.D. Seroprevalence of Toxoplasma gondii in wild boars (Sus scrofa) from Central Italy. Parasite 2013, 20, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, L.B.; Parker, S.E.; Gajadhar, A.A. Performance of commercial ELISA and agglutination test kits for the detection of anti-Toxoplasma gondii antibodies in serum and muscle fluid of swine infected with 100, 300, 500 or 1000 oocysts. Vet. Parasitol. 2012, 190, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Wallander, C.; Frössling, J.; Vågsholm, I.; Burrells, A.; Lundén, A. “Meat juice” is not a homogeneous serological matrix. Foodborne Pathog. Dis. 2015, 12, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Juránková, J.; Hůrková-Hofmannová, L.; Volf, J.; Baláž, V.; Piálek, J. Efficacy of magnetic capture in comparison with con-ventional DNA isolation in a survey of Toxoplasma gondii in wild house mice. Eur. J. Protistol. 2014, 50, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef] [PubMed]

| Animal Species | No. Farms Analysed | No. Farms Positive | Prevalence (%) (95%CI) | No. Animals Analysed | No. Animals Positive | Prevalence (%) (95%CI) |
|---|---|---|---|---|---|---|
| Sheep | 29 | 27 | 93.1 (75.8–98.8) | 390 | 221 | 56.7 (51.6–61.2) |
| Goats | 26 | 21 | 80.8 (60.0–92.7) | 241 | 114 | 47.3 (40.9–53.8) |
| Cattle | 25 | 17 | 68.0 (46.4–84.3) | 296 | 48 | 16.2 (12.3–21.0) |
| Water buffaloes | 24 | 11 | 45.8 (26.2–66.8) | 200 | 43 | 21.5 (16.1–28.0) |
| TOTAL | 104 | 76 | 73.1 (63.3–81.1) | 1.127 | 426 | 37.8 (35.0–40.7) |
| Animal Species | Age | No. Animals Analysed | No. Animals Positive | Prevalence (%) (95%CI) |
|---|---|---|---|---|
| Sheep | Youngs Adults | 78 312 | 32 190 | 41.0 (30.2–52.7) 60.9 (55.2–66.3) |
| Goats | Youngs Adults | 39 202 | 17 97 | 43.6 (28.2–60.2) 48.0 (41.0–55.1) |
| Cattle | Youngs Adults | 62 234 | 19 42 | 30.6 (19.9–43.8) 17.9 (13.4–23.6) |
| Water buffaloes | Youngs Adults | 9 191 | 0 43 | 0.0 22.5 (16.9–29.2) |
| Animal Species | Variable | Standard Error | Wald’s Chi-Square | Odds Ratio | p-Value |
|---|---|---|---|---|---|
| Sheep | Young sheep (<12 months) | 0.592 | 5.852 | 0.239 | 0.016 |
| Presence of cats | 0.341 | 11.381 | 3.164 | 0.001 | |
| Goats | Presence of cats | 0.308 | 15.074 | 3.306 | 0.000 |
| Abortion | 0.284 | 6.502 | 2.064 | 0.011 | |
| Cattle | Control rodent measures | 0.608 | 13.602 | 0.106 | 0.000 |
| Water buffaloes | Abortion | 0.476 | 4.796 | 2.837 | 0.029 |
| Control rodent measures | 0.792 | 35.350 | 0.009 | 0.000 |
| Animal Species | No. Animals Analysed | ELISA Test | Real-Time PCR | |||
|---|---|---|---|---|---|---|
| Serum (No. Animals Positive) | Myocardium (No. Animals Positive) | Diaphragm (No. Animals Positive) | Myocardium (No. Animals Positive) | Diaphragm (No. Animals Positive) | ||
| Sheep | 50 | 48 | 47 | 45 | 1 | 0 |
| Goats | 50 | 49 | 48 | 46 | 0 | 0 |
| Cattle | 45 | 6 | 8 | 7 | 0 | 0 |
| Water buffaloes | 48 | 4 | 3 | 1 | 0 | 0 |
| Pigs | 218 | 12 | 12 | 10 | 2 | 0 |
| Animal Species | Serum vs. Myocardium (Cohen’s ĸ) | Serum vs. Diaphragm (Cohen’s ĸ) |
|---|---|---|
| Sheep | 0.790 | 0.545 |
| Goats | 0.658 | 0.380 |
| Cattle | 0.831 | 0.910 |
| Water Buffaloes | 0.846 | 0.379 |
| Pigs | 1 | 0.904 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepe, P.; Bosco, A.; Capuano, F.; Baldi, L.; Giordano, A.; Mancusi, A.; Buonanno, M.; Morena, L.; Pinto, R.; Sarnelli, P.; et al. Towards an Integrated Approach for Monitoring Toxoplasmosis in Southern Italy. Animals 2021, 11, 1949. https://doi.org/10.3390/ani11071949
Pepe P, Bosco A, Capuano F, Baldi L, Giordano A, Mancusi A, Buonanno M, Morena L, Pinto R, Sarnelli P, et al. Towards an Integrated Approach for Monitoring Toxoplasmosis in Southern Italy. Animals. 2021; 11(7):1949. https://doi.org/10.3390/ani11071949
Chicago/Turabian StylePepe, Paola, Antonio Bosco, Federico Capuano, Loredana Baldi, Angela Giordano, Andrea Mancusi, Marialuisa Buonanno, Luigi Morena, Renato Pinto, Paolo Sarnelli, and et al. 2021. "Towards an Integrated Approach for Monitoring Toxoplasmosis in Southern Italy" Animals 11, no. 7: 1949. https://doi.org/10.3390/ani11071949
APA StylePepe, P., Bosco, A., Capuano, F., Baldi, L., Giordano, A., Mancusi, A., Buonanno, M., Morena, L., Pinto, R., Sarnelli, P., Cringoli, G., & Rinaldi, L. (2021). Towards an Integrated Approach for Monitoring Toxoplasmosis in Southern Italy. Animals, 11(7), 1949. https://doi.org/10.3390/ani11071949







