Simple Summary
Microbial resistance to antibiotics is a constant threat to livestock farming, and unreasonable use of antibiotics has increased the prevalence of infectious diseases in humans and animals. Antimicrobial peptides derived from black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), have great potential as alternatives to antibiotics for prophylaxis and treatment of diseases in animals because they have extensive antimicrobial properties and a lower tendency to induce resistance. Additionally, several studies have shown that Hermetia illucens larvae can participate in a circular economy by digesting organic waste alone and then promoting the growth performance of domestic animals fed the larvae. Therefore, antimicrobial peptides from Hermetia illucens are promising candidate for replacement of antibiotics in livestock farming.
Abstract
Functional antimicrobial peptides (AMPs) are an important class of effector molecules of innate host immune defense against pathogen invasion. Inability of microorganisms to develop resistance against the majority of AMPs has made them alternatives to antibiotics, contributing to the development of a new generation of antimicrobials. Due to extensive biodiversity, insects are one of the most abundant sources of novel AMPs. Notably, black soldier fly insect (BSF; Hermetia illucens (Diptera: Stratiomyidae)) feeds on decaying substrates and displays a supernormal capacity to survive under adverse conditions in the presence of abundant microorganisms, therefore, BSF is one of the most promising sources for identification of AMPs. However, discovery, functional investigation, and drug development to replace antibiotics with AMPs from Hermetia illucens remain in a preliminary stage. In this review, we provide general information on currently verified AMPs of Hermetia illucens, describe their potential medical value, discuss the mechanism of their synthesis and interactions, and consider the development of bacterial resistance to AMPs in comparison with antibiotics, aiming to provide a candidate for substitution of antibiotics in livestock farming or, to some extent, for blocking the horizontal transfer of resistance genes in the environment, which is beneficial to human and animal welfare.
1. Introduction
Growth of the world population and improvement of living standards in developing countries is linked to growing consumption and demand for animal-derived protein sources and increasing requirements for protein desperately need enhanced livestock production []. The golden age of antibiotics began with their discovery. Therefore, livestock farmers irrationally used antibiotics in animal feed to improve animal production performance and to ensure a high yield of animal-derived protein sources []. However, the use of antibiotics in animal diets resulted in dissemination of drug resistance among bacteria and decreased quality of meat products []. Humans will gradually approach the lack of medicines to cure the infections, and this lack will eventually present a risk to worldwide public health []. The use of antibiotics in livestock has been banned in many countries due to adverse consequences [,,,]. Thus, extensive studies are aiming to identify reliable alternatives to antibiotics [,,].
Recent studies demonstrated that insects are a sustainable source for animal feed in many countries around the world due to their ability to provide nutritional ingredients []. Insects have a higher feed conversion efficiency and can exploit the nutrients of the diet better than animals []. Moreover, insects produce lower ammonia emission and greenhouse gases than those produced by traditional livestock [,]. On the other hand, insects as feed can also indirectly ameliorate the environmental footprint of vertebrate meat production []. Feeding insects with human-inedible organic wastes, and then rearing poultry with these insects, facilitates an increase in protein content in these animals [], which achieves a circular economy [] of wastes and reduces the consumption of starting material and energy to promote animal growth. This approach will be conducive to overcoming the future paucity of adequate, nutritious, and healthy food in the future.
Black soldier fly (BSF), Hermetia illucens (Diptera: Stratiomyidae), is a warm-climate [] and innocuous insect [,] that rapidly colonizes decomposing waste substances, such as food scraps or kitchen waste [], straw [,], manure [,]. The variety of substances and efficiency of their consumption by BSF larvae is higher than that by Drosophila melanogaster, Apis mellifera, and Bombyx mori []. Habitats of BSF larvae are characterized by abundance of various microorganisms. BSF is able to live in adverse environments, indicating that BSF has an innate immune system that can produce various substances, such as peptides [], that protect against bacteria, fungi, and viruses [,,]. A defensin-like peptide 4 (DLP4) was recently detected and extracted from various immune tissues of BSF larvae. The survival rate of mice infected with methicillin-resistant Staphylococcus aureus (MRSA) treated with DLP4 at the doses ranging from 3 mg/kg to 7.5 mg/kg was 80–100% []. This finding demonstrated that DLP4 is a promising novel candidate antimicrobial peptide (AMP) against MRSA infections. AMPs from BSF have been at the epicenter of research for decades and are expected to be able to substitute for antibiotics in poultry feed and, to some extent, block the horizontal transfer of resistance genes in the environment [].
The present review summarizes the studies on AMPs from Hermetia illucens reported in the databases, such as National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/, accessed on 28 June 2021), antimicrobial peptide database (APD, http://aps.unmc.edu/AP/, accessed on 28 June 2021) and KEGG pathway database (https://www.kegg.jp/kegg-bin/show_pathway?map01503, accessed on 28 June 2021), and provides a comprehensive and structured overview by comparing AMPs from BSF with those from other insect species in the context of six aspects: medicinal value, diversity, mechanism of action, immune-induced signaling pathway, bacterial resistance, and applications in livestock production. Ultimately, relevant information on AMPs from BSF provides an important basis for the development of alternatives to antibiotics to block antibiotic pollution of the environment.
2. Medicinal Value of Antimicrobial Peptides
Edible insects have been consumed in China for more than two thousand years. Insects are also a source of various natural substances that can exploit natural bioactive ingredients in medical, veterinary, and agricultural applications [,]. Insect toxins are compounds with many biological activities and may be used as drugs for alleviation of pain, treatment of certain diseases and even cancer therapy []. Insects are extensively used in conventional medicine worldwide []. The number of insect patent applications has rapidly increased since 2010, indicating that the demand and utilization of insect applications continue to increase in China [].
In Chinese medicine, approximately 300 insect species are used to produce 1700 conventional medications [,]. Ants are famous medicinal species, their mandibles are used in surgery to staple wounds and ant-generated substances accelerate wound healing []. However, Drosophila melanogaster is the most common insect studied over the last few years. For example, the asexual blood stage of Plasmodium falciparum parasites causes a series of clinical manifestation of malaria, such as anemia and fever, sometimes resulting in death []. Tonk et al. [] discovered that low concentrations of AMPs (Drosocin, Mtk-1, and Mtk-2) from the fruit fly Drosophila melanogaster had low hemolytic effects on mouse and pig erythrocytes but significantly inhibited the growth of Plasmodium falciparum. Hence, insect-derived AMPs can be considered candidates for antiparasitic drugs.
The value of insects in medicinal applications is still being explored; for example, BSF larvae before the prepupal stage can be exploited as a high-quality source of protein and oil and a high-content source of chitin, AMPs, and melanin [,]. Proteins have been used in the production of aquaculture and poultry feeds because of their high digestibility. Practice indicates that the use of protein derived from the BSF in feed significantly reduces the incidence of diarrhea in breeding animals, improves the growth performance of the animals, and thus promotes the development of breeding history [,,]. High oil content is another valuable property of BSF. The content of oil obtained from Hermetia illucens larvae is considerably influenced by feeding materials, however, the content of crude lipid in these larvae is far greater than that in other insects or animal feed sources, such as soybean flour and fish meal []. AMPs extracted from Hermetia illucens larvae are the principal components of medical value. Li et al. [] demonstrated that defensin-like peptides 2 and 4 (DLP2 and DLP4) decreased disseminated bacterial burden by over 95% in the spleen and kidneys, reduced serum levels of proinflammatory cytokine, increased the levels of anti-inflammatory cytokine, and repaired lung and spleen injury. This finding suggests that DLP2 and DLP4 extracted from Hermetia illucens are promising candidates against staphylococcal infections. Therefore, AMPs from Hermetia illucens may be an important subject of investigations in the biomedical field.
3. Diversity of Antimicrobial Peptides in Insects
Identified AMPs are very diverse and hard to categorize, and their classification is generally based of secondary structure. Insects account for ninety percent of total quantity of animals on Earth, however, AMPs derived from insects correspond to only approximately ten percent of more than 2830 AMPs listed in the Antimicrobial Peptide Database []; thus, additional functional AMPs can be identified in insect species []. AMPs with antibacterial activity [] were initially detected in the hemolymph of giant silk moth pupae, Samia Cynthia, in 1974, then, an insect AMP of the cecropin type was purified from the hemolymph of immunized Cecropia moth pupae, Hyalophora cecropia, in 1980 []. Subsequently, over 150 insect AMPs have been extracted, purified, and identified []. Amino acid sequences and structural characteristics of, insect AMPs are used to define four broad categories: (a) α-helical structural peptides (e.g., moricin and ceropin), (b) glycine-rich peptides (e.g., gloverin and attacin), (c) proline-arginine-rich peptides (e.g., apidaecin, metchnikowin, and drosocin), and (d) cysteine-rich peptides (e.g., drosomycin and defensin) []. Most active AMPs are composed of 20–50 residues and are synthesized from inactive precursor proteins via limited proteolysis [,]. AMPs of the same classes or subclasses display different biological properties in different insect species. For instance, cecropin A, from Anopheles gambiae, has both antibacterial and antifungal activities; however, cecropin A from silk moths has only antibacterial activity, and this difference results from differences in the structure and size, which impact the activity of these AMPs toward various microorganisms []. Antimicrobial effects may involve enhanced innate immune reactions, and AMPs play a selective immunoregulatory role in infection by participating in wound healing and angiogenesis [].
Isolation and identification of antimicrobial peptides from Hermetia illucens has been progressing in recent years (Table 1) [,,,,,]. Structural similarity or unique sequences of defensin and cecropin AMPs enabled investigation of these AMPs in greater detail compared to other types of AMPs from Hermetia illucens. Defensins contain six conserved cysteines and are the most widespread AMPs in insects []. Insect defensins commonly include an N-terminal loop, an antiparallel β-sheet and an α-helix, generating a “loop-helix-sheet” or “cysteine-stabilized alpha beta (CSαβ)” structure []. Li et al. [] demonstrated that Hermetia illucens defensins DLP2 and DLP4 form a typical CSαβ structure and have a significant antibacterial effects against MRSA, increasing the survival rate of mice challenged with MRSA. Soon-IK et al. [] studied the cecropin family of AMPs from Hermetia illucens and demonstrated that inhibition of Escherichia. coli activity by cecropin-like peptide 1 (CLP1), containing the N-terminal helix, was 50-fold greater than that produced by ampicillin. This result demonstrated that N-terminal primary structure of cecropin may be important for antibacterial function. Furthermore, diverse unknown structures and antimicrobial functions of AMPs from Hermetia illucens in host defense systems, in almost all living organisms in nature should be explored. Scientists firmly consider that these AMPs to have promising practical applications in the future [].

Table 1.
Antimicrobial peptides from Hermetia illucens.
4. Mechanism of Action of Antimicrobial Peptides
The structures of AMPs are diverse, however, the biological mechanism of the effects of AMPs is related to the destruction of the bacterial cell membrane. The great majority of AMPs contain a cationic structure that binds to anionic lipopolysaccharides (LPS), to teichoic acids, and lipoteichoic acids (Figure 1 part 1). AMPs eventually destroy the integrity of the envelope by forming ion channels or transmembrane pores to trigger leakage of the cell contents that kills the cells []. Specific mechanisms of membrane lysis can be categorized into four models []: the toroidal pore, carpet-like, barrel-stave, and unstructured ring pore models (Figure 1 part 2). Lipid components of the membrane surface are the primary targets of AMPs. According to the toroidal pore model (Figure 1 part 2a), AMPs bind to the lipid components and form pores, ultimately resulting in disruption of bacterial membrane. According to the second model (Figure 1, part 2b), AMPs enshroud the cell membrane in a carpet-like mode, this mechanism requires a high concentration of AMPs and leads to cell membrane lysis. Cecropin-type AMPs act via this mechanism []. For example, Sato et al. [] demonstrated that continuous accumulation of cecropin-type AMPs at the bacterial lipid bilayer results in the formation of a “carpet-like” peptide structure on the membrane surface. This “carpet-like” structure has inherent detergent-like lytic properties, which dissolve the membranes. The barrel stave model represents the third mechanism (Figure 1, part 2c) of action of AMPs, in this model, the peptides combine with the cell envelope and enter the hydrophobic core of the phospholipid bilayer, resulting in leakage of intracellular substances and a reduction in the membrane potential. AMPs that damage the membranes via this mechanism include such as ceratotoxins [] and amphotericin B [], eventually cause cell death. The fourth mechanism (Figure 1 part 2d) involve damage of the cell envelope due to the formation of “unstructured ring pores”; i.e., AMPs line up in a ring-like structure that can be attached to the membrane at various angles [].
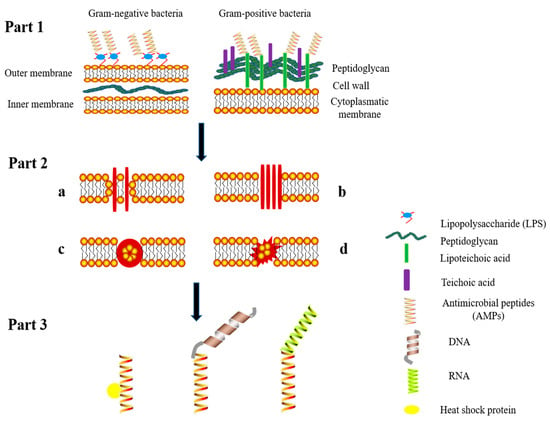
Figure 1.
Mechanism of action of antimicrobial peptides on bacterial cells. This figure presents a scheme of the models of action of AMPs: binding to the bacterial cell membrane (Part 1), possible effect resulting in the destruction of bacterial cell membrane (Part 2) and interactions of AMPs with intracellular substances (Part 3). Part 1: AMPs bind to lipopolysaccharides (LPS) of Gram-negative bacteria and to lipoteichoic or teichoic acid of Gram-positive bacteria and penetrate the cell wall. Part 2: Then the AMPs destroy the membrane structure via four pathways (a) toroidal model, (b) carpet-like model, (c) barrel-stave model, and (d) unstructured ring pores. Part 3: antibacterial activity of AMPs is mediated by interactions with heat shock proteins, DNA and RNA.
In addition to inducing membrane damage, some AMPs can spontaneously traversing cell membranes, interact with intracellular molecules and thus disrupt intracellular metabolic processes (Figure 1 part 3). Inside the cell, AMPs, such as drosocin and pyrrhocoricin, mainly interact with the bacterial chaperone DnaK (70 kDa) [], which is likely to inhibit protein folding in the cells and lead to metabolic disorders []. Additionally, DNA may be a target. The N-terminal region of AMP lactoferricin has been shown to incorporate into specific DNA domains where it can regulate transcription [].
The interaction of high concentrations of AMPs with biological membranes induces an antimicrobial effect due to permeabilization or disruption the cell envelope. However, suitable low concentrations of AMPs affect microbial activity or viability due to interactions with intracellular small molecules [].
5. AMP Induction of Immune Signaling Pathways in Insects
Induction of synthesis and secretion of numerous AMPs, lectins, lysozymes, and protein inhibitors upon invasion with exogenous pathogens are the main functions of humoral immunity in insects [,,,]. AMPs are important response factors of natural humoral immunity that play a role in the initial defense strategy against exogenous invading pathogens. Insects have a wide variety of AMP molecules; however, insect cells mainly produce specific AMPs through the Toll-like signaling and immune deficiency (IMD) pathway (Figure 2). The Toll pathway is largely activated by Gram-positive bacteria, fungi, and virulence factors (such as proteases). During various pathogenic infections, proteins encoded by spaetzle associated genes are recognized by Toll-like receptors on the cell membranes. Binding of spaetzle induces binding of activated Toll-like receptor to the primary response protein MyD88 via cytosolic intracellular homology domain known as Toll/IL-1R (TIR) [,,]. Activation of upstream interactions induces integration of MyD88, interleukin-1 receptor-associated kinase 4 (Tube) and interleukin-1 receptor-associated kinase 1 (Pell) to form a MyD88-Tube-Pelle heterotrimeric complex via death domain (DD)-mediated interactions [,,]. Subsequent, signal results in nuclear translocation of the NF-κB transcription factors Dorsal and/or Dif and the phosphorylation and degradation of the IκB inhibitor Cactus []. In the absence of a signal, Cactus binds to the NF-κB transcription factors Dorsal and/or Dif in a context-dependent manner and inhibits their activity and nuclear localization. Therefore, nuclear translocation of both Dorsal and Dif requires Cactus degradation []. Finally, the transcription factors Dorsal/Dif translocate into the nucleus and activate the secretion of corresponding AMPs to achieve the defense response []. On the other hand, the IMD pathway in insects responds to Gram-negative bacteria and fungi. Peptidoglycan (PGN) monomers or polymers from Gram-negative bacteria and secretions from fungi stimulate PGN recognition protein LC (PGRP-LC) and induce a series of cascade reactions []; the N-terminus of the nuclear factor Relish translocates to the nucleus and induces the transcriptional upregulation of AMP expression, which ultimately leads to the formation of AMPs in insects []. Additionally, these two signaling pathways act independently and lead to the induction of AMP production by transferring essential proteins into the nucleus [].
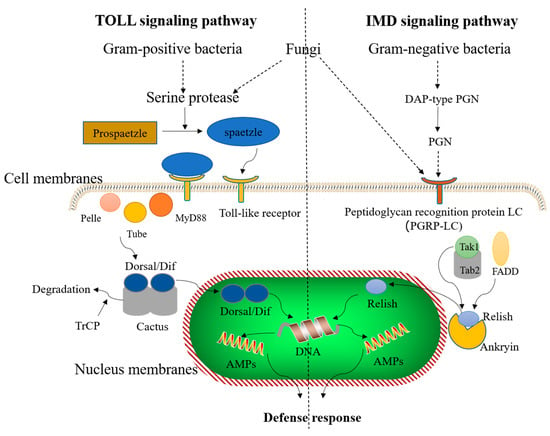
Figure 2.
The immune-induced signaling pathway of insect antimicrobial peptides (AMPs). The Toll signaling pathway is activated by Gram-positive bacteria and fungi, and the immune deficiency (IMD) signaling pathway is activated by Gram-negative bacteria and fungi. These two signaling pathways act independently and AMP production is induced by transport of a series of necessary proteins into the nucleus.
Currently, exact mechanism of production of AMPs in Hermetia illucens larvae is unknown. However, Huang et al. [] demonstrated that treatment of Hermetia illucens with Duox-TLR3 interference RNA deactivates the NF-κB signaling pathway, downregulates AMP expression, and reduces inhibitory effects on zoonotic pathogens. This finding demonstrated that Hermetia illucens utilizes the Toll pathway to regulate the expression of AMPs and subsequently inhibit pathogens in adverse environments. Furthermore, the suppression of the activities of Toll-like receptor 2 (TLR2) and 4 (TLR4) considerably diminishes NO production by dipterose-BSF, suggesting that dipterose-BSF provokes the immune function of various cytokines in Hermetia illucens macrophages via TLRs [].
In practice, survival of BSF larvae in environments contaminated with pathogens and conversion of organic wastes into protein- or fat-rich biomass, which can be used as feed substrates for livestock imply that BSF larvae may contain a variety of abundant AMPs that protect the larvae against infection by invading pathogenic bacteria. However, the mechanisms of antimicrobial effects of BSF-derived AMPs are unclear. Therefore, mechanistic investigations of antimicrobial properties of BSF-derived AMPs, which enable the replacement of antibiotics in livestock farming with BSF-derived AMPs are important subjects for future studies.
6. Bacterial Resistance to Insect AMPs
Bacterial resistance to antibiotic substances generally depends on drug inactivation and mutations or modifications of the target sites. Reduced accumulation of pharmacologically active substances due to limited uptake (for example, in Gram-negative bacteria) or improved excretion (for example, in Gram-positive bacteria) represents a significant mechanism of resistance to some types of antibacterial agents [,]. Another resistance mechanism is based on specific growth modes of bacteria, for instance, biofilm formation []. This mechanism easily induces bacterial multidrug resistance and leads to a series of disease outbreaks and large economic losses in stockbreeding [].
Unlike common antibiotics, such as kanamycin, ampicillin, and ciprofloxacin, which trigger a three- to fourfold augmentation of bacterial mutation rates, cationic antimicrobial peptides do not enhance the mutation rate in bacteria []. Moreover, Rodríguez-Rojas et al. demonstrated that stress-mediated channels enhanced the mutation rates of Escherichia coli only upon exposure to common antibiotics. In contrast, AMPs (cecropin A and melittin) extracted from insects did not induce bacterial stress pathways []. Therefore, these findings provide a new perspective suggesting that AMPs provides a distinct advantage to prevent the development of drug resistance because AMPs do not stimulate adaptation of bacteria to these immune defenses.
Horizontal transfer of bacterial resistance genes is a common and distinctive pattern of acquisition of antibiotic resistance acquisition. This process involves consolidation of drug resistance genes into DNA to create various clusters responsible for resistances of bacterial species in the environment. The mechanism of bacterial resistance to AMPs from BSF has not been described in detail. However, the development of bacterial resistance may involve inactivation of antibiotics by structural modification, alterations in the targets of antibiotics, and rapid removal of antibiotics from bacterial cells by an efflux pump-mediated mechanism []. This explanation for the development of bacterial drug resistance may be associated with AMPs entering the cells. Changes in the cell envelope are one of the main mechanisms of bacterial resistance against AMPs []. In the case of Gram-positive bacteria, relevant studies demonstrated that an increase in the levels of D-alanine esters in teichoic acids alters the charge on the surface of the cell wall, which facilitates the development of resistance of Staphylococcus aureus to vancomycin and defensins [,]. Gram-negative bacteria can avoid the effects of defensins via modification of their external cell envelope by acylating lipid A in the lipopolysaccharide layer to induce AMP resistance []. On the other hand, the production of a polysaccharide capsule is a mechanism of resistance of Klebsiella pneumoniae against defensins [,]. Enzymatic antibiotic inactivation is another common mechanism of resistance, which involves enzymes produced by resistant bacteria. This resistance mechanism includes modifications of a various antibiotic molecules by transfer of functional groups, such as acetyl, phosphoryl, ADP-ribosyl and glycosyl moieties []. The most representative enzymes include β-lactamases, which hydrolyze the C-N bond and thus decrease antibiotic activity []. Unfortunately, only a few studies investigated the degradation of insect AMPs by bacterial enzymes. The results indicated that Staphylococcus aureus resists human α-defensins by generating staphylokinase, which neutralizes bactericidal effects of these AMPs []. Additionally, proteases degrading AMPs which lack terminal charged residues, have been identified in Escherichia coli [] and Salmonella enterica serovar Typhimurium []. On the other hand, antimicrobial peptides can manifest lytic effects on eukaryotic cells, hence, intracellular proteins may be released due to peptide-dependent lysis []. Thus, these factors may limit the use of AMPs.
Recently, natural composites containing multiple insect AMPs were shown to induce bacterial resistance at a considerably lower rate than that of individual peptides and small-molecule antibiotics []. The authors extracted AMP complexes (defensins, cecropins, and diptericins), which belong to the families of cytomembrane-disrupting/permeabilizing peptides, from the blow fly Calliphora vicina infected with a mixture of Escherichia coli D31 and Micrococcus luteus A270. Moreover, the authors analyzed the changes in the resistance using Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii strains under selective stress of AMPs compound and control groups of antibiotics. All tested bacteria easily acquired drug resistance to antibiotics; in contrast, indications of the development of resistance to the AMP complex were not detected. This finding demonstrated that natural AMP complexes may provide novel solutions to the drug resistance problem. Similarly, BSF can be stimulated to secrete many kinds of AMPs in response to hostile environments containing a multitude of microbial species []. Similarly, Antonio et al. [] identified 57 putatively active peptides in BSF by machine learning bioinformatics algorithms.
The risk of the development of bacterial resistance against AMPs from BSF is considered relatively low, however, subsequent experimental validation of this hypothesis is needed with regard to potential risk of applications of these peptides.
7. Application of BSF-Derived AMPs in Livestock Production
BSF has been investigated for the ability to convert organic waste material to high-quality protein, inhibit certain detrimental bacteria and vermin, and furnish possible chemical precursors for synthesis of biodiesel and for possible use as a feed source for diverse animals []. The body composition of BSF larvae is determined by quality and quantity of feed intake []. For example, BSF larvae fed pig manure have higher protein content than those fed cow dung [,,]; however, a diet based on grain waste leads to an even higher protein level []. The same is true for crude fat levels. Nguyen et al. [] demonstrated that BSF larvae reared on liver and fish had higher fat and protein content than those reared on chicken meal. Moreover, the body composition undergoes considerable changes during larval development. For instance, crude protein content has been shown to decrease during the progress through the larval instar stage, namely, the crude protein content (dry matter) of five-day-old larvae was the highest (61%) and that of 15-day-old (44%) and 20-day-old (42%) larvae was the lowest []. The changes in the amino acid content in dried BSF larvae were also associated with the larval diet. For example, the amino acid content of BSF larvae fed cattle manure tended to be slightly higher than that of the larvae fed pig dung or chicken dung [,]. Apparently, the lysine content (6–8%) was exceedingly high in BSF larvae compared with that in characterized animal feeds []. For example, the levels of some essential amino acids in BSF larvae reared on pig slurry are consistent with those in soybean powder, especially the levels of leucine, threonine, phenylalanine and lysine []. Moreover, comparison with soybean meal indicated that BSF larvae contain higher levels of tryptophan, alanine, methionine, and histidine and lower levels of arginine. BSF larvae have a high protein content and contain many essential nutrient ingredients in the vast majority of animal diet compositions, which can impact the taste or digestibility of BSF larval meal [].
Currently, the health and productivity benefits of poultry are major concerns in the poultry feeding industry. Pathogenic microorganisms, such as bacteria, viruses, and parasites, remain important factors affecting livestock health []. For example, viral or bacterial diarrhea in pigs [], mastitis in dairy cows caused by Staphylococcus aureus [] and coccidiosis in chickens infected with Eimeria tenella [] can cause economic losses for farmers. Therefore, the growth performance of livestock poultry is directly influenced by feeding management, feeding environment, and diseases. Furthermore, AMPs whose production is induced in BSF larvae have certain advantageous characteristics, such as low molecular weight, high thermal stability, broad antimicrobial spectrum, and specific mechanism of action. In particular, a significant antimicrobial effect on drug-resistant bacteria has been demonstrated. Certain immune recognition function of BSF AMPs have also been shown these AMPs do not act on normal eukaryotic cells and only act on prokaryotic cells and pathological eukaryotic cells, and these properties can reduce the side effects of clinical treatment (such as an increase in hemoglobin (HGB) and hematocrit (HCT) in the blood, which contribute to enhanced oxygen-binding capacity and transport of the oxygen to the tissues of the body) []. A study demonstrated the lack of adverse effects on growth performance, serum indexes, diarrhea rate or nutrient digestibility in weaned piglets fed BSF larva meal when prepupa meal was used to replace an the appropriate proportion of soybean meal, fish meal, and plasma protein meal []. Furthermore, nutrient digestibility, growth performance, and immune capacity of weaned piglets were improved when BSF larvae fed kitchen waste were used to replace fish meal and soybean meal []. Thus, BSF meal will be a promising feed source.
The footprint of BSF in the entire ecosystem, is characterized by the nutrient recycling ability of BSF. On the other hand, Insects emit less ammonia and greenhouse gases than other animals []. Additionally, rearing of BSF larvae requires less land and less water than rearing of other animals []. Moreover, Liu et al. [,] demonstrated that BSF larvae effectively degrade the parent compound oxytetracycline (OTC) due to metabolic ability and function of intestinal microorganisms of BSF larvae. Moreover, OTC was not detected in the larval tissue and did not significantly influences the BSF larvae themselves because of their biological properties. Thus, OTC degradation by BSF larvae is an economical and practical means to decrease or remove antibiotic residues in the environment.
To ensure the yield of animals in the livestock industry, farmers add antibiotics to the feed to reduce the incidence rate of animal diseases []. However, these actions result in the presence of large quantities of antibiotic residues in the environment, which destroy the original ecological balance and pose a threat to public health [,]. Shin et al. determined the cDNA sequence of a BSF-derived AMP (attacin-like) by rapid amplification of cDNA ends-polymerase chain reaction (RACE-PCR) and DNA sequence analysis []. Moreover, the BSF-derived AMP gene (attacin) was expressed in the form of an inclusion body in a prokaryotic host, which contributed to reduced toxicity of this AMP to prokaryotic hosts and stabilized the expression of AMPs, demonstrating favorable antimicrobial activity against Gram-positive and Gram-negative bacteria. AMPs with a simple structures are considered to be non-absorbable in the gut and are delivered in the bacterial targets, which have sufficient affinity []. AMPs from insects are produced by ribosomes and consist of natural amino acids, hence, their activity in the animal digestive system has no systemic effects on the animals []. Moreover, cationic properties of AMPs preferentially influence negatively charged cells, such as microorganisms or cancer cells. However, the impact of AMPs on positively charged eukaryotic cells is limited []. In contrast to antibiotics, BSF-derived AMPs may provide a defensive effect that protects the animals from infections by pathogenic microorganisms and alter cellular behavior in response to external damage []. Ultimately, application of BSF-derived AMP in livestock production may be considered safe [].
The practical application of AMPs from BSF is also hampered by the cost of peptide purification and production, susceptibility of AMPs to proteolytic degradation, which has been reviewed previously [], and allergic reactions of the animals to AMPs []. Leni et al. [] identified two immunoreactive protein fragments in BSF protein hydrolysate, and suggested that tropomyosin is a potential allergen. However, they also confirmed that enzymatic hydrolysis is an effective strategy to reduce allergenic risk of BSF []. Accumulation of heavy metals (such as cadmium) and in the ecological restoration of BSF also limit its use in animal feed production []. However, Lalander et al. [] and Gao et al. [] assessed the accumulation of five types of antibiotics, including roxithromycin, trimethoprim, sulfamethazine, sulfamethoxazole, and sulfamonomethoxine (0.1–10 mg/kg). The results indicated the lack of accumulation of these antibiotics in BSF larvae. Conversely, sulfadiazine accumulated in BSF larvae when antibiotic concentrations ranged from 1 to 10 mg/kg. These studies indicated that antibiotics accumulate in BSF larvae upon treatment of organic substrates with high levels of these compounds. Thus, potential barriers, such as bioaccumulation of medical drugs, heavy metals, and natural toxins, can be controlled and addressed in mass rearing setups through quality control of their rearing substrates.
8. Conclusions and Prospects
BSF larvae have broad potential applications for the development and can be used as a resource due to rapid reproduction, large biomass, extensive feeding, harmlessness of adults, and high absorption and conversion rates. At the same time, BSF is a saprophytic resource insect that can feed on livestock excrement and household garbage to produce high-value animal protein feed. The BSF represents a new treatment mode for waste resource utilization with low energy consumption and high output value, which is conducive to the improvement of ecological environment. Moreover, some studies reported good antimicrobial properties of BSF-derived AMPs. The mechanisms of action of AMPs in insects have evolved over hundreds of years, and AMPs are conserved, indicating that the risk for the development of bacterial resistance to BSF-derived AMPs may be low. Hence, BSF-derived AMPs may be promising alternatives to antibiotics in the livestock industry required due to a global problem of increasing bacterial resistance to antibiotics. Therefore, recent, applications of BSF-derived AMPs have become an important subject in biology, agricultural science, medicine science, food, and feed industries. However, exploration of AMPs from BSF is in early stages, and investigations of the mechanisms by which BSF-derived AMPs inhibit pathogenic bacteria and interact with the resistance genes are lacking, although some studies on AMP extraction from Hermetia illucens and antibacterial activities have been carried out.
Abuse of antibiotics in various areas results in unsolved scientific questions about drug resistance in the environment caused by antibiotic contamination. Recent, detection and identification of AMPs from BSF were accelerated due to extraordinary superiority of these agents. Therefore, potential use of these AMPs as alternatives to antibiotics requires in-depth research. Future, studies on BSF AMPs and their modes of action should explore the following aspects: (a) validation of AMPs from BSF using various immune induction approaches; (b) identification of AMPs by pathogen recognition receptors and downstream reaction cascades leading to the production of BSF AMPs; (c) studies of the molecular mechanism of action of BSF AMPs against pathogenic microorganisms; (d) characterization of interactions between Hermetia illucens AMPs and resistance genes in antibiotic-resistant microorganisms; and (e) evaluation and application of BSF AMPs as alternatives to antibiotics.
Author Contributions
This work is the result of a common effort, H.Y. was responsible for supervision and conceptualization of the work. J.X. contributed to data collection and analysis, the interpretation of the results and the initial draft writing. C.G. contributed to critical review, editing of the manuscript, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (grant number 2020YFC1806900), and the National Natural Science Foundation of China (grant number41976151).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data access can be requested on demand from the corresponding author.
Conflicts of Interest
We declare that none of the authors have any conflict of interest.
References
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting Global Feed Protein Demand: Challenge, Opportunity, and Strategy. Annu. Rev. Anim. Biosci. 2019, 7, 17.01–17.23. [Google Scholar] [CrossRef]
- Nguyen, N.; Nguyen, C.; Guy, T.; Juan, C.M. Antimicrobial Usage and Antimicrobial Resistance in Animal Production in Southeast Asia: A Review. Antibiotics 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.D.; Panda, A. Rational Use of Antimicrobials in Animal Production:A Prerequisite to Stem the Tide of Antimicrobial Resistance. Curr. Sci. 2017, 113, 1846–1857. [Google Scholar] [CrossRef]
- López-Gálvez, G.; López-Alonso, M.; Pechova, A.; Mayo, B.; Dierick, N.; Gropp, J. Alternatives to antibiotics and trace elements (copper and zinc) to improve gut health and zootechnical parameters in piglets: A review. Anim. Feed. Sci. Technol. 2020, 114727. [Google Scholar] [CrossRef]
- Jones, H.E.; O’Connell White, K.; Norman, W.V.; Guilbert, E.; Lichtenberg, E.S.; Paul, M. First trimester medication abortion practice in the United States and Canada. PLoS ONE 2017, 12, e0186487. [Google Scholar] [CrossRef] [Green Version]
- Phillips, I. Withdrawal of growth-promoting antibiotics in Europe and its effects in relation to human health. Int. J. Antimicrob. Agents 2007, 30, 101–107. [Google Scholar] [CrossRef]
- Bedford, M. Removal of antibiotic growth promoters from poultry diets: Implications and strategies to minimise subsequent problems. World’s Poult. Sci. J. 2000, 56, 347–365. [Google Scholar] [CrossRef]
- Flynn, W.T. The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals; Center for Veterinary Medicine (HFV-1), Food and Drug Administration, US Department of Health and Human Services: Washington, DC, USA, 2012. [Google Scholar]
- El-Hack, A.; Mohamed, E.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M. Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed. Animals 2020, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Agnolucci, M.; Daghio, M.; Mannelli, F.; Secci, G.; Buccioni, A. Use of chitosan and tannins as alternatives to antibiotics to control mold growth on PDO Pecorino Toscano cheese rind. Food Microbiol. 2020, 92, 103598. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, H.; Shariatmadari, F.; Torshizi, M.A.K.; Rahimi, S.; Masoudi, A.A.; Zaboli, G.; Hedayat-Evrigh, N. Plant extract supplementation as a strategy for substituting dietary antibiotics in broiler chickens exposed to low ambient temperature. Arch. Anim. Nutr. 2020, 74, 206–221. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Review: Feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oonincx, D.G.; van Broekhoven, S.; van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2019, 10, e0144601, Correction in PLoS ONE 2019, 14, e0222043. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Joost, V.I.; Heetkamp, M.J.W.; Henry, V.D.B.; Van, L.J.J.A.; Arnold, V.H.; Hansen, I.A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Spranghers, T.; Noyez, A.; Schildermans, K.; De Clercq, P. Cold Hardiness of the Black Soldier Fly (Diptera: Stratiomyidae). J. Econ. Entomol. 2017, 110, 1501–1507. [Google Scholar] [CrossRef]
- Cickova, H.; Newton, G.L.; Lacy, R.C.; Kozanek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Craig, S.D.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef] [Green Version]
- Green, T.R.; Popa, R. Enhanced ammonia content in compost leachate processed by black soldier fly larvae. Appl. Biochem. Biotechnol. 2012, 166, 1381–1387. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Wu, L.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H.; Chapman, S.J. Pretreatment is an important method for increasing the conversion efficiency of rice straw by black soldier fly larvae based on the function of gut microorganisms. Sci. Total Environ. 2021, 762. [Google Scholar] [CrossRef] [PubMed]
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop. Med. Int. Health 2013, 19, 14–22. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbruegg, C.; Lindstroem, A.; Vinneras, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pacif. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, H.J.; Goo, T.W.; Quan, F.S. Novel antibacterial peptides induced by probiotics in Hermetia illucens (Diptera: Stratiomyidae) larvae. Entomol. Res. 2018, 48, 237–247. [Google Scholar] [CrossRef]
- Osama, E.; Dingzhong, Z.; Qi, S.; Aziz, S.A.; Minmin, C.; Longyu, Z.; Ziniu, Y.; Jibin, Z.; Humberto, L.M. Screening, Expression, Purification and Functional Characterization of Novel Antimicrobial Peptide Genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sus. Tain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Sun, J. Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response. Front. Immunol. 2020, 11, 2030. [Google Scholar] [CrossRef]
- Li, Z.; Mao, R.; Teng, D.; Hao, Y.; Chen, H.; Wang, X.; Wang, X.; Yang, N.; Wang, J. Antibacterial and immunomodulatory activities of insect defensins-DLP2 and DLP4 against multidrug-resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 12124. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Broeck, J.V.; Schoofs, L.; De Loof, A. Insect neuropeptides and their receptors: New leads for medical and agricultural applications. Trends Endocrinol. Metab. 1997, 8, 321–326. [Google Scholar] [CrossRef]
- Mlcek, J.; Borkovcova, M.; Rop, O.; Bednarova, M. Biologically active substances of edible insects and their use in agriculture, veterinary and human medicine. JCEA 2014, 15, 225–237. [Google Scholar] [CrossRef]
- Jin, G.; Weinberg, A. Human antimicrobial peptides and cancer. Semin. Cell Dev. Biol. 2019, 88, 156–162. [Google Scholar] [CrossRef]
- Mylonakis, E.; Podsiadlowski, L.; Muhammed, M.; Vilcinskas, A. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos. Trans. R. Soc. B. 2016, 371, 20150290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Liu, Y.; Suo, W.; Zhao, R.; Fan, H. Effects of the antimicrobial peptide of Tenebrio molitor Linnaeus on cell cycle of K562 and inhibitory effects of that on cell proliferation compared with hydroxyurea. Chin. J. Vector Biol. Control. 2010, 21, 324–326. [Google Scholar]
- Ratcliffe, N.A.; Mello, C.B.; Garcia, E.S.; Butt, T.M.; Azambuja, P. Insect natural products and processes: New treatments for human disease. Insect Biochem. Mol. Biol. 2011, 41, 747–769. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, M.; Ding, W.; Chen, X. Overview of edible insect resources and common species utilisation in China. J. Insects Food. Feed 2020, 6, 13–25. [Google Scholar] [CrossRef]
- Schiappa, J.; Van Hee, R. From ants to staples: History and ideas concerning suturing techniques. Acta Chir. Belg. 2012, 112, 395–402. [Google Scholar] [CrossRef]
- Hemingway, J.; Shretta, R.; Wells, T.N.; Bell, D.; Djimdé, A.A.; Achee, N.; Qi, G. Tools and strategies for malaria control and elimination: What do we need to achieve a grand convergence in malaria? PLoS Biol. 2016, 14, e1002380. [Google Scholar] [CrossRef] [PubMed]
- Tonk, M.; Pierrot, C.; Cabezas-Cruz, A.; Rahnamaeian, M.; Khalife, J.; Vilcinskas, A. The Drosophila melanogaster antimicrobial peptides Mtk-1 and Mtk-2 are active against the malarial parasite Plasmodium falciparum. Parasitol. Res. 2019, 118, 1993–1998. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H. Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae). Animals 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Ushakova, N.; Dontsov, A.; Sakina, N.; Bastrakov, A.; Ostrovsky, M. Antioxidative properties of melanins and ommochromes from black soldier fly Hermetia illucens. Biomolecules 2019, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Diener, S.; Zurbruegg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, P.; Martinez-Sanchez, A.; Rojo, S. The effects of larval diet on adult life-history traits of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Eur. J. Entomol. 2013, 110, 461–468. [Google Scholar] [CrossRef]
- Yi, H.; Chowdhury, M.; Huang, Y.; Yu, X. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [Green Version]
- Boman, H.G.; Nilsson-Faye, I.; Paul, K.; Rasmuson, T. Insect immunity I. Characteristics of an inducible cell-free antibacterial reaction in hemolymph of Samia cynthia pupae. Infect. Immun. 1974, 10, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Hultmark, D.; STEINER, H.; RASMUSON, T.; BOMAN, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Zool. Res. 2010, 31, 27–34. [Google Scholar] [CrossRef]
- Imler, J.L.; Hoffmann, J.A. Signaling mechanisms in the antimicrobial host defense of Drosophila. Curr. Opin. Mocrobiol. 2000, 3, 16–22. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2010, 198, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Zyowska, M.; Wyszynska, A.; Jagusztyn-Krynicka, E.K. Antimicrobial peptides - Defensins. Postep. Mikrobiol. 2011, 50, 223–234. [Google Scholar]
- Park, S.-I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Park, S.-I.; Yoe, S.M. Defensin-like peptide3 from black solder fly: Identification, characterization, and key amino acids for anti-Gram-negative bacteria. Entomol. Res. 2017, 47, 41–47. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Antimicrobial Activity of an Extract of Hermetia illucens Larvae Immunized with Lactobacillus casei against Salmonella Species. Insects 2020, 11, 704. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.-I. Novel attacin from Hermetia illucens: cDNA cloning, characterization, and antibacterial properties. Prep. Biochem. Biotechnol. 2019, 49, 279–285. [Google Scholar] [CrossRef]
- Park, S.-I.; Kim, J.-W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Čeřovský, V.; Bém, R. Lucifensins, the insect defensins of biomedical importance: The story behind maggot therapy. Pharmaceuticals 2014, 7, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Cornet, B.; Bonmatin, J.-M.; Hetru, C.; Hoffmann, J.A.; Ptak, M.; Vovelle, F. Refined three-dimensional solution structure of insect defensin A. Structure 1995, 3, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1999, 1462, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Jozefiak, A.; Engberg, R.M. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed. Sci. 2017, 26, 87–99. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessin, Y.; Saint, N.; Marri, L.; Marchini, D.; Molle, G. Antibacterial activity and pore-forming properties of ceratotoxins: A mechanism of action based on the barrel stave model. Biochim. Biophys. Acta 2004, 1667, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Umegawa, Y.; Yamagami, M.; Suzuki, T.; Tsuchikawa, H.; Hanashima, S.; Matsumori, N.; Murata, M. The perpendicular orientation of amphotericin B methyl ester in hydrated lipid bilayers supports the barrel-stave model. Biochemistry 2019, 58, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Duclohier, H. How do channel-and pore-forming helical peptides interact with lipid membranes and how does this account for their antimicrobial activity? Mini-Rev. Med. Chem. 2002, 2, 331–342. [Google Scholar] [CrossRef]
- Nicolas, P. Multifunctional host defense peptides: Intracellular-targeting antimicrobial peptides. FEBS 2009, 276, 6483–6496. [Google Scholar] [CrossRef]
- Cytryńska, M.; Rahnamaeian, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Züchner, T.; Sacheau, G.; Innis, C.A.; Vilcinskas, A. Proline-rich antimicrobial peptides in medicinal maggots of Lucilia sericata interact with bacterial DnaK but do not inhibit protein synthesis. Front. Pharmacol 2020, 11, 532. [Google Scholar] [CrossRef]
- He, J.; Furmanski, P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature 1995, 373, 721–724. [Google Scholar] [CrossRef]
- Singh, C.; Vaishna, R.; Kakkar, A.; Arunkumar, K.; Nagaraju, J. Characterization of antiviral and antibacterial activity of B ombyx mori seroin proteins. Cell. Microbiol. 2014, 16, 1354–1365. [Google Scholar] [CrossRef]
- Kurata, S. Recognition and elimination of diversified pathogens in insect defense systems. Mol. Divers. 2006, 10, 599–605. [Google Scholar] [CrossRef]
- Kurata, S.; Ariki, S.; Kawabata, S.-i. Recognition of pathogens and activation of immune responses in Drosophila and horseshoe crab innate immunity. Immunobiology 2006, 211, 237–249. [Google Scholar] [CrossRef]
- Royet, J.; Reichhart, J.-M.; Hoffmann, J.A. Sensing and signaling during infection in Drosophila. Curr. Opin. Immunol. 2005, 17, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Medzhitov, R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 12654–12658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauszig-Delamasure, S.; Bilak, H.; Capovilla, M.; Hoffmann, J.A.; Imler, J.-L. Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nat. Immunol. 2002, 3, 91–97. [Google Scholar] [CrossRef]
- Sun, H.; Bristow, B.N.; Qu, G.; Wasserman, S.A. A heterotrimeric death domain complex in Toll signaling. Proc. Natl. Acad. Sci. USA 2002, 99, 12871–12876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, T.; Towb, P.; Wasserman, S.A.; Sprang, S.R. Three-dimensional structure of a complex between the death domains of Pelle and Tube. Cell 1999, 99, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Moncrieffe, M.C.; Grossmann, J.G.; Gay, N.J. Assembly of oligomeric death domain complexes during Toll receptor signaling. J. Biol. Chem. 2008, 283, 33447–33454. [Google Scholar] [CrossRef] [Green Version]
- Moynagh, P.N. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 2008, 30, 33–42. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila toll signaling pathway. J. Immunol 2011, 186, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, W.; Gao, X.; Li, W.; Qi, S.; Guo, D.; Ajayi, O.E.; Ding, S.-W.; Wu, Q. lncRNA sensing of a viral suppressor of RNAi activates non-canonical innate immune signaling in Drosophila. Cell Host Microbe 2020, 27, 115–128. [Google Scholar] [CrossRef]
- Kaneko, T.; Goldman, W.E.; Mellroth, P.; Steiner, H.; Fukase, K.; Kusumoto, S.; Harley, W.; Fox, A.; Golenbock, D.; Silverman, N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 2004, 20, 637–649. [Google Scholar] [CrossRef] [Green Version]
- Sarvari, M.; Mikani, A.; Mehrabadi, M. The innate immune gene Relish and Caudal jointly contribute to the gut immune homeostasis by regulating antimicrobial peptides in Galleria mellonella. Dev. Comp. Immunol. 2020, 110, 103732. [Google Scholar] [CrossRef] [PubMed]
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings:functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, Y.; Zhan, S.; Tomberlin, J.K.; Huang, D.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Dual oxidase Duox and Toll-like receptor 3 TLR3 in the Toll pathway suppress zoonotic pathogens through regulating the intestinal bacterial community homeostasis in Hermetia illucens L. PLoS ONE 2020, 15, e0225873. [Google Scholar] [CrossRef]
- Fariz Zahir Ali, M.; Ohta, T.; Ido, A.; Miura, C.; Miura, T. The dipterose of black soldier fly (Hermetia illucens) induces innate immune response through toll-like receptor pathway in mouse macrophage RAW264. 7 cells. Biomolecules 2019, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug. Resist. Update 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. B. 2016, 371, 20150292. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Makarova, O.; Rolff, J. Antimicrobials, stress and mutagenesis. PLoS Pathog 2014, 10, e1004445. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.F.; Gohar, U.F.; Tahir, S.F.; Mukhtar, H.; Pornpukdeewattana, S.; Nukthamna, P.; Moula Ali, A.M.; Bavisetty, S.C.B.; Massa, S. Antimicrobial resistance: More than 70 years of war between humans and bacteria. Crit. Rev. Microbiol. 2020, 46, 578–599. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Vuong, C.; Otto, M.; Götz, F. The d-Alanine Residues ofStaphylococcus aureus Teichoic Acids Alter the Susceptibility to Vancomycin and the Activity of Autolytic Enzymes. Antimicrob. Agents Chemother. 2000, 44, 2845–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, Y.; Shai, Y. Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: Role in bacterial resistance and prevention of sepsis. Biochim. Biophys. Acta 2006, 1758, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- McCoy, A.J.; Liu, H.; Falla, T.J.; Gunn, J.S. Identification of Proteus mirabilisMutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 2001, 45, 2030–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Del. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Jin, T.; Bokarewa, M.; Foster, T.; Mitchell, J.; Higgins, J.; Tarkowski, A. Staphylococcus aureus Resists Human Defensins by Production of Staphylokinase, a Novel Bacterial Evasion Mechanism. J. Immunol. 2004, 172, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Guina, T.; Yi, E.C.; Wang, H.; Hackett, M.; Miller, S.I. A PhoP-Regulated Outer Membrane Protease of Salmonella enterica Serovar Typhimurium Promotes Resistance to Alpha-Helical Antimicrobial Peptides. J. Bacteriol. 2000, 182, 4077–4086. [Google Scholar] [CrossRef] [Green Version]
- Starr, C.G.; Wimley, W.C. Antimicrobial peptides are degraded by the cytosolic proteases of human erythrocytes. Biochim. Biophys. Acta 2017, 1859, 2319–2326. [Google Scholar] [CrossRef]
- Chernysh, S.; Gordya, N.; Suborova, T. Insect Antimicrobial Peptide Complexes Prevent Resistance Development in Bacteria. PLoS ONE 2015, 10, e0130788. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Karnik, S.; Barai, R.S.; Jayaraman, V.K.; Idicula-Thomas, S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010, 38, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed–a review. J. Insects. Food. Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Newton, G.; Sheppard, D.; Watson, D.; Burtle, G.; Dove, C.; Tomberlin, J.; Thelen, E. The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. In Proceedings of the Symposium on the state of the science of Animal Manure and Waste Management, San Antonio, TX, USA, 5–7 January 2005; pp. 5–7. [Google Scholar]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquacult. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Newton, L.; Sheppard, C.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005; p. 17. [Google Scholar]
- Oonincx, D.; Van Huis, A.; Van Loon, J. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects. Food. Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Rachmawati, R.; Buchori, D.; Hidayat, P.; Hem, S.; Fahmi, M.R. Perkembangan dan kandungan nutrisi larva Hermetia illucens (Linnaeus)(Diptera: Stratiomyidae) pada bungkil Kelapa Sawit. J. Entomol. Res. 2015, 7, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Newton, G.; Booram, C.; Barker, R.; Hale, O. Dried Hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Newton, G.; Sheppard, D.; Burtle, G. Research Briefs: Black Soldier Fly Prepupae—A Compelling Alternative to Fish Meal and Fish Oil. Aquaculture 2008, 24, 103–109. [Google Scholar]
- Kroeckel, S.; Harjes, A.-G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Mesonero-Escuredo, S.; Strutzberg-Minder, K.; Casanovas, C.; Segalés, J. Viral and bacterial investigations on the aetiology of recurrent pig neonatal diarrhoea cases in Spain. Porcine Health Manag. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Zaatout, N.; Ayachi, A.; Kecha, M.; Kadlec, K. Identification of staphylococci causing mastitis in dairy cattle from Algeria and characterization of Staphylococcus aureus. J. Appl. Microbiol. 2019, 127, 1305–1314. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Qasem, M.A.; Al-Shaebi, E.M.; Murshed, M.; Mares, M.M.; Dkhil, M.A. Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella. J. King Saud Univ Sci. 2020, 32, 2207–2211. [Google Scholar] [CrossRef]
- Driemeyer, H. Evaluation of Black Soldier Fly (Hermetia illucens) Larvae as an Alternative Protein Source in Pig Creep Diets in Relation to Production, Blood and Manure Microbiology Parameters; Stellenbosch University: Stellenbosch, South Africa, 2016. [Google Scholar]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.B.; Bergagna, S.; Sardi, L. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechno. 2019, 10, 12. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2017, 235, 33–42. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Chapman, S.J.; Su, J.; Wang, C. Changes in gut bacterial communities and the incidence of antibiotic resistance genes during degradation of antibiotics by black soldier fly larvae. Environ. Int. 2020, 142, 105834. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, H.; Wang, C. Black Soldier Fly Larvae Can Effectively Degrade Oxytetracycline Bacterial Residue by Means of the Gut Bacterial Community. Front. Microbiol. 2021, 12, e663972. [Google Scholar] [CrossRef]
- Dyar, O.J.; Zhang, T.; Peng, Y.; Sun, M.; Sun, C.; Yin, J.; Ding, L.; Sun, C.; Wang, Y.; Sun, Q. Knowledge, attitudes and practices relating to antibiotic use and antibiotic resistance among backyard pig farmers in rural Shandong province, China. Prev. Vet. Med. 2020, 175, 104858. [Google Scholar] [CrossRef]
- Khan, A.; Aziz, H.; Khan, N.; Hasan, M.; Ahmed, S.; Farooqi, I.; Dhingra, A.; Vambol, V.; Changani, F.; Yousefi, M. Impact, disease outbreak and the eco-hazards associated with pharmaceutical residues: A Critical review. Int. J. Environ. Sci. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Huang, F.; An, Z.; Moran, M.J.; Liu, F. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009−2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Peptide antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef]
- Andrä, J.; Berninghausen, O.; Leippe, M. Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med. Microbiol. Immunol. 2001, 189, 169–173. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial peptides of fish: Innocuous alternatives to antibiotics. Rev. Aquacult. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Wang, X.; Xu, X.; Cai, R.; Xie, S. Effects of heavy metals on the bioaccumulation, excretion and gut microbiome of black soldier fly larvae (Hermetia illucens). Ecotoxicol. Environ. Saf. 2020, 192, 110323. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Senecal, J.; Calvo, M.G.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinnerås, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Deng, W.; Gao, Z.; Li, M.; Liu, W.; Wang, X.; Zhu, F. Effect of sulfonamide pollution on the growth of manure management candidate Hermetia illucens. PLoS ONE 2019, 14, e0216086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).