Cell Source-Dependent In Vitro Chondrogenic Differentiation Potential of Mesenchymal Stem Cell Established from Bone Marrow and Synovial Fluid of Camelus dromedarius
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Collection and Culture of Bone Marrow and Synovial Fluid Derived Mesenchymal Stem Cells
2.3. Proliferation and Cell Cycle Assay
2.4. In Vitro Differentiation into Trilineage and Cytochemical Staining
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.6. Glycosaminoglycan (GAG) Contents
2.7. Statistical Analysis
3. Results
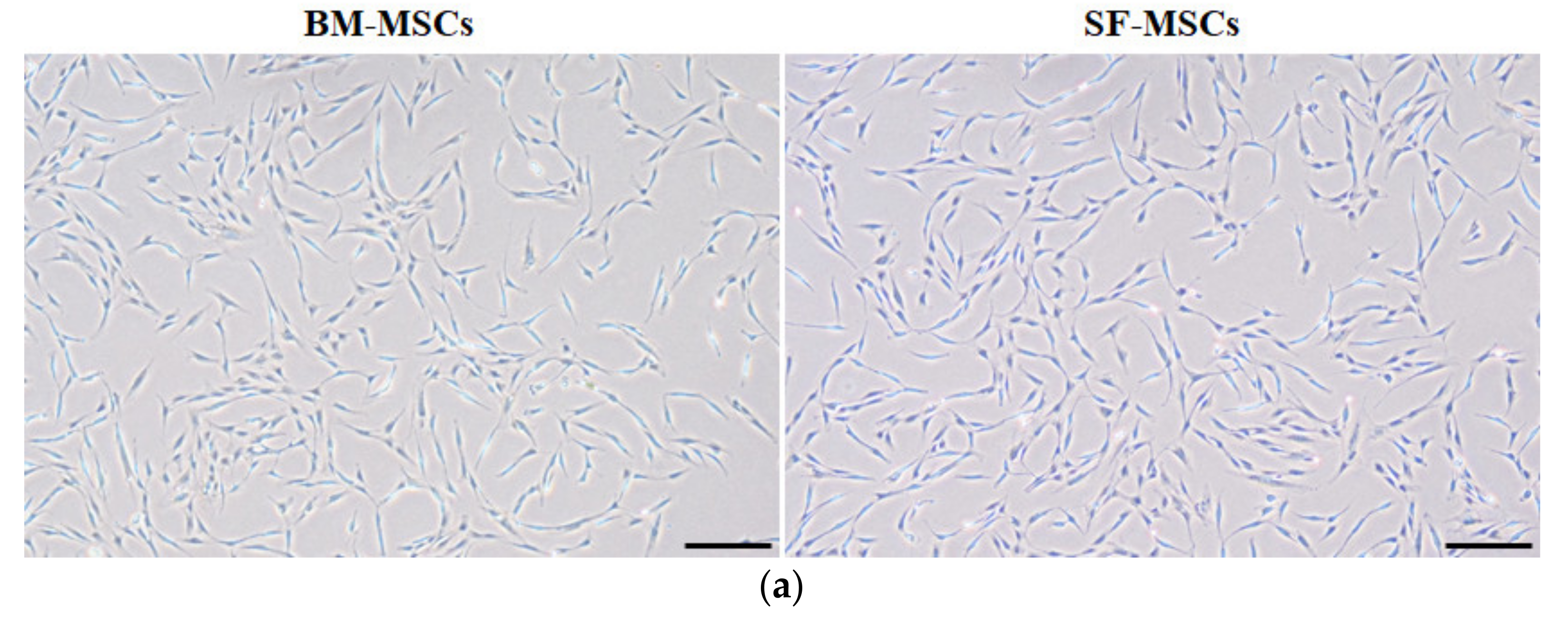
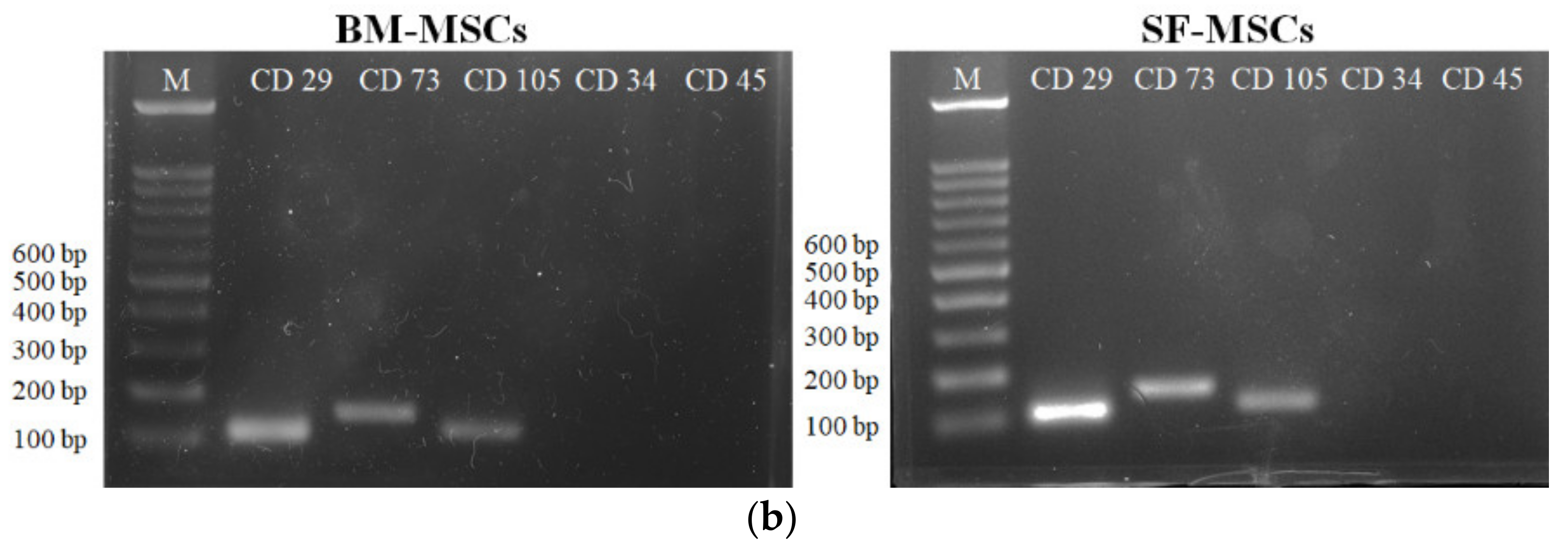
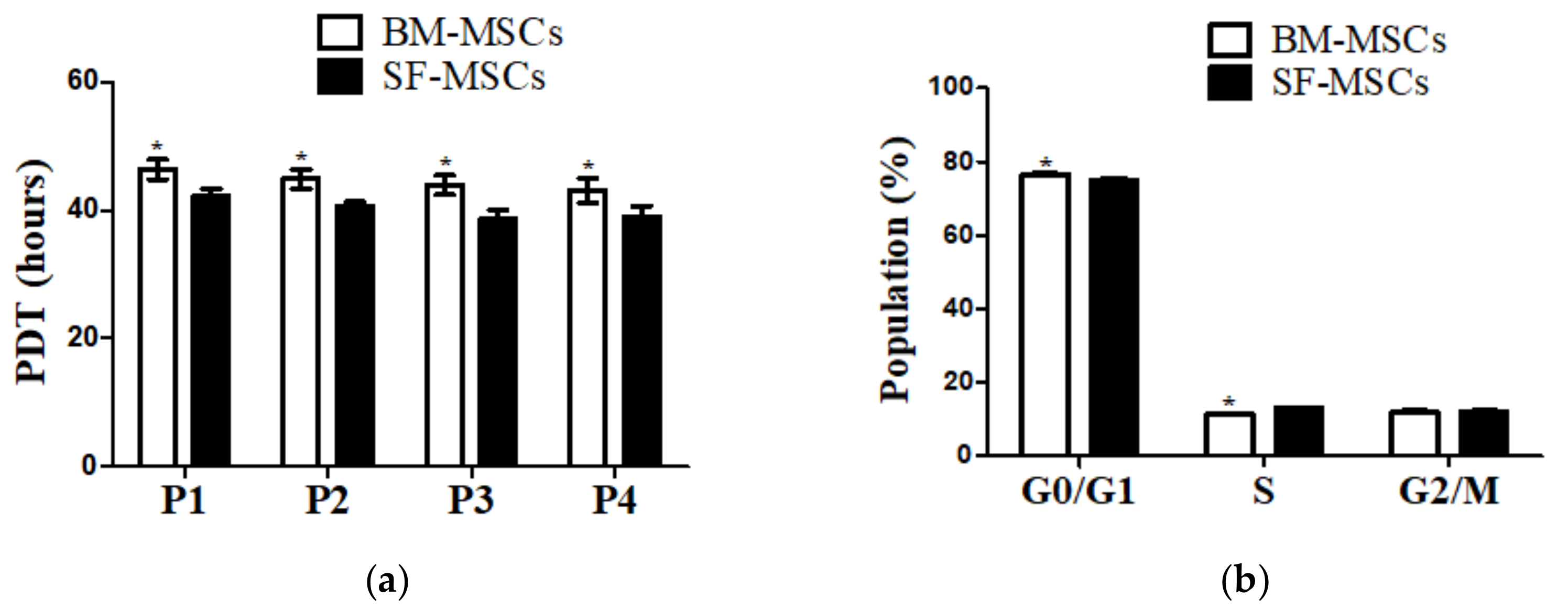
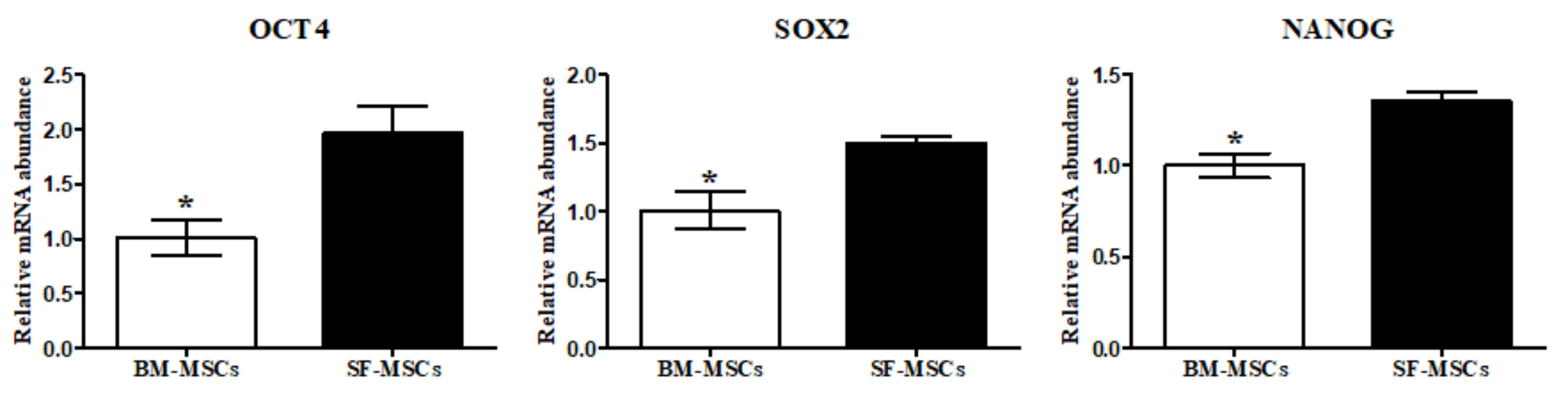
3.1. Establishment of MSCs Derived from Bone Marrow and Synovial Fluid
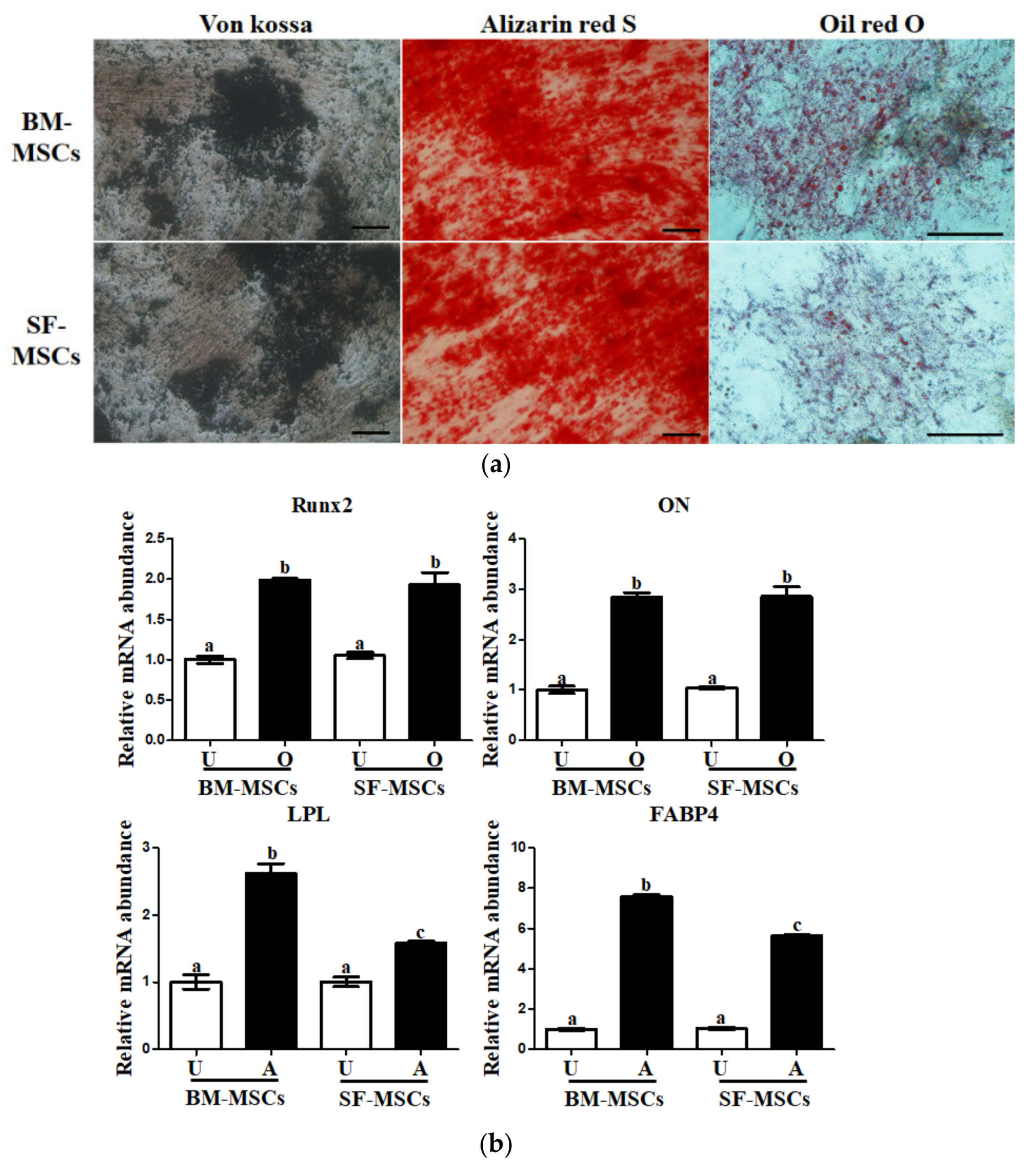
3.2. In Vitro Osteogenic and Adipogenic Lineage Differentiation Potential of MSCs
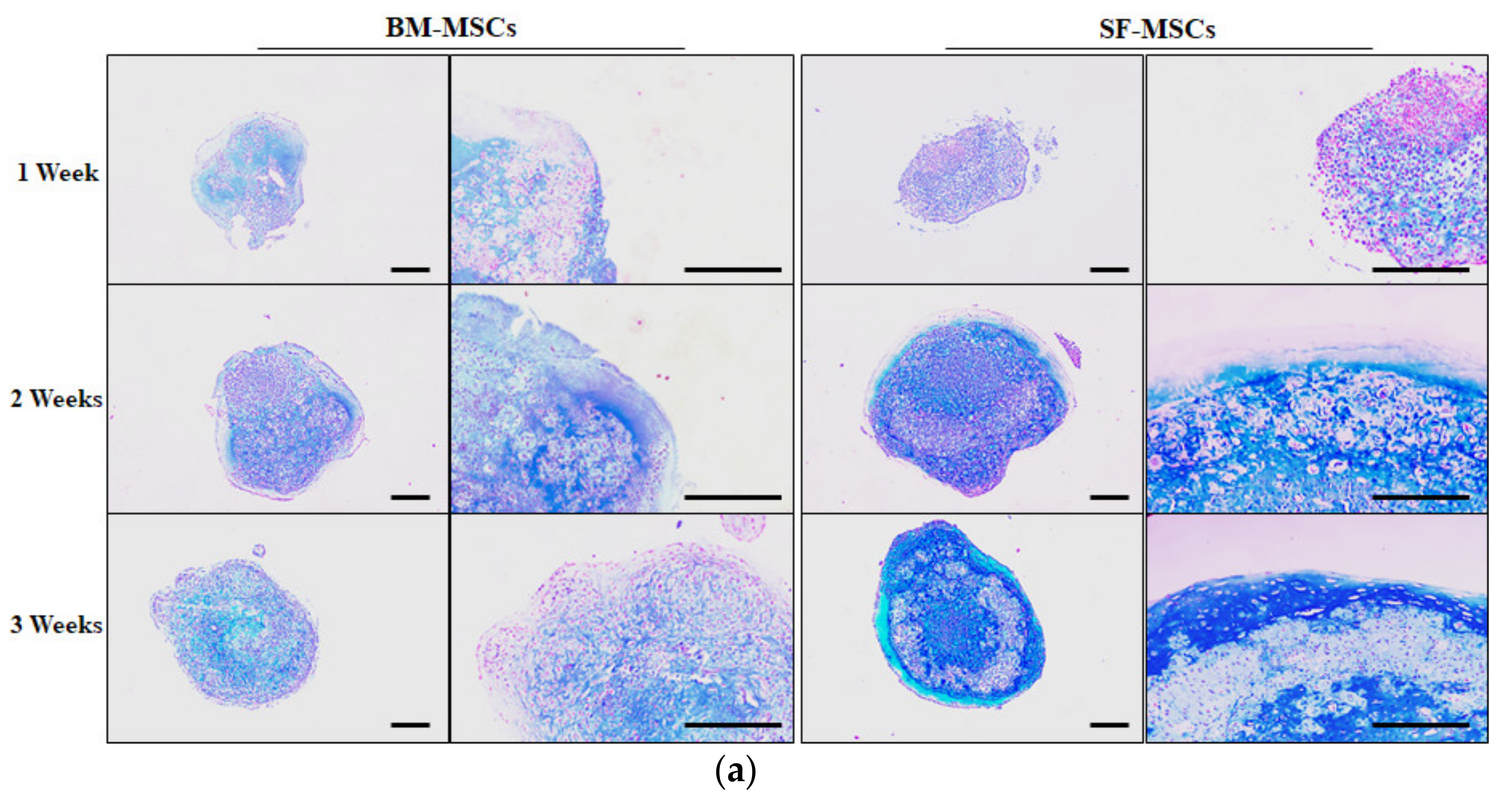
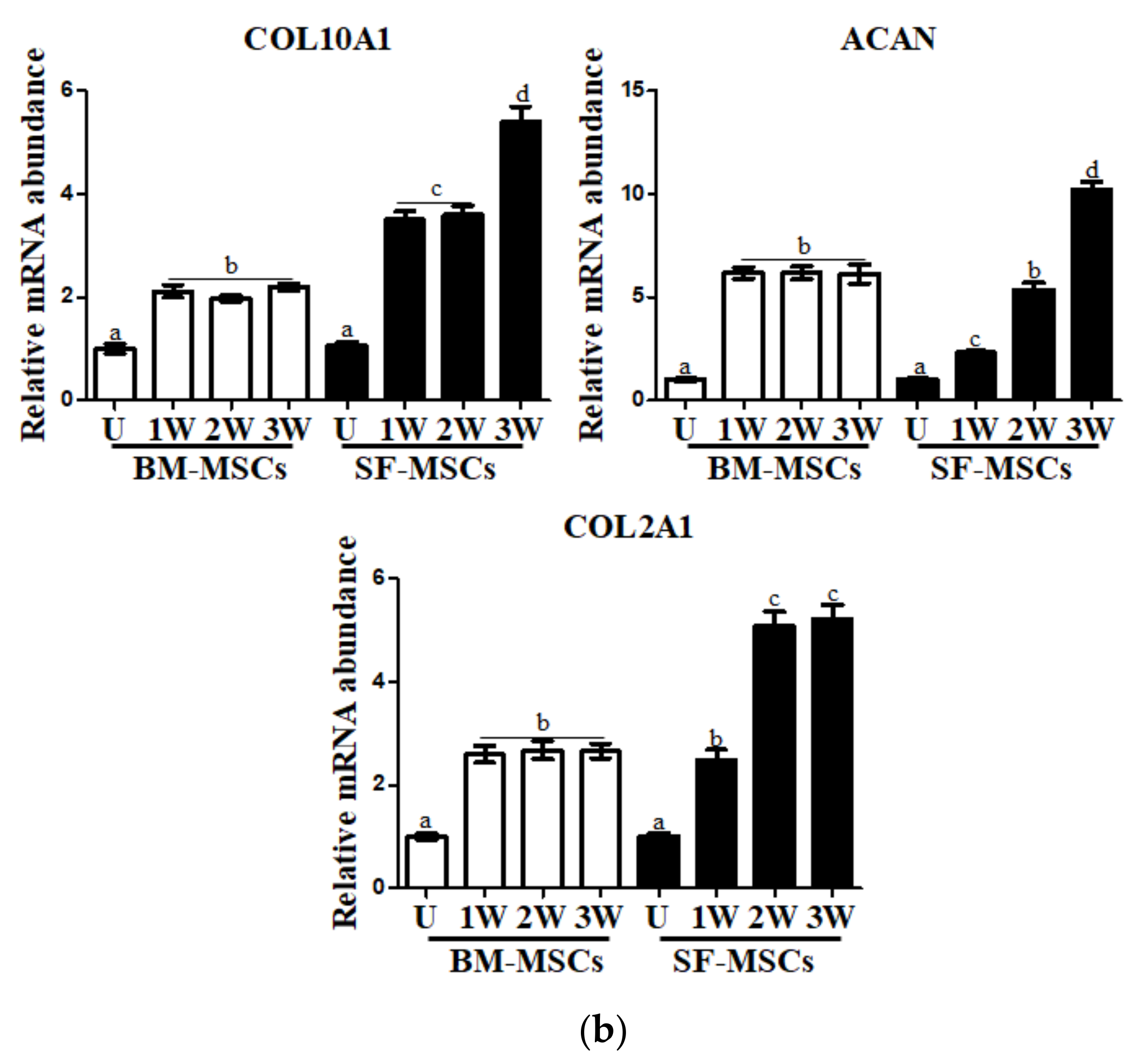
3.3. In Vitro Chondrogenic Differentiation Capacity of MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Bertuglia, A.; Pagliara, E.; Grego, E.; Ricci, A.; Brkljaca-Bottegaro, N. Pro-inflammatory cytokines and structural biomarkers are effective to categorize osteoarthritis phenotype and progression in Standardbred racehorses over five years of racing career. BMC Vet. Res. 2016, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Al-Rukibat, R.K.; Bani Ismail, Z.A.; Al-Zghoul, M.B. Cytologic analysis of synovial fluid in clinically normal tarsal joints of young camels (Camelus dromedarius). Vet. Clin. Pathol. 2006, 35, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar]
- Ge, Z.; Hu, Y.; Heng, B.C.; Yang, Z.; Ouyang, H.; Lee, E.H.; Cao, T. Osteoarthritis and Therapy. Arthritis Care Res. 2006, 55, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Chard, J.; Dieppe, P. Glucosamine for osteoarthritis: Magic, hype, or confusion? It’s probably safe-but there’s no good evidence that it works. BMJ 2001, 322, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, W.; Skinner, J.A.; Gooding, C.R.; Carrington, R.W.; Flanagan, A.M.; Briggs, T.W.R.; Bentley, G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J. Bone Jt. Surg. Br. 2005, 87, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.S.; Kim, C.W.; Jung, D.W. Management of focal Chondral lesion in the knee joint. Knee Surg. Relat. Res. 2011, 23, 185–196. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.R.; Park, H.J.; Choi, B.H.; Min, B.H. Potential predictive markers for proliferative capacity of cultured human articular chondrocytes: PCNA and p21. Artif. Organs. 2005, 29, 393–398. [Google Scholar] [CrossRef]
- Khang, G.; Kim, S.H.; Kim, M.S.; Rhee, J.M.; Lee, H.B. Recent and future directions of stem cells for the application of regenerative medicine. Tissue Eng. Regen. Med. 2007, 4, 441–470. [Google Scholar]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.B.; Bharti, D.; Kim, S.B.; Bok, E.Y.; Lee, S.Y.; Ho, H.J.; Lee, S.L.; Rho, G.J. Hematological patterns and histopathological assessment of Miniature Pigs in the experiments on human mesenchymal stem cell transplantation. Int. J. Med. Sci. 2021, 18, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.B.; Jeong, Y.I.; Jeong, Y.W.; Hossein, M.S.; Tinson, A.; Singh, K.K.; Hwang, W.S. Comparative study of biological characteristics, and osteoblast differentiation of mesenchymal stem cell established from camelus dromedarius skeletal muscle, dermal skin, and adipose tissues. Animals 2021, 11, 1017. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Yu, J.M.; Wu, X.; Gimble, J.M.; Guan, X.; Freitas, M.A.; Bunnell, B.A. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell 2011, 10, 66–79. [Google Scholar] [CrossRef]
- Chang, W.; Park, S.I.; Jun, S.; Lee, E.; Ham, H.; Bae, Y.; Kim, R.; Park, M.; Chung, Y.; Im, N.; et al. Therapeutic potential of autologous mesenchymal stem cells derived from synovial fluid in patients with degenerative arthritis. Anim. Cells Syst. 2013, 17, 315–324. [Google Scholar] [CrossRef][Green Version]
- De Sousa, E.B.; Casado, P.L.; Moura Neto, V.; Duarte, M.E.L.; Aguiar, D.P. Synovial fluid and Synovial membrane mesenchymal stem cells: Latest discoveries and therapeutic perspectives. Stem Cell Res. Ther. 2014, 5, 112. [Google Scholar] [CrossRef]
- Neybecker, P.; Henrionnet, C.; Pape, E.; Mainard, D.; Galois, L.; Loeuille, D.; Gillet, P.; Pinzano, A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 329. [Google Scholar] [CrossRef]
- Zayed, M.; Newby, S.; Misk, N.; Donnell, R.; Dhar, M. Xenogenic implantation of equine synovial fluid-derived mesenchymal stem cells leads to articular cartilage regeneration. Stem Cells Int. 2018, 2018, 1073705. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Hah, Y.-S.; Ock, S.-A.; Lee, J.-H.; Jeon, R.-H.; Park, J.-S.; Lee, S.-I.; Rho, N.-Y.; Rho, G.-J.; Lee, S.-L. Cell source-dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp. Cell Res. 2015, 333, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Park, J.-S.; Jang, S.-J.; Lee, S.-C.; Lee, H.; Lee, J.-H.; Rho, G.-J.; Lee, S.-L. Isolation and cellular phenotyping of mesenchymal stem cells derived from synovial fluid and bone marrow of minipigs. J. Vis. Exp. 2016, 113, 54077. [Google Scholar] [CrossRef]
- Son, Y.B.; Jeong, Y.I.; Hwang, K.C.; Jeong, Y.W.; Hwang, W.S. Mitochondrial metabolism assessment of lycaon-dog fetuses in interspecies somatic cell nuclear transfer. Theriogenology 2021, 165, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.H.; Wilson, E.L.; Peffers, M.J.; Roberts, S.; Simpson, D.M.; Richardson, J.B.; Gallacher, P.; Wright, K.T. Autologous chondrocyte implantation-derived synovial fluids display distinct responder and non-responder proteomic profiles. Arthritis Res. Ther. 2017, 19, 150. [Google Scholar] [CrossRef]
- Minas, T.; Gomoll, A.H.; Solhpour, S.; Rosenberger, R.; Probst, C.; Bryant, T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin. Orthop. Relat. Res. 2010, 468, 147–157. [Google Scholar] [CrossRef]
- Neybecker, P.; Henrionnet, C.; Pape, E.; Grossin, L.; Mainard, D.; Galois, L.; Loeuille, D.; Gillet, P.; Pinzano, A. Respective stemness and chondrogenic potential of mesenchymal stem cells isolated from human bone marrow, synovial membrane, and synovial fluid. Stem Cell Res. Ther. 2020, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Hung, S. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle 2012, 11, 3711–3712. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Turrubiarte, M.; Olmeo, C.; Accornero, P.; Baratta, M.; Martignani, E. Analysis of mesenchymal cells (MSCs) from bone marrow, synovial fluid and mesenteric, neck and tail adipose tissue sources from equines. Stem Cell Res. 2019, 37, 101442. [Google Scholar] [CrossRef]
- Jones, E.A.; Crawford, A.; English, A.; Henshaw, K.; Mundy, J.; Corscadden, D.; Chapman, T.; Emery, P.; Hatton, P.; McGonagle, D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single cell level. Arthiritis Rheum. 2008, 58, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular interactions and signaling in cartilage development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef]
- Sandell, L.J.; Adler, P. Developmental patterns of cartilage. Front. Biosci. 1999, 4, 731–742. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, S.; Oh, C.; Ju, J.; Song, W.K.; Yoo, Y.J.; Huh, T.; Chun, J. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J. Biol. Chem. 2002, 277, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mulloy, B. Glycosaminoglycans and proteoglycans. Pharmaceuticals 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Melrose, J. Glycosaminoglycan and proteoglycan biotherapeutics in articular cartilage protection and repair strategies: Novel approaches to visco-supplementation in orthobiologics. Adv. Ther. 2019, 2, 1900034. [Google Scholar] [CrossRef]
- Lozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar]

| Gene name (Symbol) | Primers Sequence | Product Size (bp) | Anneal. Temp (°C) |
|---|---|---|---|
| POU class 5 homeobox 1 (OCT4) | F: CGAGAGGATTTTGAGGCTGC R: GAGTACAGTGTGGTGAAGTGAG | 122 | 60 |
| Sex determining region Y-box 2 (SOX2) | F: CTCGCAGACCTACATGAACG R: TGGGAGGAAGAGGAAACCAC | 144 | 60 |
| Nanog homeobox (NANOG) | F: AGCACAGAGAAGCAGGAAGA R: CCACCGCTTACATTTCATTC | 213 | 60 |
| Runt-related transcription factor 2 (Runx2) | F: GACAGAAGCTTGATGACTCT R: GTAATCTGACTCTGTCCTTG | 166 | 60 |
| Osteocalcin (ON) | F: AGTGAGATGGTGAAGAGACT R: TAGGTTGTGCCGTAGAAG | 176 | 60 |
| Lipoprotein lipase (LPL) | F: GAGAGTGTTACCTACACCAA R: GCCTTTACTCTGATCTTCTC | 248 | 60 |
| Fatty acid-binding protein 4 (FABP4) | F: GTGACCATCAGTGTGAATG R: GCACCTCCTTCTAAAGTTAC | 152 | 60 |
| The type X collagen gene (COL10A1) | F: TATCCAGCTATAGGCAGTC R: TCGTAGGTGTACATTACAGG | 194 | 60 |
| Aggrecan (ACAN) | F: TGTGGAGGGTGTTACTGAAC R: GACTGATGACCCTTCTACCC | 154 | 60 |
| Collagen type II alpha 1 chain (COL2A1) | F: GTGGTGACAAAGGTGAAAAA R: AGCCTTCTCATCAAATCCTC | 154 | 60 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | F: GCTGAGTACGTTGTGGAGTC R: TCACGCCCATCACAAACATG | 133 | 60 |
| Integrin beta-1 (CD 29) | F: CTTGCGTTGCTGCTGATTTG R: TTCTTGCGTGTCCCATTTGG | 105 | 60 |
| 5′-nucleotidase (CD 73) | F: CAACCTCAGACATGCCGATG R: GTCAAAGGTGCCTCCAAAGG | 154 | 60 |
| Endoglin (CD 105) | F: TCCTCCAGACCTCCAACTCT R:CCCAAATTCAGTTGGCAGCT | 115 | 60 |
| Cluster of differentiation 34 molecule (CD 34) | F: GGTCTTGGCCAACAGAACAG R: CAGCTTCGACGGTTCATCAG | 216 | 60 |
| Protein tyrosine phosphatase, receptor type, C (CD 45) | F: AACTCTTGGCATTTGGCGTT R: TTCTGCCTACACTCAAGGGG | 220 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.-B.; Jeong, Y.I.; Jeong, Y.W.; Hossein, M.S.; Olsson, P.O.; Tinson, A.; Singh, K.K.; Lee, S.-Y.; Hwang, W.S. Cell Source-Dependent In Vitro Chondrogenic Differentiation Potential of Mesenchymal Stem Cell Established from Bone Marrow and Synovial Fluid of Camelus dromedarius. Animals 2021, 11, 1918. https://doi.org/10.3390/ani11071918
Son Y-B, Jeong YI, Jeong YW, Hossein MS, Olsson PO, Tinson A, Singh KK, Lee S-Y, Hwang WS. Cell Source-Dependent In Vitro Chondrogenic Differentiation Potential of Mesenchymal Stem Cell Established from Bone Marrow and Synovial Fluid of Camelus dromedarius. Animals. 2021; 11(7):1918. https://doi.org/10.3390/ani11071918
Chicago/Turabian StyleSon, Young-Bum, Yeon Ik Jeong, Yeon Woo Jeong, Mohammad Shamim Hossein, Per Olof Olsson, Alex Tinson, Kuhad Kuldip Singh, Sang-Yun Lee, and Woo Suk Hwang. 2021. "Cell Source-Dependent In Vitro Chondrogenic Differentiation Potential of Mesenchymal Stem Cell Established from Bone Marrow and Synovial Fluid of Camelus dromedarius" Animals 11, no. 7: 1918. https://doi.org/10.3390/ani11071918
APA StyleSon, Y.-B., Jeong, Y. I., Jeong, Y. W., Hossein, M. S., Olsson, P. O., Tinson, A., Singh, K. K., Lee, S.-Y., & Hwang, W. S. (2021). Cell Source-Dependent In Vitro Chondrogenic Differentiation Potential of Mesenchymal Stem Cell Established from Bone Marrow and Synovial Fluid of Camelus dromedarius. Animals, 11(7), 1918. https://doi.org/10.3390/ani11071918






