Chicken Mesenchymal Stem Cells and Their Applications: A Mini Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Characterization of Chicken MSCs
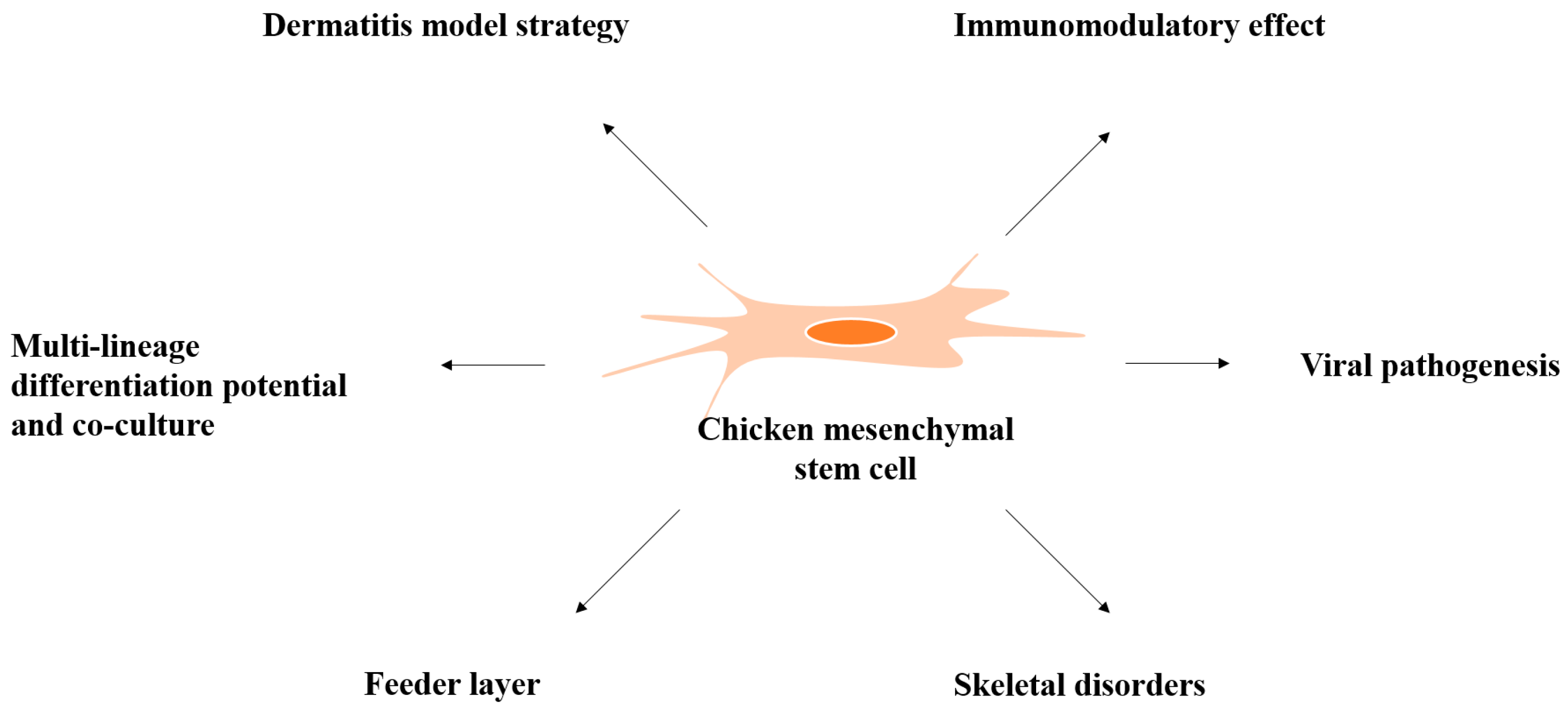
3. Biological Properties of Chicken MSCs
3.1. Three-Dimensional (3D) Culture and MSC Interactions
3.2. Feeder Cells Layer
3.3. Infectious Bursal Disease Virus
3.4. Skeletal Diseases
3.5. Probiotics and Prebiotics
3.6. Chicken Dermatitis
3.7. Meat “In Vitro”
3.8. Cryopreservation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Fornari, M.B.; Zanella, R.; Ibelli, A.M.; Fernandes, L.T.; Cantão, M.E.; Thomaz-Soccol, V.; Ledur, M.C.; Peixoto, J.O. Unraveling the associations of osteoprotegerin gene with production traits in a paternal broiler line. SpringerPlus 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- Crigler, L.; Kazhanie, A.; Yoon, T.J.; Zakhari, J.; Anders, J.; Taylor, B.; Virador, V.M. Isolation of a mesenchymal cell population from murine dermis that contains progenitors of multiple cell lineages. FASEB J. 2007, 21, 2050–2063. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Le Drévo, M.A.; Moreau, M.F.; Guillet, C.; Baslé, M.F.; Chappard, D. Isolation of osteoprogenitors from murine bone marrow by selection of CD11b negative cells. Cytotechnology 2008, 58, 163. [Google Scholar] [CrossRef][Green Version]
- Kar, S.; Mitra, S.; Banerjee, E.R. Isolation and culture of embryonic stem cells, mesenchymal stem cells, and dendritic cells from humans and mice. Methods. Mol. Biol. 2015, 1516, 145–152. [Google Scholar] [CrossRef]
- Kumar, K.; Agarwal, P.; Das, K.; Mili, B.; Madhusoodan, A.P.; Kumar, A.; Bag, S. Isolation and characterization of mesenchymal stem cells from caprine umbilical cord tissue matrix. Tissue Cell 2016, 48, 653–658. [Google Scholar] [CrossRef]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef]
- Krešić, N.; Šimić, I.; Lojkić, I.; Bedeković, T. Canine adipose derived mesenchymal stem cells transcriptome composition alterations: A step towards standardizing therapeutic. Stem Cells Int. 2017. [Google Scholar] [CrossRef]
- Nakamura, M.; Nishida, H.; Yoshizaki, K.; Akiyoshi, H.; Hatoya, S.; Sugiura, K.; Inaba, T. Canine mesenchymal stromal cell-conditioned medium promotes survival and neurite outgrowth of neural stem cells. J. Vet. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Munoz, J.L.; Greco, S.J.; Patel, S.A.; Sherman, L.S.; Bhatt, S.; Bhatt, R.S.; Shrensel, J.A.; Guan, Y.Z.; Xie, G.; Ye, J.H.; et al. Feline bone marrow-derived mesenchymal stromal cells (MSCs) show similar phenotype and functions with regards to neuronal differentiation as human MSCs. Differentiation 2012, 84, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Thiede, M.A.; Mosca, J.D.; Moorman, M.; Gerson, S.L. Phenotypic and functional comparison of cultures of marrow--derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell. Physiol. 1998, 176, 57–66. [Google Scholar] [CrossRef]
- Eleuteri, S.; Fierabracci, A. Insights into the secretome of mesenchymal stem cells and its potential applications. Inter. J. Mol. Sci. 2020, 20, 4597. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal stem cell migration and tissue repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Zannetti, A.; Benga, G.; Brunetti, A.; Napolitano, F.; Avallone, L.; Pelagalli, A. Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells 2020, 9, 2678. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Sharma, J.M. Susceptibility of chicken mesenchymal stem cells to infectious bursal disease virus. J. Virol. Methods. 2009, 160, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; Chen, C.; Waters, E.; West, F.D.; Kim, W.K. Isolation and differentiation of mesenchymal stem cells from broiler chicken compact bones. Front. Physiol. 2019, 9, 1892. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Hou, L.; Ma, Y.; Chen, L.; Zhang, M.; Guan, W. Isolation and characterization of mesenchymal stem cells from chicken bone marrow. Cell Tissue Bank. 2013, 14, 437–451. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.J.; Ji, H.; Guan, W.; Zhao, Y. Isolation, culture, and characterization of chicken lung-derived mesenchymal stem cells. Can. J. Vet. Res. 2018, 82, 225–235. [Google Scholar]
- Teresa Conconi, M.; Di Liddo, R.; Tommasini, M.; Calore, C.; Paolo Parnigotto, P. Phenotype and differentiation potential of stromal populations obtained from various zones of human umbilical cord: An overview. J. Tissue Eng. Regen. Med. 2011, 4, 6–20. [Google Scholar] [CrossRef]
- Lin, C.S.; Xin, Z.C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109. [Google Scholar] [CrossRef]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert. Rev. Respir. Med. 2020, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef]
- Lavial, F.; Acloque, H.; Bertocchini, F.; MacLeod, D.J.; Boast, S.; Bachelard, E.; Montillet, G.; Thenot, S.; Sang, H.M.; Stern, C.D.; et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 2007, 134, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; O’Brien, T.D.; Goyal, S.M.; Sharma, J.M. Isolation and characterization of chicken lung mesenchymal stromal cells and their susceptibility to avian influenza virus. Dev. Comp. Immunol. 2010, 34, 474–479. [Google Scholar] [CrossRef]
- Bai, C.; Li, X.; Hou, L.; Zhang, M.; Guan, W.; Ma, Y. Biological characterization of chicken mesenchymal stem/progenitor cells from umbilical cord Wharton’s jelly. Mol. Cell. Biochem. 2013, 376, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Glennie, S.; Dyson, J.; Scott, D.; Laylor, R.; Simpson, E.; Dazzi, F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003, 101, 3722–3729. [Google Scholar] [CrossRef]
- Maitra, B.; Szekely, E.; Gjini, K.; Laughlin, M.J.; Dennis, J.; Haynesworth, S.E.; Koç, O.N. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004, 33, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, G.E.; Rachmilewitz, J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef]
- Groh, M.E.; Maitra, B.; Szekely, E.; Koç, O.N. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Ex. Hematol. 2005, 33, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase–mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Tthromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Kocamaz, E.; Gok, D.; Cetinkaya, A.; Tufan, A.C. Implication of C-type natriuretic peptide-3 signaling in glycosaminoglycan synthesis and chondrocyte hypertrophy during TGF-β1 induced chondrogenic differentiation of chicken bone marrow-derived mesenchymal stem cells. J. Mol. His. 2012, 43, 497–508. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Mazzoni, A.; Bronte, V.; Visintin, A.; Spitzer, J.H.; Apolloni, E.; Serafini, P.; Zanovello, P.; Segal, D.M. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002, 168, 689–695. [Google Scholar] [CrossRef]
- Mais, A.; Klein, T.; Ullrich, V.; Schudt, C.; Lauer, G. Prostanoid pattern and iNOS expression during chondrogenic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 2006, 98, 798–809. [Google Scholar] [CrossRef]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.F.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Luigi, G.; Pistoia, M.V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Djouad, F.; Plence, P.; Bony, C.; Tropel, P.; Apparailly, F.; Sany, J.; Jorgensen, C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003, 102, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.B.; Scadden, D.T. The hematopoietic stem cell in its place. Nat. Immunol. 2006, 7, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Roderburg, C.; Wein, F.; Diehlmann, A.; Frankhauser, M.; Schubert, R.; Eckstein, V.; Ho, A.D. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells 2007, 25, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Leisten, I.; Kramann, R.; Ferreira, M.S.V.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knüchel, R.; Schneider, R.K. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Walenda, T.; Bokermann, G.; Ferreira, M.S.V.; Piroth, D.M.; Hieronymus, T.; Neuss, S.; Zenke, M.; Ho, A.D.; Müller, A.M.; Wagner, W. Synergistic effects of growth factors and mesenchymal stromal cells for expansion of hematopoietic stem and progenitor cells. Exp. Hematol. 2011, 39, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Walenda, T.; Bork, S.; Horn, P.; Wein, F.; Saffrich, R.; Diehlmann, A.; Eckstein, V.; Ho, A.D.; Wagner, W. Co--culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J. Cell Mol. Med. 2010, 14, 337–350. [Google Scholar] [CrossRef]
- Rustad, K.C.; Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Major, M.R.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012, 33, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, X.; Yuan, Y.; Song, Z.; Zhang, L.; Wang, X.; Li, T. Effect of cell culture using chitosan membranes on stemness marker genes in mesenchymal stem cells. Mol. Med. Rep. 2013, 7, 1945–1949. [Google Scholar] [CrossRef]
- Su, N.; Gao, P.L.; Wang, K.; Wang, J.Y.; Zhong, Y.; Luo, Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 2017, 141, 74–85. [Google Scholar] [CrossRef]
- Qian, C.; Zhou, Z.; Han, H.; Zhao, C.; Jin, X.; Zhao, H.; Zhang, Y.; Chen, W.; Yang, N.; Li, Z. Influence of microgravity on the concentration of circulating primordial germ cells in Silky chicken offspring. J. Poult. Sci. 2009, 47, 65–70. [Google Scholar] [CrossRef][Green Version]
- Naeemipour, M.; Dehghani, H.; Bassami, M.; Bahrami, A. Expression dynamics of pluripotency genes in chicken primordial germ cells before and after colonization of the genital ridges. Mol. Reprod. Dev. 2013, 80, 849–861. [Google Scholar] [CrossRef]
- Tonus, C.; Cloquette, K.; Ectors, F.; Piret, J.; Gillet, L.; Antoine, N.; Grobet, L. Long term-cultured and cryopreserved primordial germ cells from various chicken breeds retain high proliferative potential and gonadal colonisation competency. Reprod. Fertil. Dev. 2016, 28, 628–639. [Google Scholar] [CrossRef]
- Li, D.; Chen, Z.; Chen, S.; Ji, H.; Zhan, X.; Luo, D.; Luo, H.; Wang, B. Chicken Mesenchymal Stem Cells as Feeder Cells Facilitate the Cultivation of Primordial Germ Cells from Circulating Blood and Gonadal Ridge. Stem Cell Discov. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Xie, H.; Sun, L.; Zhang, L.; Liu, T.; Chen, L.; Zhao, A.; Gao, F.; Zou, P.; Li, Q.; Guo, A.J.; et al. Mesenchymal stem cell-derived microvesicles support ex vivo expansion of cord blood-derived CD34+ cells. Stem Cells 2016. [Google Scholar] [CrossRef]
- Iacono, M.L.; Anzalone, R.; La Rocca, G.; Baiamonte, E.; Maggio, A.; Acuto, S. Wharton’s jelly mesenchymal stromal cells as a feeder layer for the ex vivo expansion of hematopoietic stem and progenitor cells: A review. Stem. Cell. Rev. Rep. 2017, 13, 35–49. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chu, T.Y.; Ding, D.C. WNT/β-Catenin signaling pathway regulates non-tumorigenesis of human embryonic stem cells co-cultured with human umbilical cord mesenchymal stem cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zmrhal, V.; Slama, P. Current knowledge about interactions between avian dendritic cells and poultry pathogens. Dev. Comp. Immunol. 2020, 104, 103565. [Google Scholar] [CrossRef] [PubMed]
- Tippenhauer, M.; Heller, D.E.; Weigend, S.; Rautenschlein, S. The host genotype influences infectious bursal disease virus pathogenesis in chickens by modulation of T cells responses and cytokine gene expression. Dev. Comp. Immunol. 2013, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Pathak, D.C.; Ramamurthy, N.; Maity, H.K.; Chellappa, M.M. Infectious bursal disease virus in chickens: Prevalence, impact, and management strategies. Vet. Med. Res. Rep. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Elankumaran, S.; Heckert, R.A.; Moura, L. Pathogenesis and tissue distribution of a variant strain of infectious bursal disease virus in commercial broiler chickens. Avian Dis. 2002, 46, 169–176. [Google Scholar] [CrossRef]
- Kabell, S.; Handberg, K.J.; Kusk, M.; Bisgaard, M. Detection of infectious bursal disease virus in various lymphoid tissues of experimentally infected specific pathogen free chickens by different reverse transcription polymerase chain reaction assays. Avian Dis. 2005, 49, 534–539. [Google Scholar] [CrossRef]
- Kim, I.J.; You, S.K.; Kim, H.; Yeh, H.Y.; Sharma, J.M. Characteristics of bursal T lymphocytes induced by infectious bursal disease virus. J. Virol. 2000, 74, 8884–8892. [Google Scholar] [CrossRef] [PubMed]
- Rautenschlein, S.; Yeh, H.Y.; Njenga, M.K.; Sharma, J.M. Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch. Virol. 2002, 147, 285–304. [Google Scholar] [CrossRef]
- Ruby, T.; Whittaker, C.; Withers, D.R.; Chelbi-Alix, M.K.; Morin, V.; Oudin, A.; Young, J.R.; Zoorob, R. Transcriptional profiling reveals a possible role for the timing of the inflammatory response in determining susceptibility to a viral infection. J. Virol. 2006, 80, 9207–9216. [Google Scholar] [CrossRef]
- Eldaghayes, I.; Rothwell, L.; Williams, A.; Withers, D.; Balu, S.; Davison, F.; Kaiser, P. Infectious bursal disease virus: Strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 2006, 19, 83–91. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Han, H.; Yuan, J.; Li, Z. Comparison of the expression of cytokine genes in the bursal tissues of the chickens following challenge with infectious bursal disease viruses of varying virulence. Virol. J. 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Heo, Y.T.; Lee, S.H.; Yang, J.H.; Kim, T.; Lee, H.T. Bone marrow cell-mediated production of transgenic chickens. Lab. Investig. 2011, 1229–1240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000, 79, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Yahyaei, B.; Gilanpour, H.; Veshkini, A. Study of the ossification centers and skeletal development of pelvic limb in quail after hatching. Adv. Environ. Biol. 2013, 2074–2081. [Google Scholar]
- Iqbal, M.; Zhang, H.; Mehmood, K.; Li, A.; Jiang, X.; Wang, Y.; Zhang, J.; Iqbal, M.K.; Rehman, M.U.; Yao, W.; et al. Icariin: A potential compound for the recovery of Tibial Dyschondroplasia affected chicken via up-regulating BMP-2 expression. Biol. Proced. 2018, 20, 1–7. [Google Scholar] [CrossRef]
- Fleming, R.H.; McCormack, H.A.; McTeir, L.; Whitehead, C.C. Incidence, pathology and prevention of keel bone deformities in the laying hen. Brit. Poult. Sci. 2004, 45, 320–330. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; Tuyttens, F.A.M.; De Reu, K.; Herman, L.; Zoons, J.; Sonck, B. Welfare assessment of laying hens in furnished cages and non-cage systems: An on-farm comparison. Anim. Welf. 2008, 17, 363–373. [Google Scholar]
- Käppeli, S.; Gebhardt-Henrich, S.G.; Fröhlich, E.; Pfulg, A.; Stoffel, M.H. Prevalence of keel bone deformities in Swiss laying hens. Br. Poult. Sci. 2011, 52, 531–536. [Google Scholar] [CrossRef]
- Wilkins, L.J.; McKinstry, J.L.; Avery, N.C.; Knowles, T.G.; Brown, S.N.; Tarlton, J.; Nicol, C.J. Influence of housing system and design on bone strength and keel bone fractures in laying hens. Vet. Rec. 2011, 169, 414. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.T.; Guerin, M.T.; Widowski, T.M. On-farm comparison of keel fracture prevalence and other welfare indicators in conventional cage and floor-housed laying hens in Ontario, Canada. Poult. Sci. 2015, 94, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.J.; Dunn, I.C.; Christensen, J.P.; Petow, S.; Kittelsen, K.; Ulrich, R. Explanations for keel bone fractures in laying hens: Are there explanations in addition to elevated egg production? Poult. Sci. 2020, 99, 4183–4194. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Lee, N.; Wang, K.C.; Soong, Y.K.; Huang, K.E. Effect of estrogen and 1α, 25 (OH) 2-vitamin D3 on the activity and growth of human primary osteoblast-like cells in vitro. Fertil. Steril. 2002, 77, 1038–1043. [Google Scholar] [CrossRef]
- Jørgensen, N.R.; Henriksen, Z.; Sørensen, O.H.; Civitelli, R. Dexamethasone, BMP-2, and 1, 25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: Validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids 2004, 69, 219–226. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Niu, X.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012, 33, 4818–4827. [Google Scholar] [CrossRef]
- Tourkova, I.L.; Liu, L.; Sutjarit, N.; Larrouture, Q.C.; Luo, J.; Robinson, L.J.; Blair, H.C. Adrenocorticotropic hormone and 1, 25-dihydroxyvitamin D 3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab. Investig. 2017, 97, 1072–1083. [Google Scholar] [CrossRef]
- Harrison, J.R.; Petersen, D.N.; Lichtler, A.C.; Mador, A.T.; Rowe, D.W.; Kream, B.E. 1, 25-Dihydroxyvitamin D3 inhibits transcription of type I collagen genes in the rat osteosarcoma cell line ROS 17/2.8. Endocrinology 1989, 125, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Chen, T.L. 1, 25-Dihydroxyvitamin D3 interaction with dexamethasone and retinoic acid: Effects on procollagen messenger ribonucleic acid levels in rat osteoblast-like cells. Mol. Endocrinol. 1989, 3, 97–104. [Google Scholar] [CrossRef]
- Van Driel, M.; Van Leeuwen, J.P. Vitamin D endocrine system and osteoblasts. Bonekey Rep. 2014, 3, 493. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dosier, C.R.; Park, J.H.; De, S.; Guldberg, R.E.; Boyan, B.D.; Schwartz, Z. Mineralization of three--dimensional osteoblast cultures is enhanced by the interaction of 1α, 25--dihydroxyvitamin D3 and BMP2 via two specific vitamin D receptors. J. Tissue. Eng. Regen. Med. 2016, 10, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seong, S.; Kim, K.; Kim, I.; Jeong, B.C.; Kim, N. Downregulation of Runx2 by 1, 25-dihydroxyvitamin D3 induces the transdifferentiation of osteoblasts to adipocytes. Int. J. Mol. Sci. 2016, 17, 770. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Xin, N.; Yuan, Y.; Zhang, Q.; Gong, P.; Wu, Y. 1α, 25-Dihydroxyvitamin D3 promotes osteogenesis by promoting Wnt signaling pathway. J. Steroid. Biochem. Mol. Biol. 2017, 174, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Broess, M.; Riva, A.; Gerstenfeld, L.C. Inhibitory effects of 1, 25 (OH) 2 vitamin D3 on collagen type I, osteopontin, and osteocalcin gene expression in chicken osteoblasts. J. Cell. Bibiochem. 1995, 57, 440–451. [Google Scholar] [CrossRef]
- Pande, V.V.; Chousalkar, K.C.; Bhanugopan, M.S.; Quinn, J.C. Super pharmacological levels of calcitriol (1, 25-(OH) 2 D3) inhibits mineral deposition and decreases cell proliferation in a strain dependent manner in chicken mesenchymal stem cells undergoing osteogenic differentiation in vitro. Poult. Sci. 2015, 94, 2784–2796. [Google Scholar] [CrossRef]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Milford, A.B.; Le Mouël, C.; Bodirsky, B.L.; Rolinski, S. Drivers of meat consumption. Appetite 2019, 141, 104313. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. The transitional dynamics of caloric ecosystems: Changes in the food supply around the world. Crit. Public Health. 2015, 25, 248–264. [Google Scholar] [CrossRef]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.A.N.; Zakeri, A.; Kamrani, B.; Pourakbari, Y. Effect of prebiotics, probiotics, acidfire, growth promoter antibiotics and synbiotic on humural immunity of broiler chickens. Global Vet. 2012, 8, 612–617. [Google Scholar]
- Maiorano, G.; Stadnicka, K.; Tavaniello, S.; Abiuso, C.; Bogucka, J.; Bednarczyk, M. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 2017, 96, 511–518. [Google Scholar] [CrossRef]
- Carrade, D.D.; Borjesson, D.L. Immunomodulation by mesenchymal stem cells in veterinary species. Com. Med. 2013, 63, 207–217. [Google Scholar]
- Lotfinegad, P. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: A hope in cell therapy. Adv. Pharm. Bull. 2014, 4, 5. [Google Scholar] [CrossRef]
- Zimmermann, K.; Haas, A.; Oxenius, A. Systemic antibody responses to gut microbes in health and disease. Gut Microbes 2012, 3, 42–47. [Google Scholar] [CrossRef]
- Li, G.; Lillehoj, H.S.; Lee, K.W.; Jang, S.I.; Marc, P.; Gay, C.G.; Ritter, G.D.; Bautista, D.A.; Phillips, K.; Neumann, A.P.; et al. An outbreak of gangrenous dermatitis in commercial broiler chickens. Avian Path. 2010, 39, 247–253. [Google Scholar] [CrossRef]
- McDevitt, R.M.; Brooker, J.D.; Acamovic, T.; Sparks, N.H.C. Necrotic enteritis; a continuing challenge for the poultry industry. World’s Poul. Sci. J. 2006, 62, 221–247. [Google Scholar] [CrossRef]
- Mataragas, M.; Skandamis, P.N.; Drosinos, E.H. Risk profiles of pork and poultry meat and risk ratings of various pathogen/product combinations. Int. J. Food Microbiol. 2008, 126, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.K.; Songer, J.G. Necrotic enteritis in chickens: A paradigm of enteric infection by Clostridium perfringens type A. Anaerobe 2009, 15, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F.; Rood, J.I.; Moore, R.J.; Titball, R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009, 17, 32–36. [Google Scholar] [CrossRef]
- Gornatti-Churria, C.D.; Crispo, M.; Shivaprasad, H.L.; Uzal, F.A. Gangrenous dermatitis in chickens and turkeys. J. Vet. Diag. Investig. 2018, 30, 188–196. [Google Scholar] [CrossRef]
- Shivaprasad, H.L. Gangrenous dermatitis in poultry. In Clostridial Diseases of Animals; Wiley-Blackwell: Ames, IA, USA, 2016; pp. 255–264. [Google Scholar]
- Dinev, I.; Denev, S.; Vashin, I.; Kanakov, D.; Rusenova, N. Pathomorphological investigations on the prevalence of contact dermatitis lesions in broiler chickens. J. Appl. Anim. Res. 2019, 47, 129–134. [Google Scholar] [CrossRef]
- Li, G.; Lillehoj, H.S.; Lee, K.W.; Lee, S.H.; Park, M.S.; Jang, S.I.; Bauchan, G.R.; Gay, C.G.; Ritter, G.D.; Bautista, D.A.; et al. Immunopathology and cytokine responses in commercial broiler chickens with gangrenous dermatitis. Avian Pathol. 2010, 39, 255–264. [Google Scholar] [CrossRef]
- Golchin, A.; Farahany, T.Z.; Khojasteh, A.; Soleimanifar, F.; Ardeshirylajimi, A. The clinical trials of mesenchymal stem cell therapy in skin diseases: An update and concise review. Curr. Stem Cell Res. Ther. 2019, 14, 22–33. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- Hoekstra, A.Y.; Chapagain, A.K. Water footprints of nations: Water use by people as a function of their consumption pattern. In Integrated Assessment of Water Resources and Global Change; Springer: Dordrecht, The Netherlands, 2006; pp. 35–48. [Google Scholar]
- Fiala, N. Meeting the demand: An estimation of potential future greenhouse gas emissions from meat production. Ecol. Econom. 2008, 67, 412–419. [Google Scholar] [CrossRef]
- Sutton, T.C. The pandemic threat of emerging H5 and H7 avian influenza viruses. Viruses 2018, 10, 461. [Google Scholar] [CrossRef]
- Park, S.E. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin. Exp. Pediatr. 2020, 63, 119. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Tzatzalos, E.; Donne, M.; Kolundzic, N.; Helgason, I.; Ilic, D. Prospects for the use of induced pluripotent stem cells in animal conservation and environmental protection. Stem Cells Transl. Med. 2019, 8, 7–13. [Google Scholar] [CrossRef]
- Datar, I.; Betti, M. Possibilities for an in vitro meat production system. Innov. Food. Sci. Emerg. Technol. 2010, 11, 13–22. [Google Scholar] [CrossRef]
- Arshad, M.S.; Javed, M.; Sohaib, M.; Saeed, F.; Imran, A.; Amjad, Z. Tissue engineering approaches to develop cultured meat from cells: A mini review. Cogent Food Agric 2017, 3, 1320814. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Fayaz, H. In Vitro meat production: Challenges and benefits over conventional meat production. J. Integr. Agric. 2015, 14, 241–248. [Google Scholar] [CrossRef]
- Will, K.; Schering, L.; Albrecht, E.; Kalbe, C.; Maak, S. Differentiation of bovine satellite cell-derived myoblasts under different culture conditions. In Vitro. Cell. Dev. Biol. Animal 2015, 51, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Ahadian, S.; Ramon--Azcon, J.; Hosseini, V.; Fujie, T.; Parthiban, S.P.; Khademhosseini, A. Three–dimensional co--culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 2017, 11, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Mehta, F.; Theunissen, R.; Post, M.J. Adipogenesis from bovine precursors. In Myogenesis; Humana Press: New York, NY, USA, 2019; pp. 111–125. [Google Scholar] [CrossRef]
- Cremonesi, F.; Corradetti, B.; Consiglio, A.L. Fetal adnexa derived stem cells from domestic animal: Progress and perspectives. Theriogenology 2001, 75, 1400–1415. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.B.; Song, Y.P.; Han, Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012, 652034. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Li, C.; Jin, D.; Guo, Y.; Guan, W.; Ma, Y.; Zhao, Q. Establishment and characterization of a fibroblast line from landrace. Artif. Cell Blood Sub. 2010, 38, 129–135. [Google Scholar] [CrossRef]
- Na, R.S.; Zhao, Q.J.; Su, X.H.; Chen, X.W.; Guan, W.J.; Ma, Y.H. Establishment and biological characteristics of Ujumqin sheep fibroblast line. Cytotechnology 2010, 62, 43–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Sources | Type of Digestive | Separation | Morphology | Confluency | Positive Markers | Negative Markers | Transcription Factors | References |
|---|---|---|---|---|---|---|---|---|
| Bone marrow | - | Ficoll-Hypaque (1.090 g/mL) | spindle-shaped | 14 days | CD44, CD90 CD105 | CD45 | PouV, Sox2, Nanog | [17] |
| Percoll solution (1.073 g/mL) | spindle-shaped | 2–3 days | CD44, CD29, CD71, CD73 | CD31, CD34 | [19] | |||
| Compact bones | 0.25% collagenase | spindle-shaped | 8–10 days | CD90, CD105, CD73, CD44 | CD31, CD34 CD45 | [18] | ||
| Lung | 0.1% collagenase | spindle-shaped | 5–7 days | CD29, CD73, CD90, CD105 | CD34, CD45 | OCT-4 | [20] | |
| 0.5 mg/mL collagenase type IV | Ficoll-Hypaque (1.090 g/mL) | spindle-shaped | 14 days | CD44, CD90, CD105 | PouV | [28] | ||
| Wharton’s jelly | 0.1% collagenase type IV | fibroblast-like shaped | 5–6 days | CD29, CD44, CD71, CD73 | CD31, CD34 | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svoradova, A.; Zmrhal, V.; Venusova, E.; Slama, P. Chicken Mesenchymal Stem Cells and Their Applications: A Mini Review. Animals 2021, 11, 1883. https://doi.org/10.3390/ani11071883
Svoradova A, Zmrhal V, Venusova E, Slama P. Chicken Mesenchymal Stem Cells and Their Applications: A Mini Review. Animals. 2021; 11(7):1883. https://doi.org/10.3390/ani11071883
Chicago/Turabian StyleSvoradova, Andrea, Vladimir Zmrhal, Eva Venusova, and Petr Slama. 2021. "Chicken Mesenchymal Stem Cells and Their Applications: A Mini Review" Animals 11, no. 7: 1883. https://doi.org/10.3390/ani11071883
APA StyleSvoradova, A., Zmrhal, V., Venusova, E., & Slama, P. (2021). Chicken Mesenchymal Stem Cells and Their Applications: A Mini Review. Animals, 11(7), 1883. https://doi.org/10.3390/ani11071883






