Relationship between Temperament and Stage of Lactation, Productivity and Milk Composition of Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Animals
2.2. Measurements
2.3. Data Analysis and Statistics
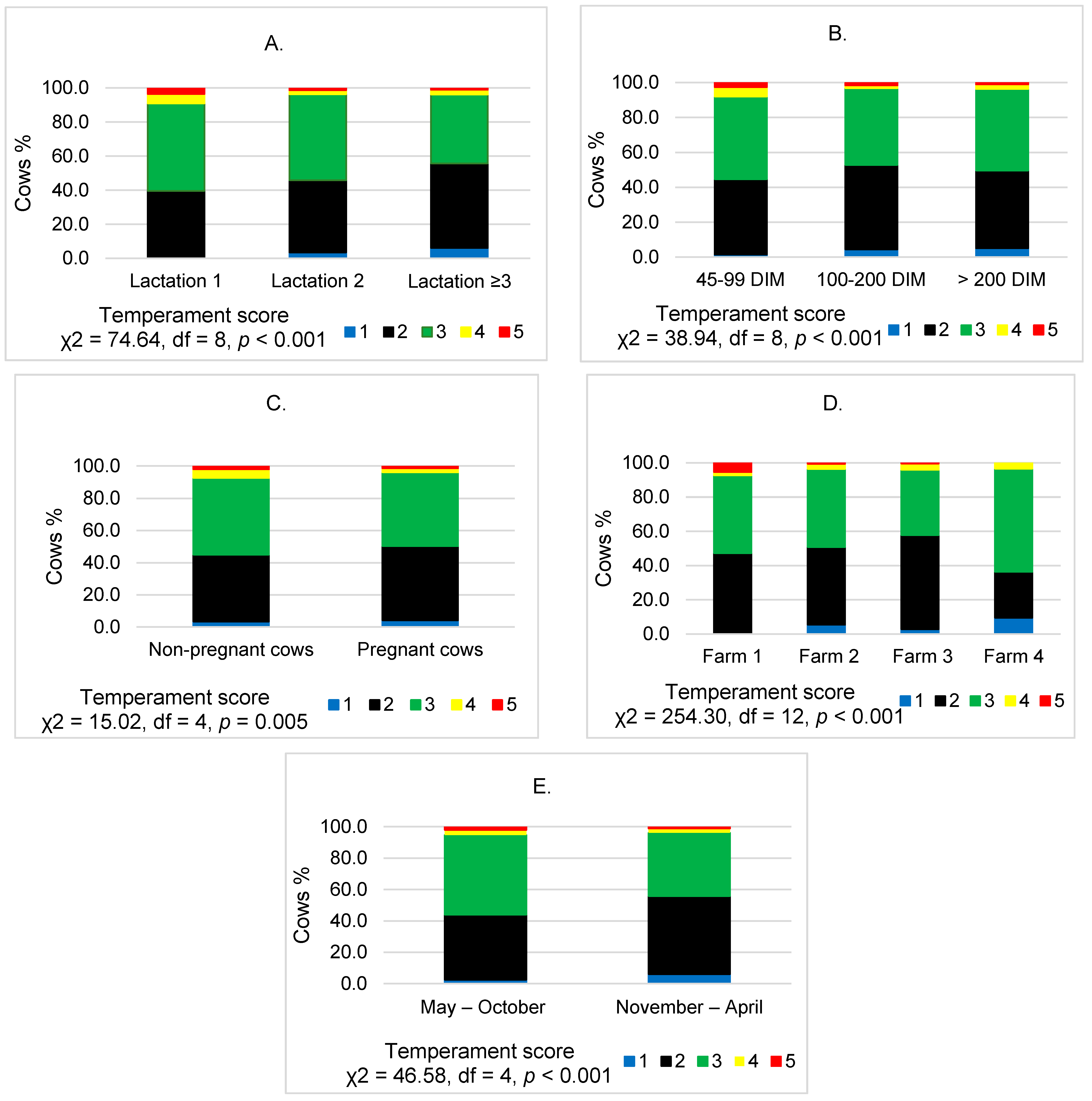
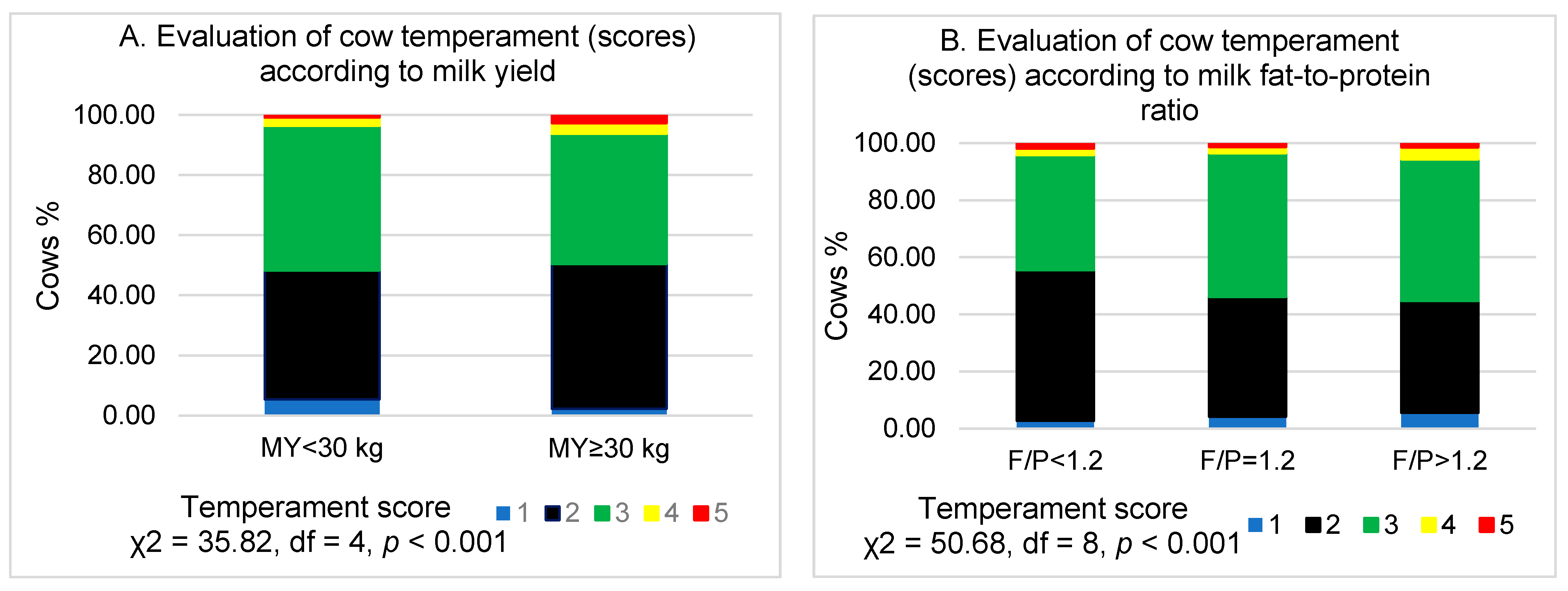
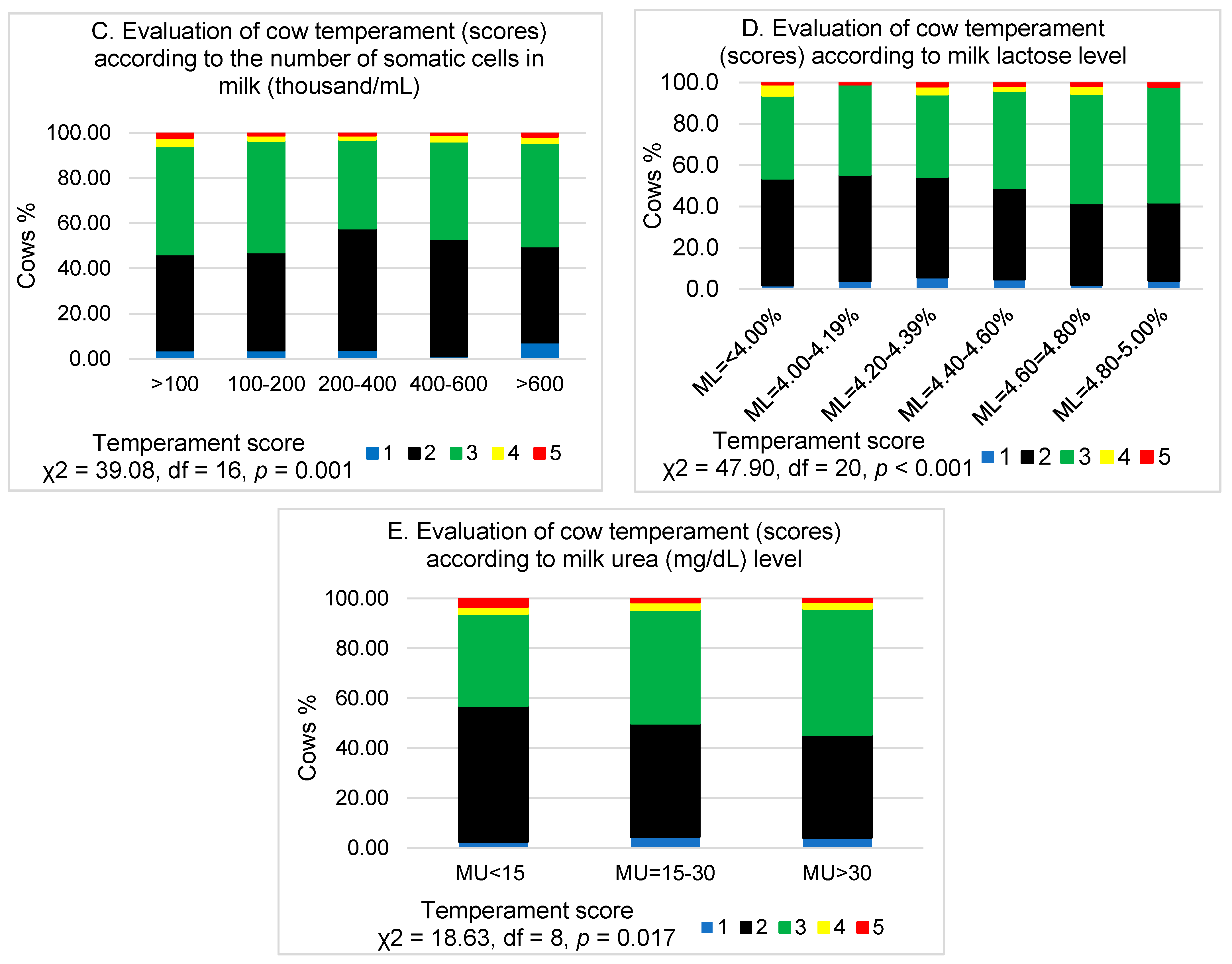
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Health and Food Safety Directorate-General; Overview Report of the Directorate-General for Health and Food Safety on a Series of Audits Carried out in 2016 in Order to Evaluate Member State Controls and Use of Indicators to Ensure the Welfare of Cattle on Dairy Farms; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-52981-8. [Google Scholar] [CrossRef]
- Danchuk, O.V.; Karposvkii, V.I.; Tomchuk, V.A.; Zhurenko, O.V.; Bobryts’ka, O.M.; Trokoz, V.O. Temperament in Cattle: A Method of Evaluation and Main Characteristics. Neurophysiology 2020, 52, 73–79. [Google Scholar] [CrossRef]
- Haskell, M.J.; Geoff, S.; Turner, S.P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 368. [Google Scholar]
- Chang, Y.; Brito, L.F.; Alvarenga, A.B.; Wang, Y. Incorporating temperament traits in dairy cattle breeding programs: Challenges and opportunities in the phenomics era. Anim. Front. 2020, 10, 29–36. [Google Scholar] [CrossRef]
- Fordyce, G.; Goddard, M.E.; Seifert, G.W. The measurement of temperament in cattle and the effect of experience and genotype. Proc. Aust. Soc. Anim. Prod. J. 1982, 4, 329–332. [Google Scholar]
- Gibbons, J.; Lawrence, A.B.; Haskell, M.J. Responsiveness of dairy cows to human approach and novel stimuli. Appl. Anim. Behav. Sci. 2009, 116, 163–173. [Google Scholar] [CrossRef]
- Gibbons, J.M.; Lawrence, A.B.; Haskell, M.J. Measuring sociability in dairy cows. Appl. Anim. Behav. Sci. 2010, 122, 84–91. [Google Scholar] [CrossRef]
- Burrow, H.M.; Seifert, G.W.; Corbet, N.J. A new technique for measuring temperament in cattle. Proc. Aust. Soc. Anim. Prod. 1988, 17, 154–157. [Google Scholar]
- King, D.A.; Pfeiffer, C.S.; Randel, R.D.; Welsh, T.H., Jr.; Oliphint, R.A.; Baird, B.E.; Savell, J.W. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci. 2006, 74, 546–556. [Google Scholar] [CrossRef]
- Breuer, K.; Hemsworth, P.H.; Barnett, J.L.; Matthews, L.R.; Coleman, G.J. Behavioural response to humans and the productivity of commercial dairy cows. Appl. Anim. Behav. Sci. 2000, 66, 273–288. [Google Scholar] [CrossRef]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; McIntyre, B.L.; Geesink, G.H.; Greenwood, P.L. Cattle temperament: Persistence of assessments and associations with productivity, efficiency, carcass and meat quality traits. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- Yu, H.; Morota, G.; Celestino, E.F., Jr.; Dahlen, C.R.; Wagner, S.A.; Riley, D.G.; Hulsman Hanna, L.L. Deciphering cattle temperament measures derived from a four-platform standing scale using genetic factor analytic modeling. Front. Genet. 2020, 11, 599. [Google Scholar] [CrossRef]
- Gauly, M.; Mathiak, H.; Hoffmann, K.; Kraus, M.; Erhardt, G. Estimating genetic variability in temperamental traits in German Angus and Simmental cattle. Appl. Anim. Behav. Sci. 2001, 74, 109–119. [Google Scholar] [CrossRef]
- Hoppe, S.; Brandt, H.R.; Konig, S.; Erhardt, G.; Gauly, M. Temperament traits of beef calves measured under field conditions and their relationships to performance. J. Anim. Sci. 2010, 88, 1982–1989. [Google Scholar] [CrossRef]
- Sewalem, A.; Miglior, F.; Kistemaker, G.J. Short communication: Genetic parameters of milking temperament and milking speed in Canadian Holsteins. J. Dairy. Sci. 2011, 94, 512–516. [Google Scholar] [CrossRef]
- Cue, R.I.; Harris, B.L.; Rendel, J.M. Genetic parameters for traits other than production in purebred and crossbred New Zealand dairy cattle. Livest. Prod. Sci. 1996, 45, 123–135. [Google Scholar] [CrossRef]
- Chang, Y.; Li, X.; Zhang, H.L.; Qi, J.G.; Guo, G.; Liu, L.; Wang, Y.C. Genetic analysis for temperament in holstein cattle in Beijing area. Acta Vet. Zootech. Sin. 2019, 50, 712–720. [Google Scholar]
- Kramer, M.; Erbe, M.; Bapst, B.; Bieber, A.; Simianer, H. Estimation of genetic parameters for novel functional traits in Brown Swiss cattle. J. Dairy Sci. 2013, 96, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Wethal, K.B.; Heringstad, B. Genetic analyses of novel temperament and milkability traits in Norwegian Red cattle based on data from automatic milking systems. J. Dairy Sci. 2019, 102, 8221–8233. [Google Scholar] [CrossRef] [PubMed]
- Juga, J. Evaluation methods of subjectively scored functional traits in Finland. Interbull Bull. 1996, 14, 155–160. [Google Scholar]
- Ali, A.K.A.; Shook, G.E. An optimum transformation for somatic cell concentration in milk. J. Dairy Sci. 1980, 63, 487. [Google Scholar] [CrossRef]
- Lansade, L.; Bouissou, M.; Erhard, H.W. Reactivity to isolation and association with conspecifics: A temperament trait stable across time and situations. Appl. Anim. Behav. Sci. 2008, 109, 355–373. [Google Scholar] [CrossRef]
- Tőzsér, J.; Maros, K.; Szentléleki, A.; Zándoki, R.; Nikodémusz, E.; Balázs, F.; Bailo, A.; Alföldi, L. Evaluation of temperament in cows of different age and bulls of different colour variety. Czech J. Anim. Sci. 2003, 48, 344–348. [Google Scholar]
- Cooke, R.F.; Schubach, K.M.; Marques, R.S.; Peres, R.F.G.; Silva, L.G.T.; Carvalho, R.S.; Cipriano, R.S.; Bohnert, D.W.; Pires, A.V.; Vasconcelos, J.L.M. Effects of temperament on physiological, productive, and reproductive responses in beef cows. J. Anim. Sci. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steen, A.; Østeras, O.; Grønstøl, H. Evaluation of additional acetone and urea analyses, and of the fat-lactose-quotient in cow milk samples in the herd recording system in Norway. J. Vet. Med. A 1996, 43, 181–191. [Google Scholar] [CrossRef]
- Reist, M.; Erdin, D.; von Euw, D.; Tschuemperlin, K.; Leuenberger, H.; Chilliard, Y.; Hammon, H.M.; Morel, C.; Philipona, C.; Zbinden, Y.; et al. Estimation of energy balance at the individual and herd level using blood and milk traits in high-yielding dairy cows. J. Dairy Sci. 2002, 85, 3314–3327. [Google Scholar] [CrossRef]
- Orban, M.; Gaal, K.; Pajor, F.; Szentleleki, A.; Poti, P.; Tozser, J.; Gulyas, L. Effect of temperament of Jersey and Holstein Friesian cows on milk production traits and somatic cell count. Archiv. Tierz. 2011, 54, 594–599. [Google Scholar] [CrossRef][Green Version]
- Fulwider, W.K.; Grandin, T.; Rollin, B.E.; Engle, T.E.; Dalsted, N.L.; Lamm, W.D. Survey of dairy management practices on one hundred thirteen North Central and North Eastern United States dairies. J. Dairy Sci. 2007, 91, 1686–1692. [Google Scholar] [CrossRef]
- Szentleleki, A.; Nagy, K.; Szeplaki, K.; Kekesi, K.; Tozser, J. Behavioural responses of primiparous and multiparous dairy cows to the milking process over an entire lactation. Ann. Anim. Sci. 2015, 15, 185–195. [Google Scholar] [CrossRef]
- Toni, F.; Vincenti, L.; Grigoletto, L.; Ricci, A.; Schukken, Y.H. Early lactation ratio of fat and protein percentage in milk is associated with health, milk production, and survival. J. Dairy Sci. 2011, 94, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Gantner, V.; Bobic, T.; Potocnik, K. Prevalence of metabolic disorders and effect on subsequent daily milk quantity and quality in Holstein cows. Arch. Anim. Breed. 2016, 59, 381–386. [Google Scholar] [CrossRef]
- Hamann, J.; Krömker, V. Potential of specific milk composition variables for cow health management. Livest. Prod. Sci. 1997, 48, 201–208. [Google Scholar] [CrossRef]
- Castillo, C.; Hernández, J.; López-Alonso, M.; Miranda Benedito, J.L. Values of plasma lipid hydroperoxides and total antioxidant status in healthy dairy cows: Preliminary observations. Arch. Tierz. 2003, 46, 227–233. [Google Scholar] [CrossRef]
- Erf, D.F.; Hansen, L.B.; Lawstuen, D.A. Inheritance and relation-ships of workability traits and yield for holsteins. J. Dairy Sci. 1992, 75, 1999–2007. [Google Scholar] [CrossRef]
| Indices | Temperament | MY (kg) | MF (%) | MP (%) | F/P | ML (%) | MU (mg%) | SCS |
|---|---|---|---|---|---|---|---|---|
| Temperament | 0.044 | −0.113 ** | 0.030 ** | 0.011 ** | 0.013 ** | −0.051 ** | 0.042 ** | 0.201 ** |
| MY (kg) | 0.043 * | 0.231 | −0.293 ** | −0.399 ** | 0.009 ** | 0.401 ** | 0.205 ** | 0.174 ** |
| MF (%) | 0.040 * | −0.488 ** | 0.310 | 0.336 ** | 0.498 ** | −0.095 ** | 0.018 ** | 0.142 ** |
| MP (%) | −0.024 | −0.485 ** | 0.535 ** | 0.342 | −0.062 ** | −0.045 ** | 0.018 ** | 0.280 ** |
| F/P | 0.073 ** | −0.250 ** | 0.815 ** | −0.039 | 0.113 ** | 0.004 ** | 0.052 ** | 0.023 ** |
| ML (%) | 0.038 * | 0.305 ** | −0.109 ** | −0.264 ** | 0.062 * | 0.431 | 0.063 ** | −0.466 ** |
| MU (mg/dL) | 0.045 * | 0.157 ** | 0.011 | −0.047 * | 0.041 * | 0.080 ** | 0.103 | −0.071 ** |
| SCS | −0.047 * | −0.311 ** | 0.108 ** | 0.212 ** | −0.020 | −0.450 ** | −0.120 ** | 0.192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanaitis, R.; Juozaitienė, V.; Jonike, V.; Čukauskas, V.; Urbšienė, D.; Urbšys, A.; Baumgartner, W.; Paulauskas, A. Relationship between Temperament and Stage of Lactation, Productivity and Milk Composition of Dairy Cows. Animals 2021, 11, 1840. https://doi.org/10.3390/ani11071840
Antanaitis R, Juozaitienė V, Jonike V, Čukauskas V, Urbšienė D, Urbšys A, Baumgartner W, Paulauskas A. Relationship between Temperament and Stage of Lactation, Productivity and Milk Composition of Dairy Cows. Animals. 2021; 11(7):1840. https://doi.org/10.3390/ani11071840
Chicago/Turabian StyleAntanaitis, Ramūnas, Vida Juozaitienė, Vesta Jonike, Vytenis Čukauskas, Danguolė Urbšienė, Algirdas Urbšys, Walter Baumgartner, and Algimantas Paulauskas. 2021. "Relationship between Temperament and Stage of Lactation, Productivity and Milk Composition of Dairy Cows" Animals 11, no. 7: 1840. https://doi.org/10.3390/ani11071840
APA StyleAntanaitis, R., Juozaitienė, V., Jonike, V., Čukauskas, V., Urbšienė, D., Urbšys, A., Baumgartner, W., & Paulauskas, A. (2021). Relationship between Temperament and Stage of Lactation, Productivity and Milk Composition of Dairy Cows. Animals, 11(7), 1840. https://doi.org/10.3390/ani11071840







