Endemic Cyprus Scops Owl Otus cyprius Readily Breeds in Artificial Nest Boxes
Abstract
Simple Summary
Abstract
1. Introduction
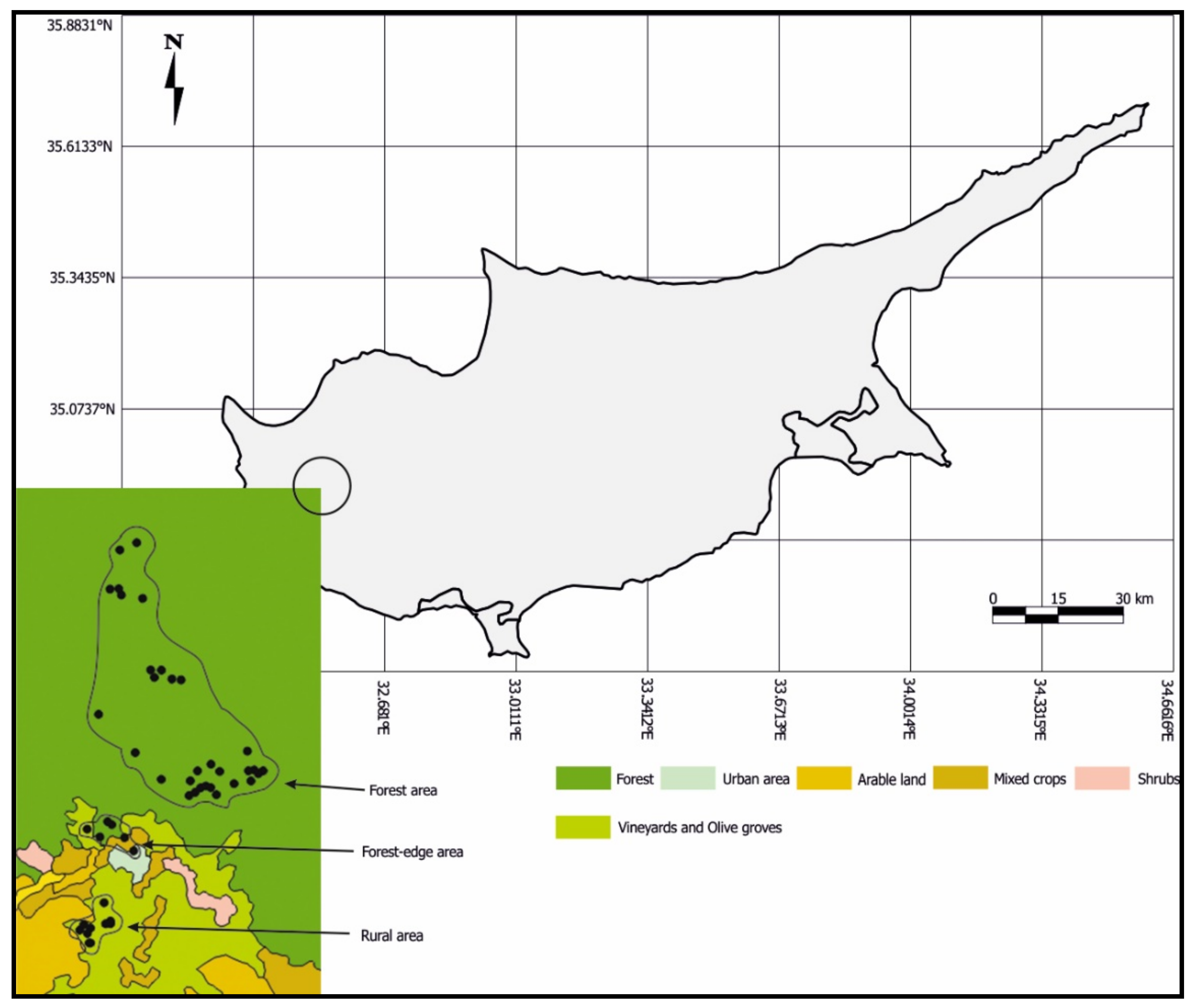
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambrechts, M.M.; Wiebe, K.L.; Sunde, P.; Solonen, T.; Sergio, F.; Roulin, A.; Møller, A.P.; López, B.C.; Fargallo, J.A.; Exo, K.-M.; et al. Nest box design for the study of diurnal raptors and owls is still an overlooked point in ecological, evolutionary and conservation studies: A review. J. Ornithol. 2012, 153, 23–34. [Google Scholar] [CrossRef]
- Vlachos, C.G.; Bakaloudis, D.E.; Kitikidou, K.; Goutner, V.; Bontzorlos, V.; Papakosta, M.A.; Chatzinikos, E. Home range and foraging habitat selection by breeding Lesser kestrels (Falco naumanni) in Greece. J. Nat. Hist. 2015, 49, 371–381. [Google Scholar] [CrossRef]
- Schaub, T.; Meffert, P.J.; Kerth, G. Nest-boxes for Common Swifts Apus apus as compensatory measures in the context of building renovation: Efficacy and predictors of occupancy. Bird Conserv. Int. 2016, 26, 164–176. [Google Scholar] [CrossRef]
- Luniak, M.; Grzeniewski, M. Nest-boxes for the Common Swift Apus apus—Experience from Poland. Ecol. Urbana 2011, 23, 3–5. [Google Scholar]
- Smallwood, J.A.; Causey, M.F.; Mossop, D.H.; Klucsarits, J.R.; Robertson, B.; Robertson, S.; Mason, J.; Maurer, M.J.; Melvin, R.J.; Dawson, R.D. Why are American kestrels (Falco sparverius) populations declining in North America? Evidence from nest box programs. J. Raptor Res. 2009, 43, 274–282. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, D.; Genot, J.-C.; Johnson, D.H. The Little Owl; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Iezekiel, S.; Yosef, R.; Bakaloudis, D.E.; Papakosta, M.A.; Vlachos, C.G.; Antoniou, A.; Zduniak, P. The endemic Cyprus Wheatear (Oenanthe cypriaca) adapts readily to artificial nest sites. Biol. Conserv. 2017, 213, 1–4. [Google Scholar] [CrossRef]
- Marti, C.D.; Wagner, P.W.; Denne, K.W. Nest boxes for the management of Barn Owls. Wildl. Soc. Bull. 1979, 7, 145–148. [Google Scholar]
- Korpimaki, E. Clutch size and breeding success in relation to nest-box size in Tenglmalm’s Owl Aegolius funereus. Holarct. Ecol. 1985, 8, 175–180. [Google Scholar]
- López, B.C.; Potrony, D.; López, A.; Badosa, E.; Bonada, A.; Saló, R. Nest-Box Use by Boreal Owls (Aegolius funereus) in the Pyrenees Mountains in Spain. J. Raptor Res. 2010, 44, 40–49. [Google Scholar] [CrossRef]
- Marchesi, L.; Sergio, F. Distribution, density, diet and productivity of the Scops Owl Otus scops in the Italian Alps. Ibis 2005, 147, 176–187. [Google Scholar] [CrossRef]
- Flint, P.; Whaley, D.; Kirwan, G.M.; Charalambides, M.; Schweizer, M.; Wink, M. Reprising the taxonomy of Cyprus Scops Owl Otus (scops) cyprius, a neglected island endemic. Zootaxa 2015, 4040, 301–316. [Google Scholar] [CrossRef]
- Flint, P. Unusual song of an individual Cyprus Scops Owl Otus cyprius (Aves: Strigidae). Zool. Middle East 2017, 63, 291–295. [Google Scholar] [CrossRef]
- Flint, P.; Richardson, C. A review of the residential status of the endemic Cyprus Scops Owl Otus cyprius. Sandgrouse 2017, 39, 177–181. [Google Scholar]
- Gill, F.; Donsker, D. (Eds.) IOC World Bird List. Version 6.3. 2016. Available online: http://worldbirdnames.org (accessed on 5 February 2021).
- BirdLife International. Species Factsheet: Otus cyprius. 2020. Available online: https://www.worldbirdnames.org/ioc-lists/crossref/ (accessed on 3 February 2021).
- Flint, P.; Stewart, P.F. The Birds of Cyprus: An Annotated Check-List, 1st ed.; BOU Check-list No. 6.; British Ornithologists’ Union: Tring, UK, 1983; 234p. [Google Scholar]
- Ieronymidou, C. Habitat Preferences of the Cyprus Scops Owl (Otus scops cyprius). Master’s Thesis, Institute of Zoology & Royal Veterinary College, University of London, London, UK, 2008. [Google Scholar]
- Ieronymidou, C. An overview of BirdLife Cyprus monitoring programmes in 2017. Cyprus Bird Rep. 2019, 173–180. [Google Scholar]
- Pomeroy, D.; Walsh, F. Numbers of three small predators in western Cyprus. Cyprus Bird Rep. 2013, 166–172. [Google Scholar]
- Hadjisterkotis, E. The effect of corvid shooting on the populations of owls, kestrels and cuckoos in Cyprus, with notes on corvid diet. Z. Jadgwiss. 2003, 49, 50–60. [Google Scholar] [CrossRef]
- Michel, V.T.; Franco, M.V.J.; Naef-Daenzer, B.; Grüebler, M.U. Intraguild predator drives forest edge avoidance of a mesopredator. Ecosphere 2016, 7, e01229. [Google Scholar] [CrossRef]
- Kauhala, K.; Talvitie, K.; Vuorisalo, T. Free-ranging house cats in urban and rural areas in the north: Useful rodent killers or harmful bird predators? Folia Zool. 2015, 64, 45–55. [Google Scholar] [CrossRef]
- Sonerud, G.A. Reduced Predation by Nest Box Relocation: Differential Effect on Tengmalm’s Owl Nests and Artificial Nests. Ornis Scand. 1993, 24, 249–253. [Google Scholar] [CrossRef]
- Charalambides, M. Cyprus Scops Owl Otus scops cyprius breeding at Kalo Khorio Orinis, in spring 2009. Cyprus Bird Rep. 2010, 153–154. [Google Scholar]
- Charalambides, M. Cyprus Scops Owl breeding in a garden at Kalo Khorio Orinis, in spring 2017. Cyprus Bird Rep. 2019, 189. [Google Scholar]
- Mayfield, H. Nesting success calculated from exposure. Wilson Bull. 1961, 73, 255–261. [Google Scholar]
- Mayfield, H. Suggestions for calculating nesting success. Wilson Bull. 1975, 87, 456–466. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hill: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Newton, I. The role of nest sites in limiting the numbers of hole-nesting birds: A review. Biol. Conserv. 1994, 70, 265–276. [Google Scholar] [CrossRef]
- Bolton, M.; Medeiros, R.; Hothersall, B.; Campos, A. The use of artificial breeding chambers as a conservation measure for cavity-nesting procellariiform: A case study of the Madeiran storm petrel (Oceanodroma castro). Biol. Conserv. 2004, 116, 73–80. [Google Scholar] [CrossRef]
- Dawson, R.D.; Lawrie, C.C.; O’Brien, E.L. The importance of microclimate variation in determining size, growth and survival of avian offspring: Experimental evidence from a cavity nesting passerine. Oecologia 2005, 144, 499–507. [Google Scholar] [CrossRef]
- Brian Davis, J.; Straub, J.N.; Wang, G.; Kaminski, R.M.; Leopold, B.D. Simulations of wood duck recruitment from nest boxes in Mississippi and Alabama. J. Wildl. Manag. 2015, 79, 907–916. [Google Scholar] [CrossRef]
- Olah, G.; Vigo, G.; Heinsohn, R.; Brightsmith, D.J. Nest-site selection and efficacy of artificial nests for breeding success of Scarlet Macaws Ara macao in lowland Peru. J. Nat. Conserv. 2014, 22, 176–185. [Google Scholar] [CrossRef]
- Denac, K.; Kmecl, P.; Koce, U. Habitat use of Eurasian Scops Owls Otus scops in an agricultural mosaic landscape. Ardea 2019, 107, 119–129. [Google Scholar] [CrossRef]
- Bavoux, C.; Burneleau, G.; Nicolau-Guillaumet, P. Aspects de la biologie de reproduction du hibou petit-duc Otus scops. Alauda 1991, 592, 65–71. [Google Scholar]
- Blanco, G.; Davila, J.L.; Septiem, J.A.L.; Ridriguez, R.; Martinez, F. Sex-biased initial eggs favours sons in the slightly size-dimorphic Scops owl (Otus scops). Biol. J. Linn. Soc. 2002, 76, 1–7. [Google Scholar] [CrossRef]
- Toyama, M.; Kotaka, N.; Koizumi, I. Breeding timing and nest predation rate of sympatric scops owls with different dietary niche breadth. Can. J. Zool. 2015, 93, 841–847. [Google Scholar] [CrossRef]
- Akatani, K.; Matsuo, T.; Masoki, T. Breeding ecology and habitat use of the Daito Scops Owl (Otus elegans interpositus) on an Oceanic Island. J. Raptor Res. 2011, 45, 315–323. [Google Scholar] [CrossRef]
- Currie, D.; Fanchette, R.; Millett, J.; Hoareau, C.; Shah, N.J. The breeding biology of the Critically Endangered Seychelles Scops-owl Otus insularis: Consequences for conservation and management. Bird Conserv. Int. 2004, 14, 123–137. [Google Scholar] [CrossRef][Green Version]
- Kryštufek, B.; Vohralík, V. Mammals of Turkey and Cyprus. Rodentia II: Cricetinae, Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae; Annales Majora: Koper, Slovenia, 2001; 372p. [Google Scholar]
- Flint, P.R.; Stewart, P.F. The Birds of Cyprus: An Annotated Check-List, 2nd ed.; BOU Check-list No. 6.; British Ornithologists’ Union: Tring, UK, 1992; 234p. [Google Scholar]
- Sergio, F.; Marchesi, L.; Pedrini, P. Conservation of Scops owl Otus scops in the Alps: Relationship with grassland management, predation risk and wider biodiversity. Ibis 2009, 151, 40–50. [Google Scholar] [CrossRef]
- Flint, P. Long-term changes in the numbers and abundance of regularly breeding land bird species on Cyprus: A review. Sandgrouse 2019, 41, 36–70. [Google Scholar]
- Panayides, P. Six aspects of land use and development activity that result in adverse effects to Cypriot wildlife resources. In Proceedings of the XXV International Congress of the International Union of Game Biologists—IUGB and IXth International Symposium Perdix 2: 182–196; Ministry of the Interior: Nicosia, Cyprus, 2005. [Google Scholar]
- Statistical Service. Agricultural Statistics 2015; Statistical Service of Cyprus: Nicosia, Cyprus, 2017.
- Christodoulou, D. The Evolution of the Rural Land Use Pattern in Cyprus; Geographical Publications: Bude, UK, 1959. [Google Scholar]
- Thirgood, J.V. Cyprus: A Chronicle of Its Forests, Land and People; University of British Columbia Press: Vancouver, BC, Canada, 1987. [Google Scholar]
- Department of Forests. Criteria and Indicators for the Sustainable Forest Management in Cyprus. 2006. Available online: www.moa.gov.cy/moa/fd/fd.nsf (accessed on 3 February 2021).
- European Environment Agency. Cyprus Land Country Fact Sheet 2012. 2017. Available online: https://www.eea.europa.eu/themes/landuse/land-cover-country-fact-sheets/cy-cyprus-landcover-2012.pdf/view (accessed on 5 February 2021).
- Hadjikyriakou, G. Forest Fire Management in Cyprus. Int. For. Fire News 2005, 33, 38–43. [Google Scholar]
- Treggiari, A.A.; Gagliardone, M.; Pellegrino, I.; Cucco, M. Habitat selection in a changing environment: The relationship between habitat alteration and Scops owl (Aves: Strigidae) territory occupancy. Ital. J. Zool. 2013, 80, 574–585. [Google Scholar] [CrossRef][Green Version]
- Malle, G.; Probst, R. Die Zwergohreule (Otus scops) in Österreich. Bestand, Ökologie und Schutz in Zentraleuropa unter Besonderer Berücksichtigung der Kärntner Arten—Schutz Projekte; Verlag des Naturwissenschaftlichen Vereins für Kärnten: Klagenfurt am Wörthersee, Austria, 2015. [Google Scholar]
- Mori, E.; Ancillotto, L.; Menchetti, M.; Strubbe, D. The early bird catches the nest: Possible competition between scops owls and ring-necked parakeets. Anim. Conserv. 2017, 20, 463–470. [Google Scholar] [CrossRef]
- Biber, E. Patterns of endemic extinctions among island bird species. Ecography 2002, 25, 661–676. [Google Scholar] [CrossRef]
- Newton, I. The Speciation and Biogeography of Birds; Academic Press: London, UK, 2003. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Steibl, S.; Laforsch, C. Disentangling the environmental impact of different human disturbances: A case study on islands. Sci. Rep. 2019, 9, 13712. [Google Scholar] [CrossRef]
- Flint, P.; McArthur, A. Is the Sardinian Warbler Sylvia melanocephala displacing the endemic Cyprus Warbler S. melanothorax on Cyprus? Sandgrouse 2014, 36, 63–109. [Google Scholar]
- Pomeroy, D.; Walsh, F.; Flint, P.; Hellicar, M.; Shaw, P. A sustained decline in Cyprus Warbler Sylvia melanothorax numbers in western Cyprus, coinciding with the colonisation of its breeding range by the Sardinian Warbler S. melanocephala. Bird Conserv. Int. 2016, 26, 436–450. [Google Scholar] [CrossRef]
- Xenophontos, M.; Cresswell, W. Reproductive success and productivity of the Cyprus Wheatear Oenanthe cypriaca, a migratory, island endemic. J. Ornithol. 2016, 157, 721–731. [Google Scholar] [CrossRef]
| Habitat | Laying Date (d) | Clutch Size | No. Day of Incubation | Hatching Day | No. of Hatchlings | No. of Fledglings |
|---|---|---|---|---|---|---|
| Forest | 125.9 (123.2–128.7); 47 | 2.4(2.1–2.6); 47 | 22.1 (21.4–22.8); 38 | 147.4 (143.9–150.8); 38 | 2.0 (1.6–2.3); 47 | 1.9 (1.6–2.2); 47 |
| Edge | 124.0 (120.1–127.8); 20 | 2.5 (1.9–3.0); 20 | 22.1 (21.3–23.8); 20 | 146.1 (142.0–150.1); 20 | 2.5 (1.9–3.0); 20 | 2.3 (1.7–2.9); 20 |
| Rural | 126.3 (122.0–130.6); 24 | 2.3 (1.9–2.7); 24 | 22.7 (21.4–23.9); 20 | 148.2 (142.9–153.4); 20 | 2.1 (1.5–2.6); 24 | 2.0 (1.5–1.5); 24 |
| all | 125.6 (123.7–127.5); 91 | 2.4 (2.2–2.6); 91 | 22.5 (22.2–22.9); 78 | 147.2 (144.9–149.5); 78 | 2.1 (1.8–2.3); 91 | 2.0 (1.8–2.3); 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iezekiel, S.; Yosef, R.; Themistokleus, C.; Bakaloudis, D.E.; Vlachos, C.G.; Antoniou, A.; Iezekiel, E.; Papakosta, M.A.; Kosicki, J.Z. Endemic Cyprus Scops Owl Otus cyprius Readily Breeds in Artificial Nest Boxes. Animals 2021, 11, 1775. https://doi.org/10.3390/ani11061775
Iezekiel S, Yosef R, Themistokleus C, Bakaloudis DE, Vlachos CG, Antoniou A, Iezekiel E, Papakosta MA, Kosicki JZ. Endemic Cyprus Scops Owl Otus cyprius Readily Breeds in Artificial Nest Boxes. Animals. 2021; 11(6):1775. https://doi.org/10.3390/ani11061775
Chicago/Turabian StyleIezekiel, Savvas, Reuven Yosef, Constantinos Themistokleus, Dimitrios E. Bakaloudis, Christos G. Vlachos, Andreas Antoniou, Eandas Iezekiel, Malamati A. Papakosta, and Jakub Z. Kosicki. 2021. "Endemic Cyprus Scops Owl Otus cyprius Readily Breeds in Artificial Nest Boxes" Animals 11, no. 6: 1775. https://doi.org/10.3390/ani11061775
APA StyleIezekiel, S., Yosef, R., Themistokleus, C., Bakaloudis, D. E., Vlachos, C. G., Antoniou, A., Iezekiel, E., Papakosta, M. A., & Kosicki, J. Z. (2021). Endemic Cyprus Scops Owl Otus cyprius Readily Breeds in Artificial Nest Boxes. Animals, 11(6), 1775. https://doi.org/10.3390/ani11061775









