Screening of Lactic Acid Bacteria with Inhibitory Activity against ETEC K88 as Feed Additive and the Effects on Sows and Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and LAB Isolation
2.2. Screening of LAB with Inhibitory Activity against ETEC K88
2.3. Physiological and Biochemical Characteristics of Selected LAB Isolates
2.4. Exopolysaccharide (EPS) Produced Capacity of Selected LAB Isolates
2.5. Cell Surface Hydrophobicity and Auto-Aggregation of Selected Strains
2.6. 16S rRNA Gene Analysis of Selected Strains
2.7. Antimicrobial Activity of Selected LAB Isolates
2.8. Survival of Selected LAB Strains in Bile Salt and Simulated Gastrointestinal Fluids
2.9. Assessment of Antibiotic Susceptibility of Selected LAB Isolates
2.10. Hemolytic Activity of Selected LAB Isolates
2.11. Animals, Diets, and Experimental Design
2.12. Data and Sample Collection of Sows and Weaned Piglets
2.13. Statistical Analysis
3. Results
3.1. Isolated LAB Strains from Piglet Fecal Samples
3.2. Inhibitory Activity of LAB Strains against ETEC K88 Isolated from Piglet Fecal Samples
3.3. Physiological and Biochemical Characteristics of Selected LAB Isolates
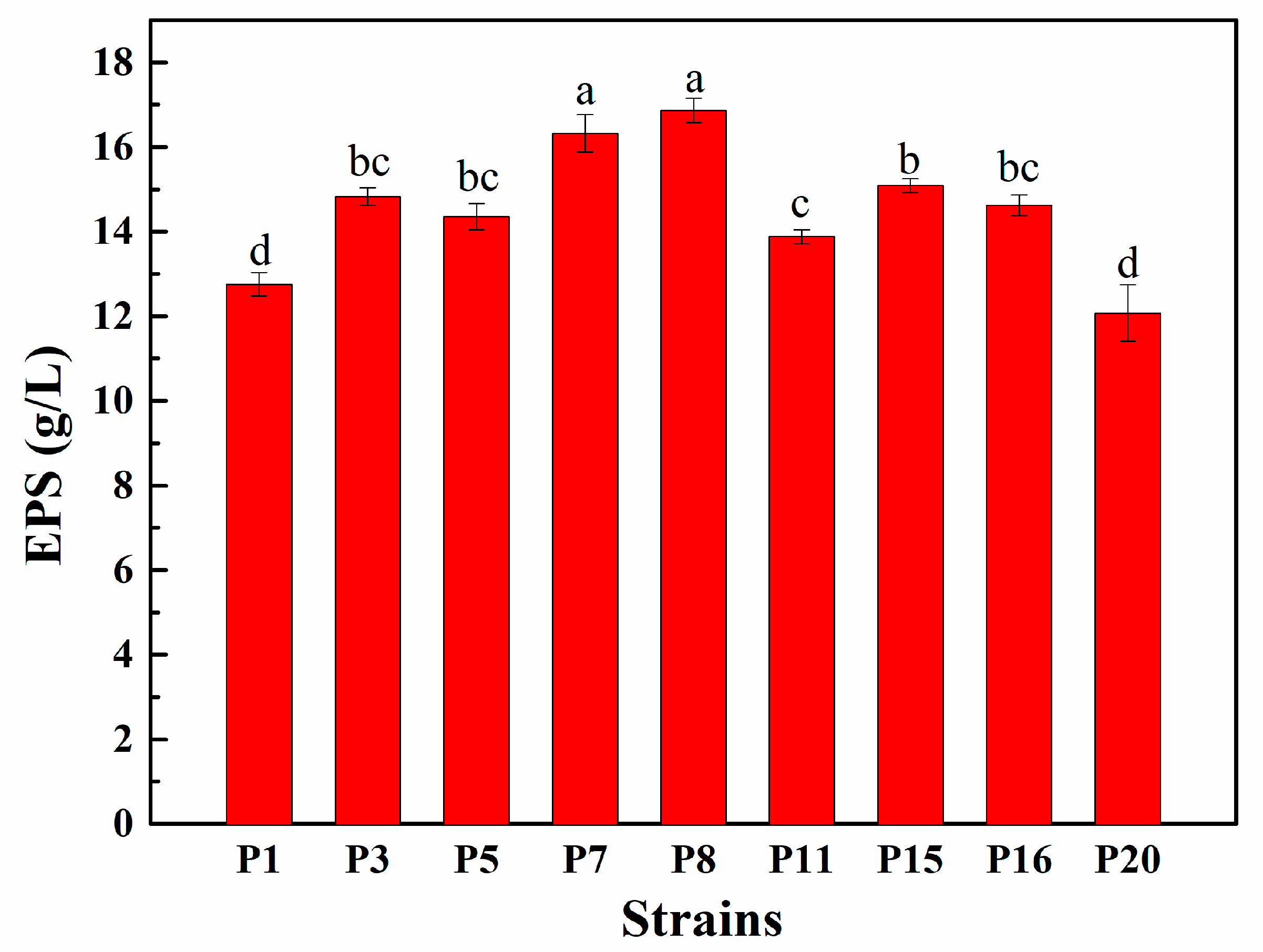
3.4. EPS Production Capacity of Selected LAB Isolates
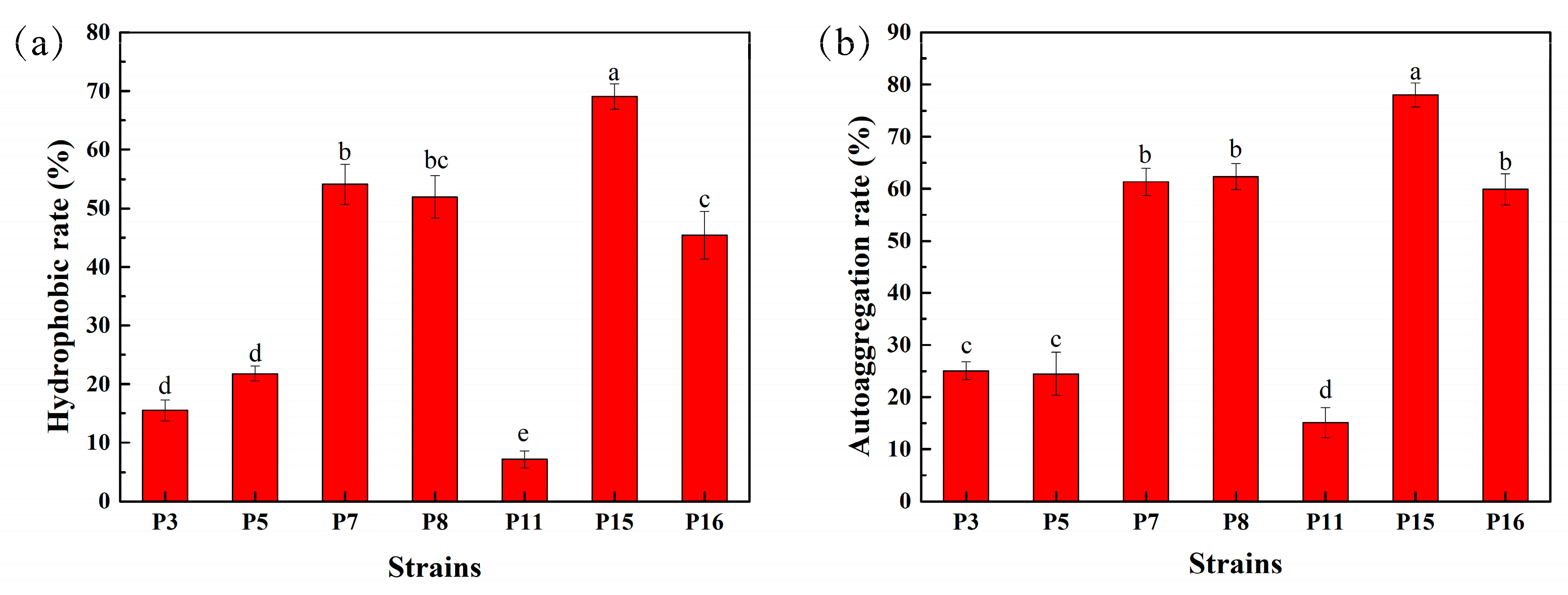
3.5. Hydrophobicity and Auto-Aggregation Ability of Selected LAB Isolates
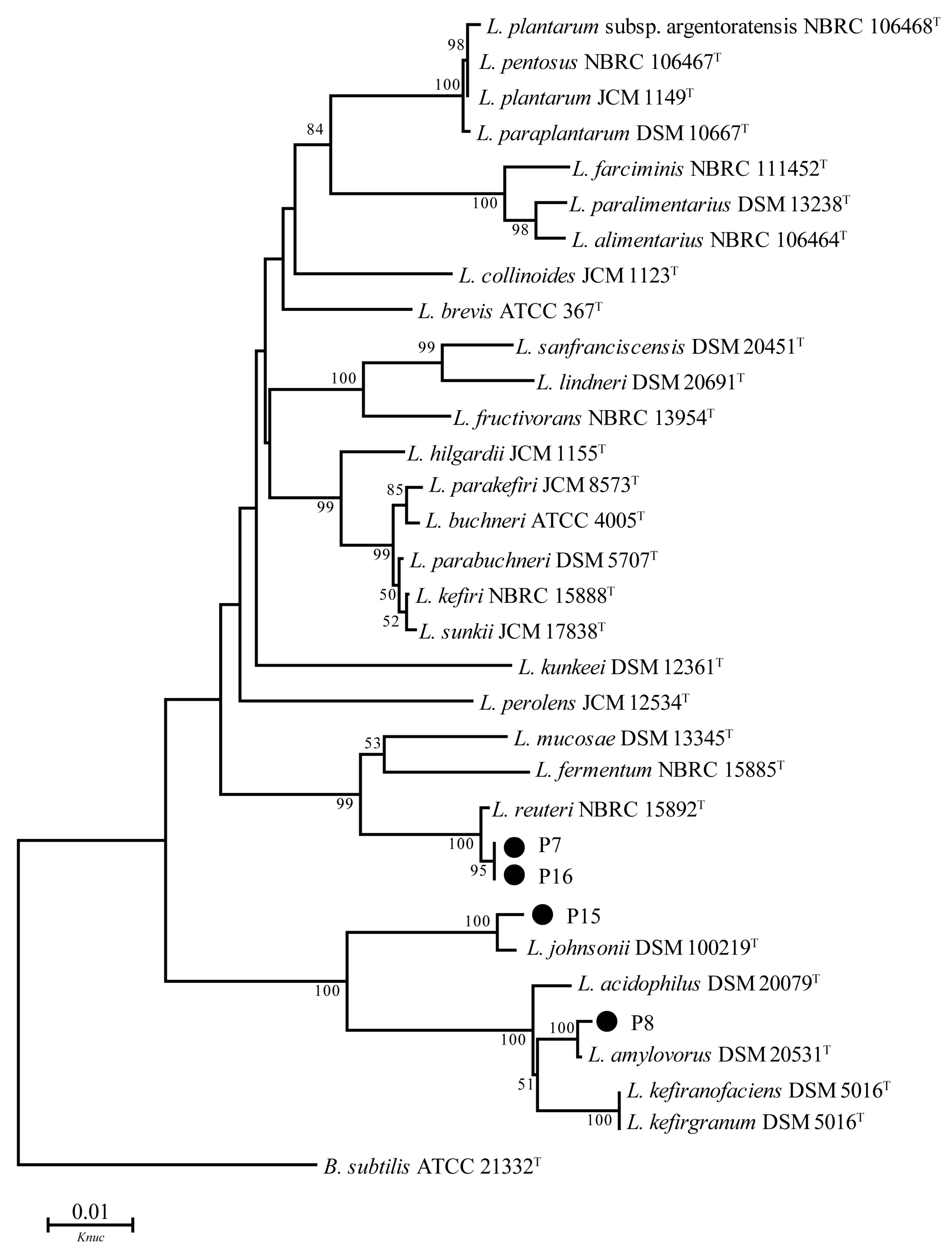
3.6. 16S DNA Sequence Analysis
3.7. Antimicrobial Spectrum Test of Selected LAB Isolates
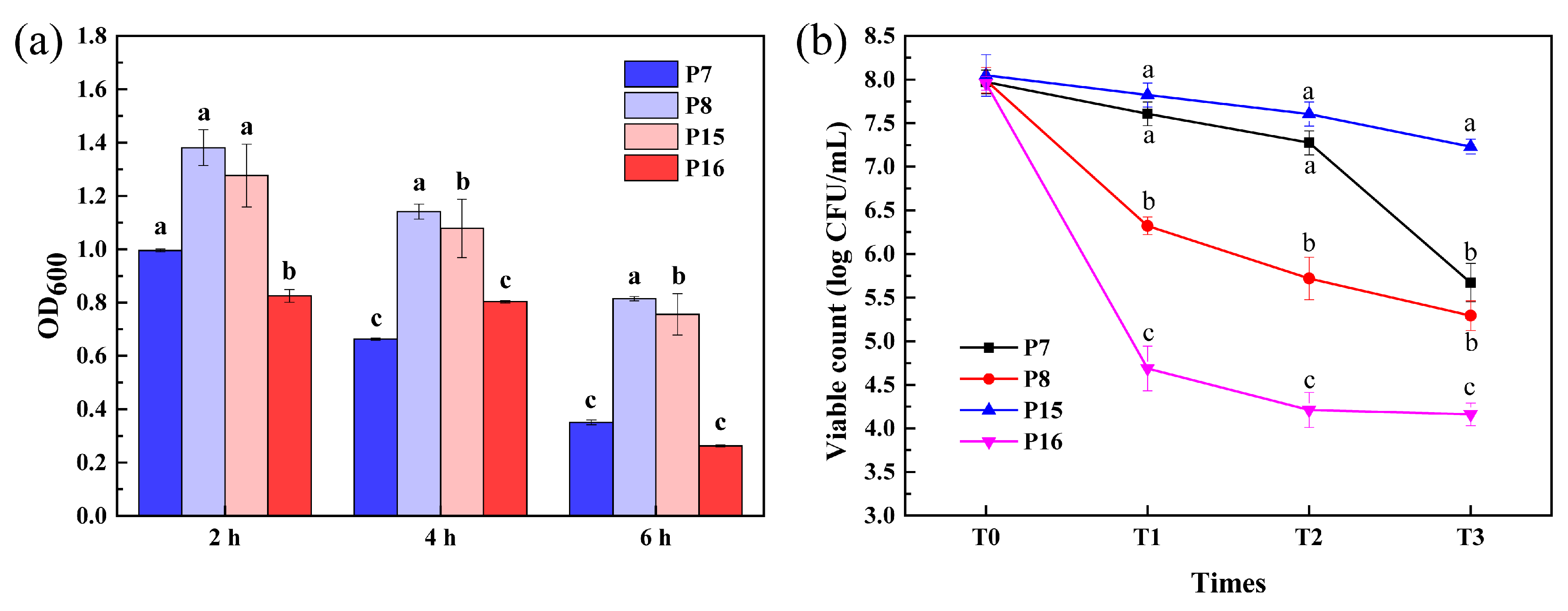
3.8. Survival of Selected LAB Isolates after Simulated Gastrointestinal Tract (GIT) Exposure
3.9. Assessment of Antibiotic Susceptibility
3.10. Hemolytic Activity of Selected LAB Isolates
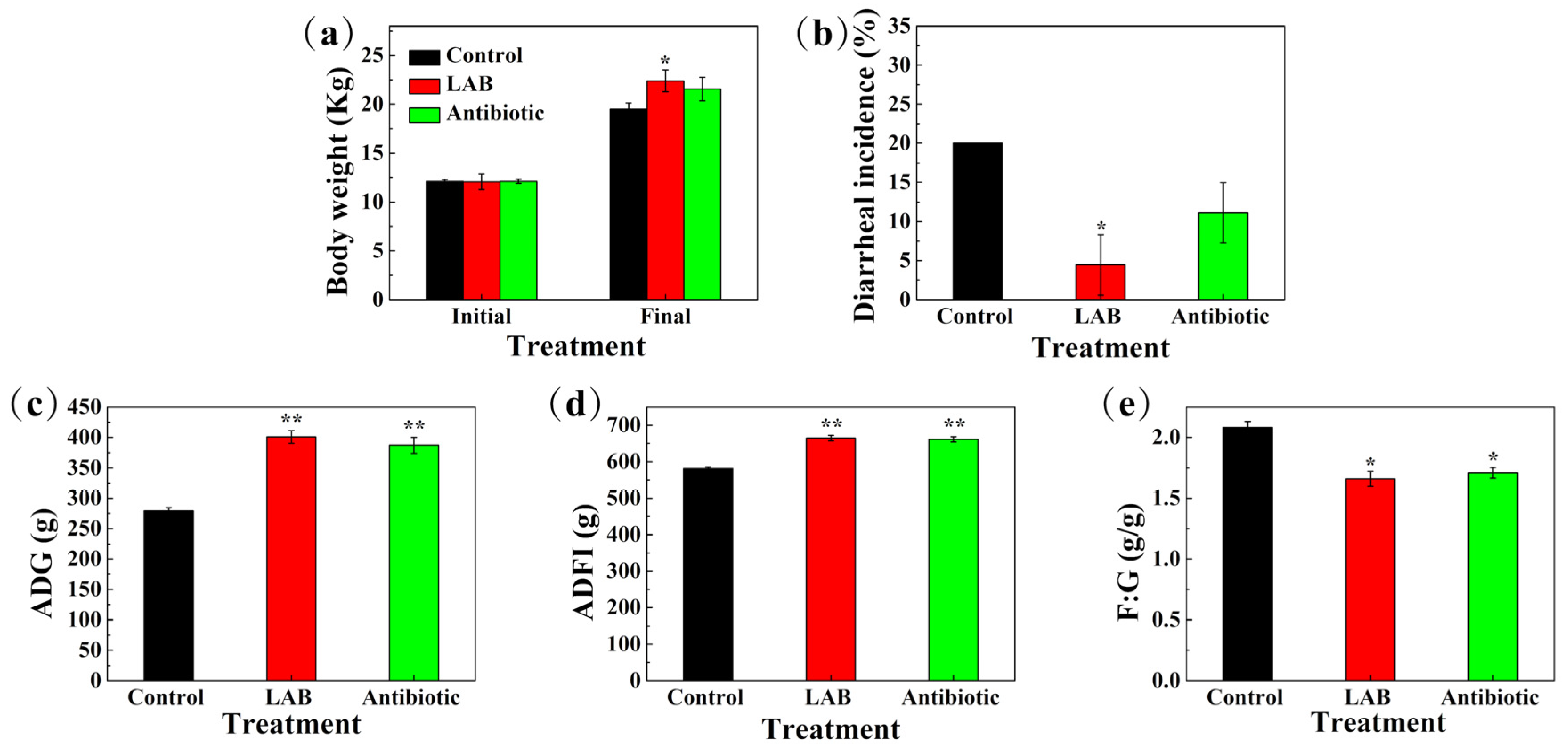
3.11. Reproductive Performance of Sows, Growth Performance of Piglets, and Incidence of Diarrhea
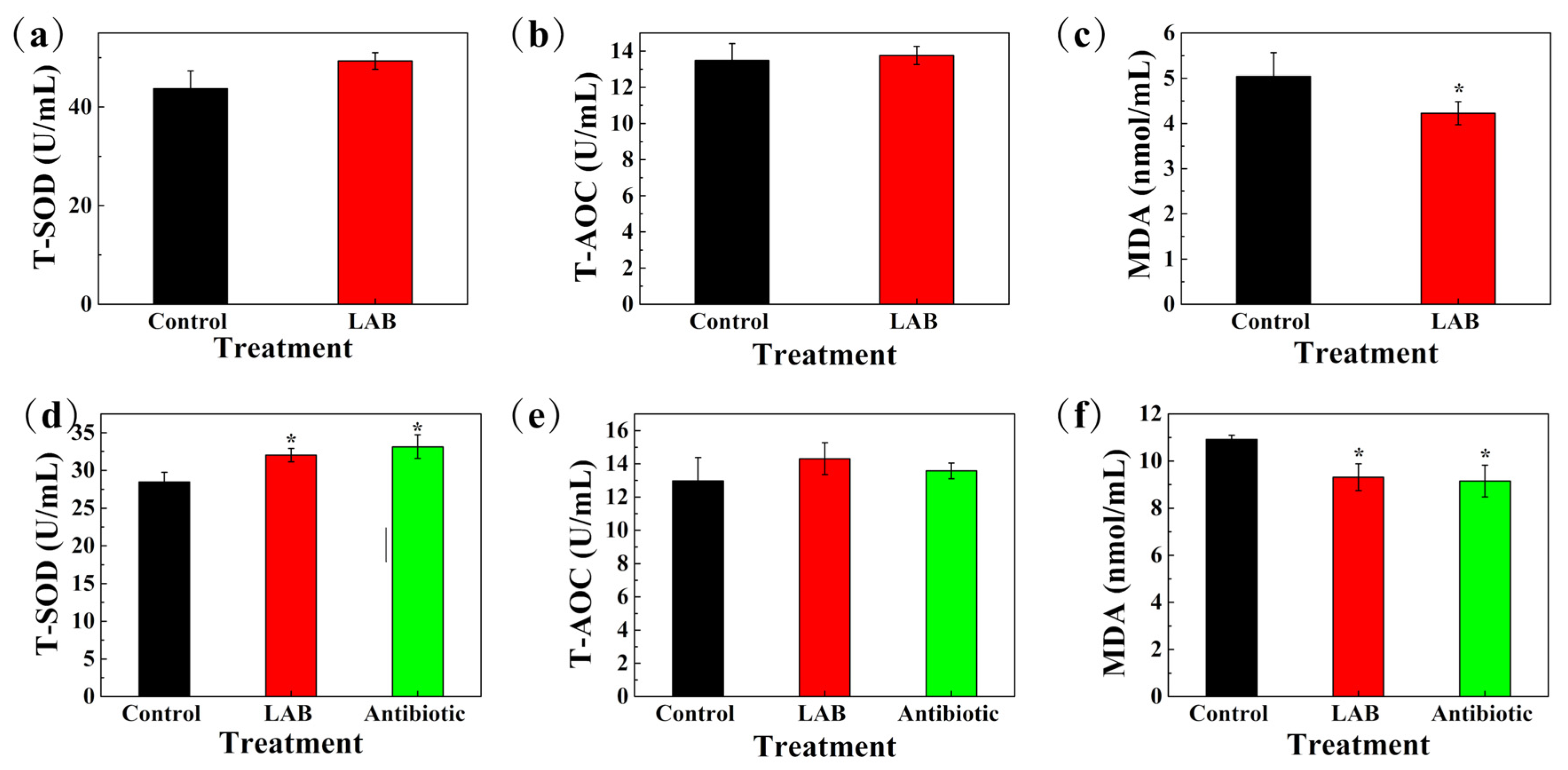
3.12. Antioxidant Capacity and Immune Indexes of Serum in Sows and Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Alison, L.S.; Timo, V.S.; Tom, J.B.; John, W.M. Effect of weaning age on nursery pig and sow reproductive performance. J. Swine Health Prod. 2008, 16, 131–137. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Menz, J.; Olsson, O.; Kummerer, K. Antibiotic residues in livestock manure: Does the EU risk assessment sufficiently protect against microbial toxicity and selection of resistant bacteria in the environment? J. Hazard. Mater. 2019, 379. [Google Scholar] [CrossRef]
- Gaggia, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141 (Suppl. 1), S15–S28. [Google Scholar] [CrossRef]
- Kaevska, M.; Lorencova, A.; Videnska, P.; Sedlar, K.; Provaznik, I.; Trckova, M. Effect of sodium humate and zinc oxide used in prophylaxis of post-weaning diarrhoea on faecal microbiota composition in weaned piglets. Vet. Med.-Czech. 2016, 61, 328–336. [Google Scholar] [CrossRef]
- Sterndale, S.O.; Evans, D.J.; Mansfield, J.P.; Clarke, J.; Sahibzada, S.; Abraham, S.; O’Dea, M.; Miller, D.W.; Kim, J.C.; Pluske, J.R. Effect of mucin 4 allele on susceptibility to experimental infection with enterotoxigenic F4 Escherichia coli in pigs fed experimental diets. J. Anim. Sci. Biotechnol. 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ordaz, A.A.; Gonzalez-Ortiz, G.; La Ragione, R.M.; Woodward, M.J.; Collins, J.W.; Perez, J.F.; Martin-Orue, S.M. Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef]
- Joghataei, M.; Shahidi, F.; Pouladfar, G.; Mortazavi, S.A.; Ghaderi, A. Probiotic potential comparison of Lactobacillus strains isolated from Iranian traditional food products and human feces with standard probiotic strains. J. Sci. Food Agri. 2019, 99, 6680–6688. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A.; da Silva Duarte, V.; de Castilhos, J.; Pakroo, S.; Lemos Junior, W.J.F.; Luchese, R.H.; Fioravante Guerra, A.; Rossi, R.C.; Righetto Ziegler, D.; Corich, V. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 2019, 54, 489–497. [Google Scholar] [CrossRef]
- Chytilova, M.; Mudronova, D.; Nemcova, R.; Gancarcikova, S.; Buleca, V.; Koscova, J.; Tkacikova, L. Anti-inflammatory and immunoregulatory effects of flax-seed oil and Lactobacillus plantarum—Biocenol LP96 in gnotobiotic pigs challenged with enterotoxigenic Escherichia coli. Res. Vet. Sci. 2013, 95, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Galle, S.; Le, M.H.; Zijlstra, R.T.; Ganzle, M.G. Feed fermentation with reuteran and levan producing Lactobacillus reuteri reduces colonization of weanling pigs by enterotoxigenic Escherichia coli. Appl. Environ. Microb. 2015, 81, 5743–5752. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Le, M.; Zijlstra, R.T.; Ganzle, M.G. Reutericyclin producing Lactobacillus reuteri modulates development of fecal microbiota in weanling pigs. Front. Microbiol. 2015, 6, 762. [Google Scholar] [CrossRef]
- Sayan, H.; Assavacheep, P.; Angkanaporn, K.; Assavacheep, A. Effect of Lactobacillus salivarius on growth performance, diarrhea incidence, fecal bacterial population and intestinal morphology of suckling pigs challenged with F4+ enterotoxigenic Escherichia coli. J. Anim. Sci. 2018, 31, 1308–1314. [Google Scholar] [CrossRef]
- Takanashi, N.; Tomosada, Y.; Villena, J.; Murata, K.; Takahashi, T.; Chiba, E.; Tohno, M. Advanced application of bovine intestinal epithelial cell line for evaluating regulatory effect of lactobacilli against heat-killed enterotoxigenic Escherichia coli mediated inflammation. BMC Microbiol. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Sirichokchatchawan, W.; Pupa, P.; Praechansri, P.; Am-In, N.; Tanasupawat, S.; Sonthayanon, P.; Prapasarakul, N. Autochthonous lactic acid bacteria isolated from pig faeces in Thailand show probiotic properties and antibacterial activity against enteric pathogenic bacteria. Microb. Pathog. 2018, 119, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Cui, M.; Wang, Y.; Jiao, Z.; Tan, Z. Ensilage of oats and wheatgrass under natural alpine climatic conditions by indigenous lactic acid bacteria species isolated from high-cold areas. PLoS ONE 2018, 13, e0192368. [Google Scholar] [CrossRef] [PubMed]
- Bachtarzi, N.; Kharroub, K.; Ruas-Madiedo, P. Exopolysaccharide-producing lactic acid bacteria isolated from traditional Algerian dairy products and their application for skim-milk fermentations. LWT-Food Sci. Technol. 2019, 107, 117–124. [Google Scholar] [CrossRef]
- Pacularu-Burada, B.; Georgescu, L.A.; Vasile, M.A.; Rocha, J.M.; Bahrim, G.E. Selection of wild lactic acid bacteria strains as promoters of postbiotics in gluten-free sourdoughs. Microorganisms 2020, 8, 643. [Google Scholar] [CrossRef]
- Wang, W.; Ma, H.; Yu, H.; Qin, G.; Tan, Z.; Wang, Y.; Pang, H. Screening of Lactobacillus plantarum subsp. plantarum with potential probiotic activities for inhibiting ETEC K88 in weaned piglets. Molecules 2020, 25, 4481. [Google Scholar] [CrossRef]
- Choudhary, J.; Dubey, R.C.; Sengar, G.; Dheeman, S. Evaluation of probiotic potential and safety assessment of Lactobacillus pentosus MMP4 isolated from mare’s lactation. Probiotics Antimicrob. 2019, 11, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Massounga Bora, A.F.; Li, X.; Zhu, Y.; Du, L. Improved viability of microencapsulated probiotics in a freeze-dried banana powder during storage and under simulated gastrointestinal tract. Probiotics Antimicrob. 2019, 11, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, S.; Cai, Y.; Li, E.; Ren, Z.; Wu, Y.; Guo, W.; Sun, Y.; Zhou, Y. Beneficial effects of a host gut-derived probiotic, Bacillus pumilus, on the growth, non-specific immune response and disease resistance of juvenile golden pompano, Trachinotus ovatus. Aquaculture 2020, 514. [Google Scholar] [CrossRef]
- Jayaraman, B.; Nyachoti, C.M. Husbandry practices and gut health outcomes in weaned piglets: A review. Anim. Nutr. 2017, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, J.G.; Lines, D.S.; Hallett, S.; Plush, K.J. A review of success factors for piglet fostering in lactation. Animals 2018, 8, 38. [Google Scholar] [CrossRef]
- Illmann, G.; Chaloupková, H.; Melišová, M. Impact of sow prepartum behavior on maternal behavior, piglet body weight gain, and mortality in farrowing pens and crates. J. Anim. Sci. 2016, 94, 3978–3986. [Google Scholar] [CrossRef]
- Le Dividich, J.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 2005, 143, 469–485. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Li, D.; Sun, J. Fecal microbiota transplantation shows marked shifts in the multi-omic profiles of porcine post-weaning diarrhea. Front. Microbiol. 2021, 12, 619460. [Google Scholar] [CrossRef]
- Pettersson, E.; Sjolund, M.; Wallgren, T.; Lind, E.O.; Hoglund, J.; Wallgren, P. Management practices related to the control of gastrointestinal parasites on Swedish pig farms. Porcine Health Manag. 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, W.; Hu, Z.; Liu, Z.; Du, T.; Dai, Y.; Xiong, T. Isolation, characterization and selection of potential probiotic lactic acid bacteria from feces of wild boar, native pig and commercial pig. Livest. Sci. 2020, 237. [Google Scholar] [CrossRef]
- Pazhoohan, M.; Sadeghi, F.; Moghadami, M.; Soltanmoradi, H.; Davoodabadi, A. Antimicrobial and antiadhesive effects of Lactobacillus isolates of healthy human gut origin on enterotoxigenic Escherichia coli (ETEC) and enteroaggregative Escherichia coli (EAEC). Microb. Pathog. 2020, 148. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333. [Google Scholar] [CrossRef]
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Safety evaluation and colonisation abilities of four lactic acid bacteria as future probiotics. Probiotics Antimicrob. 2019, 11, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Furtado, D.N.; Saad, S.M.; Tome, E.; Franco, B.D. Potential beneficial properties of bacteriocin-producing lactic acid bacteria isolated from smoked salmon. J. Appl. Microbiol. 2011, 110, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.V.; Sreenivasa, M.Y. Probiotic properties of lactic acid bacteria isolated from Neera: A naturally fermenting coconut palm nectar. Front. Microbiol. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, M.; Lin, C.; Chiou, M.; Chen, J.; Yang, K.; Wu, C. Potential probiotic of Lactobacillus strains isolated from the intestinal tracts of pigs and feces of dogs with antibacterial activity against multidrug-resistant pathogenic bacteria. Arch. Microbiol. 2020, 202, 1849–1860. [Google Scholar] [CrossRef]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Jadhav, S.E.; Dutta, N.; Kumar, A. Probiotic potential of a Lactobacillus bacterium of canine faecal-origin and its impact on select gut health indices and immune response of dogs. Probiotics Antimicrob. 2017, 9, 262–277. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016, 167, 1125–1136.e1128. [Google Scholar] [CrossRef]
- Evivie, S.E.; Abdelazez, A.; Li, B.; Lu, S.; Liu, F.; Huo, G. Lactobacillus delbrueckii subsp. bulgaricus KLDS 1.0207 exerts antimicrobial and cytotoxic effects in vitro and improves blood biochemical parameters in vivo against notable foodborne pathogens. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Amiranashvili, L.L.; Gagelidze, N.A.; Varsimashvili, K.I.; Tinikashvili, L.M.; Tolordava, L.L.; Gamkrelidze, M.D.; Amashukeli, N.V.; Makaradze, L.A. Antimicrobial susceptibility and antibiotic resistance profiles of cultivable lactic acid bacteria from intestinal tract of domestic chickens collected in Adjara. Ann. Agrar. Sci. 2016, 14, 182–186. [Google Scholar] [CrossRef]
- Remize, F.; Leneveu-Jenvrin, C.; Garcia, C. Editorial for special issue “lactic acid bacteria, biopreservation agents for fruit and vegetables”. Microorganisms 2021, 9, 939. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Brit. J. Nutr. 2013, 109 (Suppl. 2), S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Kaktcham, P.M.; Tchamani Piame, L.; Sandjong Sileu, G.M.; Foko Kouam, E.M.; Temgoua, J.B.; Zambou Ngoufack, F.; de Lourdes Perez-Chabela, M. Bacteriocinogenic Lactococcus lactis subsp. lactis 3MT isolated from freshwater Nile Tilapia: Isolation, safety traits, bacteriocin characterisation, and application for biopreservation in fish pate. Arch. Microbiol. 2019, 9, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Kaewnopparat, S.; Dangmanee, N.; Kaewnopparat, N.; Srichana, T.; Chulasiri, M.; Settharaksa, S. In vitro probiotic properties of Lactobacillus fermentum SK5 isolated from vagina of a healthy woman. Anaerobe 2013, 22, 6–13. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimaraes, J.T.; Yilmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pillidge, C.J.; Gopal, P.K.; Gill, H.S. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int. J. Food Microbiol. 2005, 98, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lavilla-Lerma, L.; Perez-Pulido, R.; Martinez-Bueno, M.; Maqueda, M.; Valdivia, E. Characterization of functional, safety, and gut survival related characteristics of Lactobacillus strains isolated from farmhouse goat’s milk cheeses. Int. J. Food Microbiol. 2013, 163, 136–145. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Hou, C.; Wang, S.; Zhang, D.; Liu, H.; Shan, D.; Wang, Y. Effects of Lactobacillus johnsonii XS4 supplementation on reproductive performance, gut environment, and blood biochemical and immunological index in lactating sows. Livest. Sci. 2014, 164, 96–101. [Google Scholar] [CrossRef]
- Betancur, C.; Martinez, Y.; Tellez-Isaias, G.; Castillo, R.; Ding, X. Effect of oral administration with Lactobacillus plantarum CAM6 strain on sows during gestation-lactation and the derived impact on their progeny performance. Mediat. Inflamm. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Zhang, Y.; Lv, Y.; Qiao, H.; Tian, M.; Cheng, L.; Chen, F.; Zhang, S.; Guan, W. Effects of maternal supplementation with fully oxidised beta-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets. Br. J. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Park, B.C.; Jung, D.Y.; Kang, S.Y.; Ko, Y.H.; Ha, D.M.; Kwon, C.H.; Park, M.J.; Han, J.H.; Jang, I.; Lee, C.Y. Effects of dietary supplementation of a zinc oxide product encapsulated with lipid on growth performance, intestinal morphology, and digestive enzyme activities in weanling pigs. Anim. Feed Sci. Technol. 2015, 200, 112–117. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, H.; Zhang, L.; Liu, M.; Qiao, X.; Cui, W.; Jiang, Y.; Wang, L.; Li, Y.; Tang, L. Probiotic properties of genetically engineered Lactobacillus plantarum producing porcine lactoferrin used as feed additive for piglets. Process Biochem. 2016, 51, 719–724. [Google Scholar] [CrossRef]
- Nordeste, R.; Tessema, A.; Sharma, S.; Kovac, Z.; Wang, C.; Morales, R.; Griffiths, M.W. Molecules produced by probiotics prevent enteric colibacillosis in pigs. BMC Vet. Res. 2017, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Casas, G.A.; Blavi, L.; Cross, T.L.; Lee, A.H.; Swanson, K.S.; Stein, H.H. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance. J. Anim. Sci. 2020, 98. [Google Scholar] [CrossRef]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with a complex of lactic acid bacteria alone or in combination with Bacillus subtilis and Saccharomyces boulardii. Livest. Sci. 2012, 143, 132–141. [Google Scholar] [CrossRef]
- Shah, A.A.; Yuan, X.; Khan, R.U.; Shao, T. Effect of lactic acid bacteria-treated King grass silage on the performance traits and serum metabolites in New Zealand white rabbits (Oryctolagus cuniculus). J. Anim. Physiol. Anim. Nutr. 2018, 102, e902–e908. [Google Scholar] [CrossRef]
- Boontiam, W.; Wachirapakorn, C.; Phaengphairee, P. Effects of hydrolyzed yeast supplementation on growth performance, immunity, antioxidant capacity, and microbial shedding in weaning pigs. Vet. World 2020, 13, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Yue, Y.; Xiong, S.; Xu, W. Enhanced protection against pulmonary mycobacterial challenge by chitosan-formulated polyepitope gene vaccine was associated with elevated pulmonary SIgA and IFN-γ (+) T cell response. Microbiol. Immunol. 2013, 57, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Kaburagi, T.; Yamano, T.; Fukushima, Y.; Yoshino, H.; Mito, N. Effect of Lactobacillus johnsonii la1 on immune function and serum albumin in aged and malnourished aged mice. Nutrition 2007, 23, 342–350. [Google Scholar] [CrossRef]
- Villena, J.; Salva, S. Immunomodulatory and protective effect of probiotic Lactobacillus casei against Candida albicans infection in malnourished mice. Microbiol. Immunol. 2011, 55, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, E.; Jarosz, L.; Gradzki, Z. Effect of multi-microbial probiotic formulation bokashi on pro- and anti-inflammatory cytokines profile in the serum, colostrum and milk of sows, and in a culture of polymorphonuclear cells isolated from colostrum. Probiotics Antimicrob. 2019, 11, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Mizumachi, K.; Aoki, R.; Ohmori, H.; Saeki, M.; Kawashima, T. Effect of fermented liquid diet prepared with Lactobacillus plantarum LQ80 on the immune response in weaning pigs. Animal 2009, 3, 670–676. [Google Scholar] [CrossRef] [PubMed]
| Items | Gestation (%) | Lactation (%) | Piglet (%) |
|---|---|---|---|
| Corn | 57.34 | 60.36 | 58.00 |
| Soybean meal | 12.80 | 23.20 | 25.00 |
| Fish meal | 0 | 3.00 | 4.00 |
| Dried whey | 4.00 | 4.50 | 5.00 |
| Wheat bran | 22.10 | 5.00 | 3.00 |
| Limestone | 1.50 | 1.40 | 1.00 |
| CaHPO4 | 0.96 | 0.99 | 2.00 |
| NaCl | 0.40 | 0.40 | 0.30 |
| L-Lysine HCl | 0.15 | 0.30 | 0.35 |
| DL-Methionine | 0 | 0.15 | 0.10 |
| Choline chloride | 0.15 | 0.15 | 0.20 |
| Threonine | 0.05 | 0 | 0.05 |
| Vitamin-mineral premix a | 0.55 | 0.55 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels | |||
| Digestible energy (MJ/kg) | 12.11 | 13.59 | 14.00 |
| Crude protein (%) | 14.66 | 17.76 | 18.50 |
| Ca (%) | 1.05 | 1.08 | 0.80 |
| Lysine (%) | 0.73 | 1.11 | 1.35 |
| Methionine (%) | 0.40 | 0.56 | 0.47 |
| Isolates | Antimicrobial Activity | Isolate | Antimicrobial Activity |
|---|---|---|---|
| P1 | +++ | P11 | ++++ |
| P2 | ++ | P12 | ++ |
| P3 | +++ | P13 | ++ |
| P4 | +++ | P14 | +++ |
| P5 | ++++ | P15 | ++++ |
| P6 | ++ | P16 | ++++ |
| P7 | +++ | P17 | +++ |
| P8 | ++++ | P18 | +++ |
| P9 | ++ | P19 | ++ |
| P10 | ++++ | P20 | +++ |
| Isolates | Temperature (°C) | NaCl (w/v, %) | pH | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 10 | 40 | 45 | 50 | 3.0 | 6.5 | 3.0 | 3.5 | 4.0 | 4.5 | 8.0 | 9.0 | 10.0 | |
| P1 | w | + | + | + | - | + | + | - | w | + | + | + | + | + |
| P3 | w | + | + | + | w | + | + | w | w | + | + | + | + | + |
| P4 | - | w | + | w | - | + | + | - | - | w | + | + | + | w |
| P5 | w | + | + | + | w | + | + | - | w | + | + | + | + | + |
| P7 | w | + | + | + | - | + | + | w | + | + | + | + | + | + |
| P8 | w | + | + | + | + | + | + | - | w | + | + | + | + | + |
| P10 | - | w | + | w | - | + | + | - | w | + | + | + | + | w |
| P11 | w | + | + | + | - | + | + | w | + | + | + | + | + | + |
| P14 | - | w | + | + | + | + | w | - | - | w | + | + | + | + |
| P15 | w | + | + | + | w | + | + | + | + | + | + | + | + | + |
| P16 | w | + | + | + | + | + | + | + | + | + | + | + | + | + |
| P17 | - | + | + | + | - | + | w | - | - | w | + | + | + | + |
| P18 | - | w | + | w | - | + | + | - | w | + | + | + | + | w |
| P20 | w | + | + | + | - | + | + | - | w | + | + | + | + | + |
| Isolates | Indicator Bacteria | ||||||
|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. subtilis | L. monocytogenes | M. luteus | S. enterica | |
| P7 | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| P8 | ++++ | + | ++ | +++ | +++ | ++ | +++ |
| P15 | +++ | ++++ | +++ | +++ | ++++ | +++ | +++ |
| P16 | ++++ | - | ++ | ++ | + | ++ | ++ |
| Isolates | CB | CZ | AM | GM | NOR | CC | P | E | C | AK |
|---|---|---|---|---|---|---|---|---|---|---|
| P7 | S | S | S | I | R | S | I | S | S | R |
| P8 | S | S | R | S | R | S | R | S | S | R |
| P15 | S | S | S | I | R | S | I | S | S | R |
| P16 | S | S | S | S | R | S | R | I | S | R |
| Items | Treatment | (Control vs. LAB) p-Value | |
|---|---|---|---|
| Control | LAB | ||
| Total born | 11.90 ± 0.35 | 12.30 ± 0.26 | 0.09 |
| Born alive | 11.20 ± 0.10 | 12.10 ± 0.21 | <0.05 |
| Weak piglets | 2.95 ± 0.29 | 2.45 ± 0.37 | <0.05 |
| Stillborn piglets | 0.27 ± 0.05 | 0.23 ± 0.02 | 0.18 |
| Total weight per litter, kg | 17.25 ± 0.82 | 18.45 ± 0.58 | <0.05 |
| Individual weight, kg | 1.45 ± 0.10 | 1.50 ± 0.52 | 0.34 |
| Live litter rate, % | 98.32 ± 1.25 | 98.35 ± 1.31 | 0.82 |
| Weak litter rate, % | 9.82 ± 3.09 | 7.44 ± 0.78 | 0.28 |
| Weaning survival rate, % | 90.10 ± 1.37 | 91.50 ± 0.72 | 0.31 |
| Estrus rate of 1–7 days, % | 80.00 ± 8.71 | 90.00 ± 4.58 | 0.31 |
| Estrus rate of 8–14 days, % | 10.00 ± 1.00 | 10.00 ± 1.73 | 1.00 |
| Conception rate during estrus, % | 70.00 ± 5.00 | 100.00 ± 0.00 | <0.01 |
| Items | Treatment in Sows | Treatment in Piglets | |||
|---|---|---|---|---|---|
| Control | LAB | Control | LAB | Antibiotic | |
| TNF-α, ng/L | 121.02 ± 1.34 b | 145.41 ± 1.16 a | 68.75 ± 0.49 b | 107 ± 2.21 a | 80.75 ± 1.92 b |
| IFN-γ, ng/L | 59.72 ± 0.87 | 60.15 ± 0.92 | 38.17 ± 0.66 | 39.52 ± 1.03 | 39.11 ± 0.84 |
| IgG, g/L | 9.17 ± 0.69 | 9.23 ± 0.28 | 3.97 ± 0.18 b | 4.45 ± 0.23 a | 4.34 ± 0.10 a |
| IgM, g/L | 2.47 ± 0.32 | 2.52 ± 0.39 | 1.15 ± 0.11 | 1.20 ± 0.05 | 1.22 ± 0.13 |
| IgA, g/L | 1.26 ± 0.09 b | 1.76 ± 0.17 a | 0.45 ± 0.03 b | 0.57 ± 0.13 a | 0.48 ± 0.08 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Ma, H.; Zhu, Y.; Ni, K.; Qin, G.; Tan, Z.; Wang, Y.; Wang, L.; Pang, H. Screening of Lactic Acid Bacteria with Inhibitory Activity against ETEC K88 as Feed Additive and the Effects on Sows and Piglets. Animals 2021, 11, 1719. https://doi.org/10.3390/ani11061719
Wang W, Ma H, Zhu Y, Ni K, Qin G, Tan Z, Wang Y, Wang L, Pang H. Screening of Lactic Acid Bacteria with Inhibitory Activity against ETEC K88 as Feed Additive and the Effects on Sows and Piglets. Animals. 2021; 11(6):1719. https://doi.org/10.3390/ani11061719
Chicago/Turabian StyleWang, Weiwei, Hao Ma, Yajie Zhu, Kuikui Ni, Guangyong Qin, Zhongfang Tan, Yanping Wang, Lei Wang, and Huili Pang. 2021. "Screening of Lactic Acid Bacteria with Inhibitory Activity against ETEC K88 as Feed Additive and the Effects on Sows and Piglets" Animals 11, no. 6: 1719. https://doi.org/10.3390/ani11061719
APA StyleWang, W., Ma, H., Zhu, Y., Ni, K., Qin, G., Tan, Z., Wang, Y., Wang, L., & Pang, H. (2021). Screening of Lactic Acid Bacteria with Inhibitory Activity against ETEC K88 as Feed Additive and the Effects on Sows and Piglets. Animals, 11(6), 1719. https://doi.org/10.3390/ani11061719






