Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Facilities and Rearing Conditions
2.2. Experimental Diets
2.3. Digestibility Trial
2.4. N and P Excretion Estimation
2.5. Chemical Analyses and Calculations
2.6. Predicted Digestibility
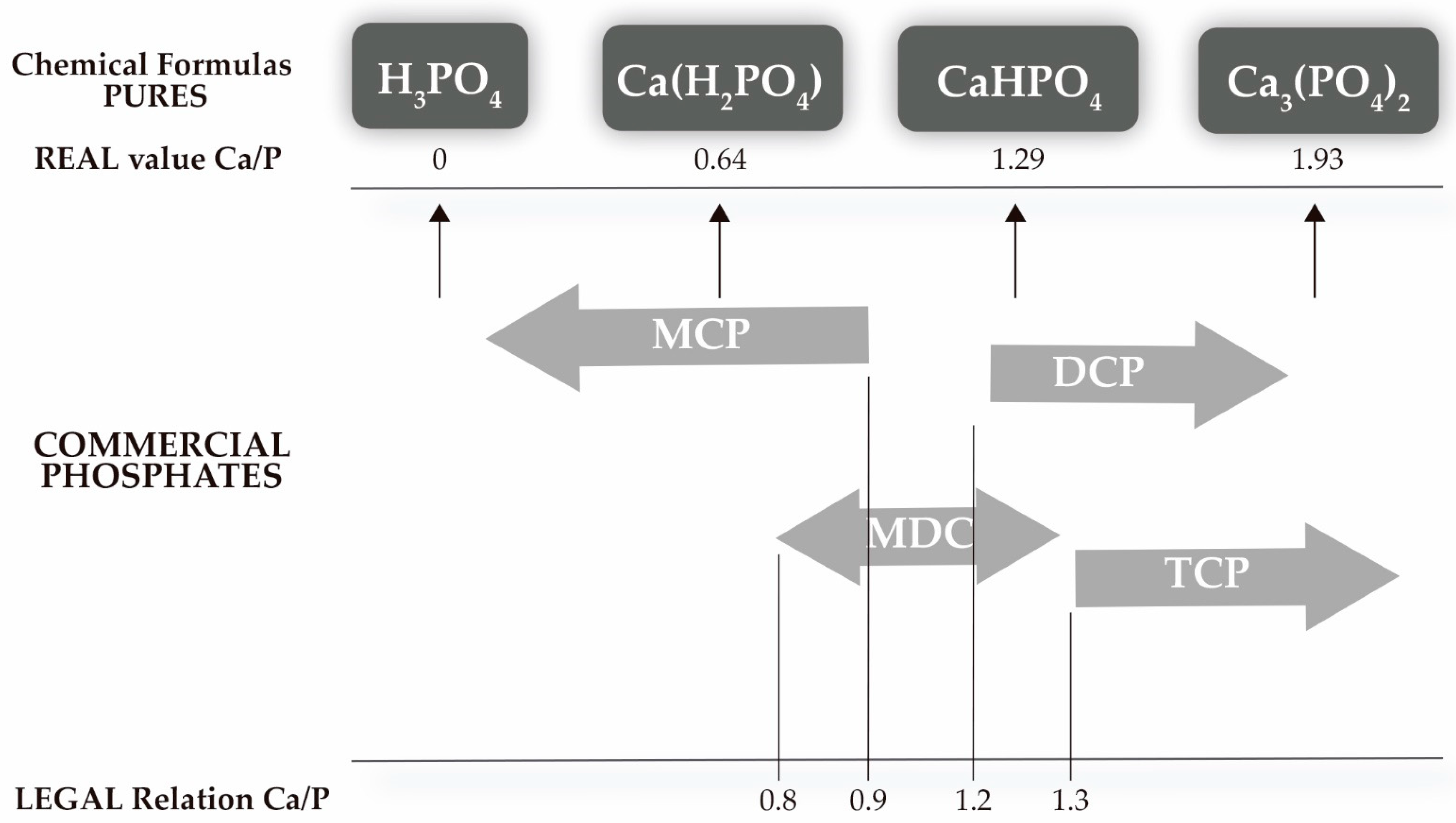
- ▪
- Percentage of P, Ca and Na: determination of the composition of the product.
- ▪
- Solubility in water: above 80% correlates with MCP, while solubility around 50% is due to the presence of MDCP.
- ▪
- Solubility in alkaline ammonium citrate: almost completely dissolves DCP but not TCP, which remains insoluble.
- ▪
- Solubility in 2% citric acid: difference in TCP (> 95%).
- ▪
- Solubility in neutral ammonium citrate: good correlation with bioavailability.
- ▪
- Percentage of CaCO3: presence of impurities and type of phosphate.
- ▪
- Humidity: allows for quantification of free water.
- ▪
- Loss 200–250 °C: estimate of hydration water.
- ▪
- Ca/P ratio: estimation of the presence of different phosphates, as well as their composition.
2.7. Statistical Analyses
3. Results
3.1. Effect of P Inorganic Sources on Digestibility
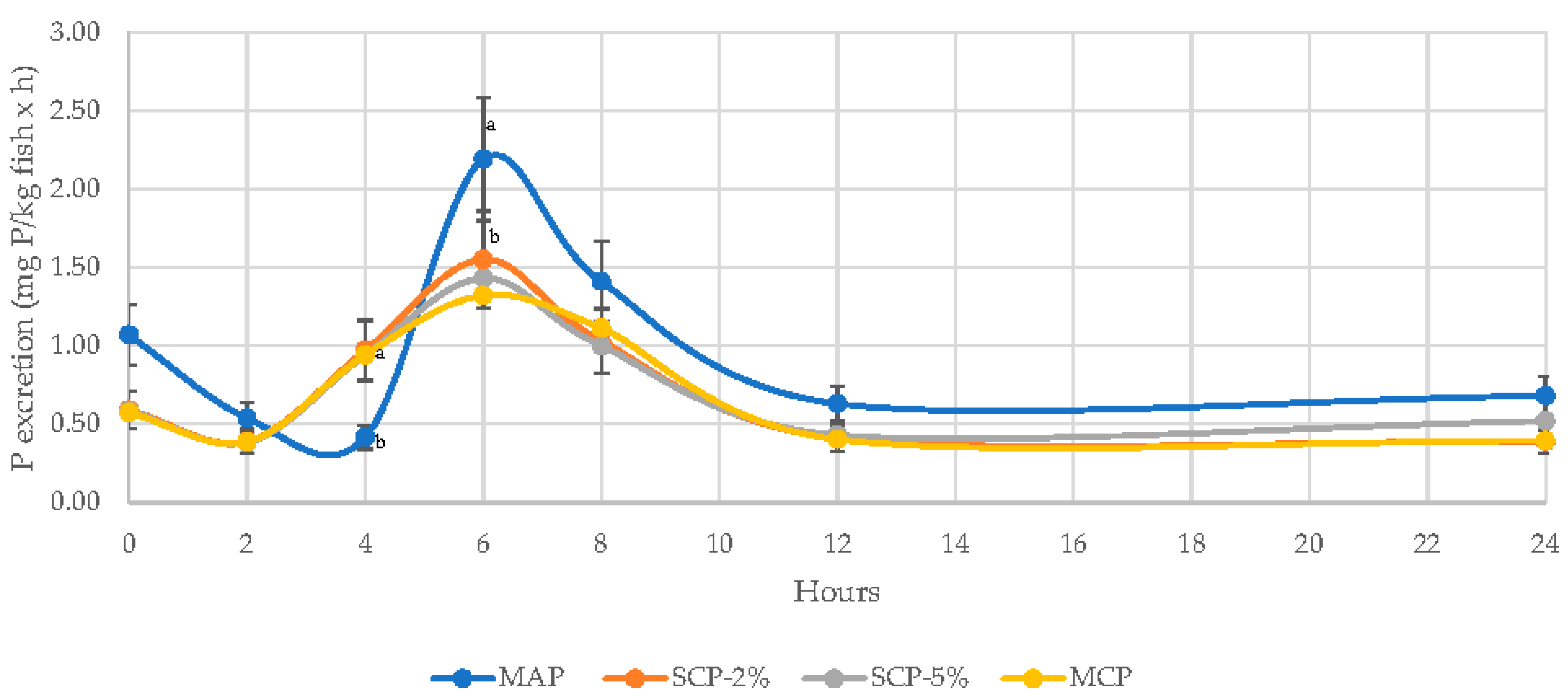
3.2. Effect of Inorganic Phosphates on N and P Excretion
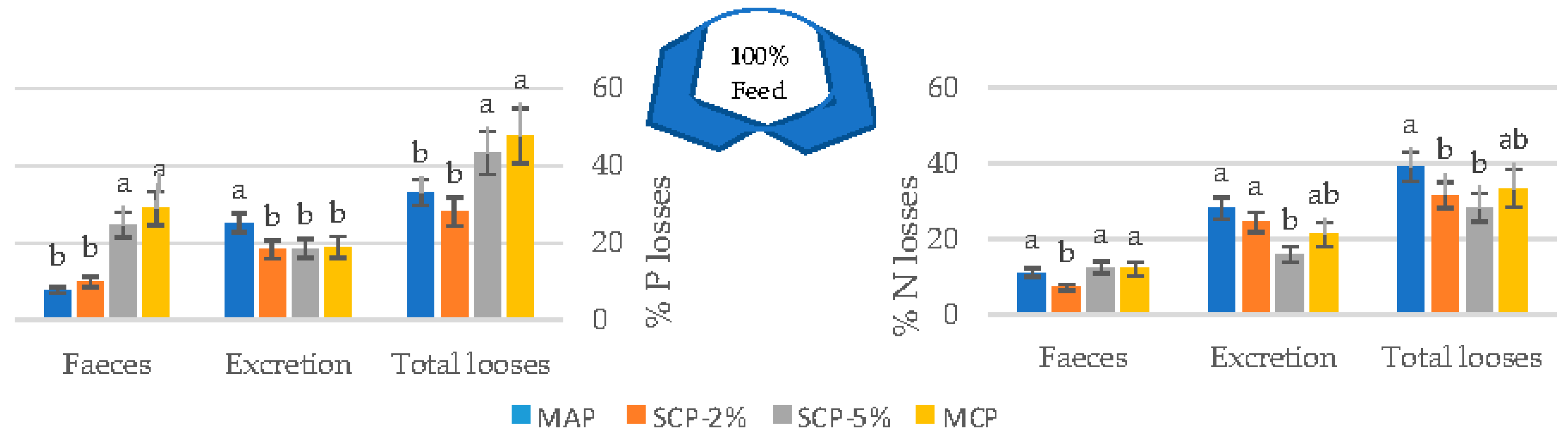
3.3. Total N and P Losses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef]
- Yogev, U.; Vogler, M.; Nir, O.; Londong, J.; Gross, A. Phosphorous recovery from a novel recirculating aquaculture system followed by its sustainable reuse as a fertilizer. Sci. Total Environ. 2020, 722, 137949. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Factors Regulating Bone Maturity and Strength in Poultry. Poult. Sci. 2000, 79, 1024–1032. [Google Scholar] [CrossRef]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2011, 20, 601–602. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Dong, F.M.; Hardy, R.W. Effects of dietary supplements on the availability of minerals in fish meal; preliminary observations. Aquaculture 1998, 160, 283–303. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Vidal, A.T.; Cerdá, M.J. A new tool for determining the optimum fish meal and vegetable meals in diets for maximizing the economic profitability of gilthead sea bream (Sparus aurata, L.) feeding. Aquac. Res. 2011, 43, 1697–1709. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Tomás-Vidal, A.; Moñino-López, A.; Jover-Cerdá, M.; Martínez-Llorens, S. Pea protein concentrate in diets for sharpsnout sea bream (Diplodus puntazzo): Effects on growth and health status. Arch. Anim. Nutr. 2016, 70, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozano, N.B.; Martínez-Llorens, S.; Tomás-Vidal, A.; Cerdá, M.J. Effect of high-level fish meal replacement by pea and rice concentrate protein on growth, nutrient utilization and fillet quality in gilthead seabream (Sparus aurata, L.). Aquaculture 2009, 298, 83–89. [Google Scholar] [CrossRef]
- Ribeiro, L.; Moura, J.; Santos, M.T.N.; Colen, R.; Rodrigues, V.; Bandarra, N.; Soares, F.; Ramalho, P.; Barata, M.; Moura, P.; et al. Effect of vegetable based diets on growth, intestinal morphology, activity of intestinal enzymes and haematological stress indicators in meagre (Argyrosomus regius). Aquaculture 2015, 447, 116–128. [Google Scholar] [CrossRef]
- Berzi-Nagy, L.; Mozsár, A.; Tóth, F.; Gál, D.; Nagy, Z.; Nagy, S.; Kerepeczki, É.; Antal, L.; Sándor, Z. Effects of Different Fish Diets on the Water Quality in Semi-Intensive Common Carp (Cyprinus carpio) Farming. Water 2021, 13, 1215. [Google Scholar] [CrossRef]
- Lall, S.P. Digestibility, metabolism and excretion in dietary phosphorus in fish. In Nutritional Strategies & Aquaculture Waste: Proceedings of the First International Symposium on Nutritional Strategies in Management of Aquaculture Waste (NSMAW); Cowey, C.B., Cho., C.Y., Eds.; Fish Nutrition Research Laboratory, University of Guelph: Guelph, ON, Canada, 1991; pp. 21–36. [Google Scholar]
- Hua, K.; Bureau, D. Modelling digestible phosphorus content of salmonid fish feeds. Aquaculture 2006, 254, 455–465. [Google Scholar] [CrossRef]
- Satoh, S.; Viyakarn, V.; Takeuchi, T.; Watanabe, T. Availability of Phosphorus in Various Phosphates to Carp and Rainbow Trout Determined by a Simple Fractionation Method. Fish. Sci. 1997, 63, 297–300. [Google Scholar] [CrossRef]
- Lemos, D.; Tacon, A.G.J. Use of phytases in fish and shrimp feeds: A review. Rev. Aquac. 2016, 9, 266–282. [Google Scholar] [CrossRef]
- Javid, I.; Fatima, M.; Shah, S.Z.H.; Afzal, M. A comparison of the effect of organic acids and dicalcium phosphate supplementation on phosphorus bioavailability, growth performance and digestive enzyme activities of Labeo rohita fingerlings. Aquac. Nutr. 2021, 27, 217–224. [Google Scholar] [CrossRef]
- El Bakali, M.; Mustapha, A. Phosphorus Waste Production in Fish Farming a Potential for Reuse in Integrated Aquaculture Agriculture. Int. J. Environ. Agric. Res. 2021, 7. [Google Scholar] [CrossRef]
- European Union Commission Regulation (EU). 2017/1017 of 15 June 2017 amending Regulation (EU) No 68/2013 on the Catalogue of feed materials. Off. J. Eur. Union 2017, 159, 48–119. [Google Scholar]
- Forbes, R.M.; Erdman, J.W. Bioavailability of Trace Mineral Elements. Annu. Rev. Nutr. 1983, 3, 213–231. [Google Scholar] [CrossRef]
- Carpintero, L.; Donadeu, A.; Dupuy, J.; Macías-Vidal, J. EPCD Index: Predictive Equations for Digestibility Comparison among Different Sources of Commercial Phosphates in Poultry. 2020. Available online: https://globalfeed.es/en/news/163-epcd-index (accessed on 21 February 2021).
- Hossain, S.; Chance, A.B.; El Kertaoui, N.; Wattiez, X.; Houndji, A.; Mandiki, S.N.M.; Kestemont, P. Dietary inorganic monophosphates in high plant ingredient-based diets influence nutrient digestibility, postprandial macro-mineral status and immune functions of juvenile rainbow trout, Oncorhynchus mykiss. Aquac. Nutr. 2020, 26, 2178–2194. [Google Scholar] [CrossRef]
- Morales, G.A.; Azcuy, R.L.; Casaretto, M.E.; Márquez, L.; Hernández, A.J.; Gómez, F.; Koppe, W.; Mereu, A. Effect of different inorganic phosphorus sources on growth performance, digestibility, retention efficiency and discharge of nutrients in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 568–574. [Google Scholar] [CrossRef]
- Cho, C. Feeding systems for rainbow trout and other salmonids with reference to current estimates of energy and protein requirements. Aquaculture 1992, 100, 107–123. [Google Scholar] [CrossRef]
- Brouwer, E. Report of sub-committee on constants and factors. In Proceedings of the 3rd Symposium on Energy Metabolism of Farm Animals, London, UK, 20 April 2015; 1965; Volume 11. [Google Scholar]
- Laining, A.; Rachmansyah; Ahmad, T.; Williams, K. Apparent digestibility of selected feed ingredients for humpback grouper, Cromileptes altivelis. Aquaculture 2003, 218, 529–538. [Google Scholar] [CrossRef]
- Bhujel, R.C. Statistics for Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 0813815878. [Google Scholar]
- Monge-Ortiz, R.; Tomás-Vidal, A.; Gallardo-Álvarez, F.; Estruch, G.; Godoy-Olmos, S.; Jover-Cerdá, M.; Llorens, S.M. Partial and total replacement of fishmeal by a blend of animal and plant proteins in diets for Seriola dumerili: Effects on performance and nutrient efficiency. Aquac. Nutr. 2018, 24, 1163–1174. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg Maryland, DC, USA, 2005; ISBN 0935584544. [Google Scholar]
- Neto, H.B.; Graner, C.A.F.; Pezzato, L.E.; Padovani, C.R. Determinação de rotina do crômio em fezes, como marcador biológico, pelo método espectrofotométrico ajustado da 1,5-difenilcarbazida. Ciência Rural. 2005, 35, 691–697. [Google Scholar] [CrossRef]
- Cho, C.Y.; Kaushik, S.J. Nutritional Energetics in Fish: Energy and Protein Utilization in Rainbow Trout (Salmo gairdneri). World Rev. Nutr. Diet. 1990, 61, 132–172. [Google Scholar] [CrossRef] [PubMed]
- Rucker, R.B.; Parker, H.E.; Rogler, J.C. Utilization of Calcium and Phosphorus from Hydrous and Anhydrous Dicalcium Phosphates. J. Nutr. 1968, 96, 513–518. [Google Scholar] [CrossRef]
- Gillis, M.B.; Edwards, H.M.; Young, R.J. Studies on the Availability of Calcium Orthophosphates to Chickens and Turkeys. J. Nutr. 1962, 78, 155–161. [Google Scholar] [CrossRef]
- Kwon, W.B.; Kim, B.G. Standardized total tract digestibility of phosphorus in various inorganic phosphates fed to growing pigs. Anim. Sci. J. 2017, 88, 918–924. [Google Scholar] [CrossRef]
- Lopez, D.A.; Stein, H.H. PSVI-8 Mineral composition of feed grade monocalcium phosphate. J. Anim. Sci. 2020, 98, 200–201. [Google Scholar] [CrossRef]
- Shastak, Y.; Rodehutscord, M. Recent developments in determination of available phosphorus in poultry. J. Appl. Poult. Res. 2015, 24, 283–292. [Google Scholar] [CrossRef]
- Glencross, B.D. A feed is still only as good as its ingredients: An update on the nutritional research strategies for the optimal evaluation of ingredients for aquaculture feeds. Aquac. Nutr. 2020, 26, 1871–1883. [Google Scholar] [CrossRef]
- Sarker, P.K.; Fukada, H.; Masumoto, T. Phosphorus availability from inorganic phosphorus sources in yellowtail (Seriola quinqueradiata Temminck and Schlegel). Aquaculture 2009, 289, 113–117. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D. Quantification of differences in digestibility of phosphorus among cyprinids, cichlids, and salmonids through a mathematical modelling approach. Aquaculture 2010, 308, 152–158. [Google Scholar] [CrossRef]
- Baeverfjord, C.; Åsgård, T. Shearer development and detection of phosphorus deficiency in Atlantic salmon, Salmo salar L., parr and post-smolts. Aquac. Nutr. 1998, 4, 1–11. [Google Scholar] [CrossRef]
- Lovell, R.T. Dietary Phosphorus Requirement of Channel Catfish (Ictalurus punctatus). Trans. Am. Fish. Soc. 1978, 107, 617–621. [Google Scholar] [CrossRef]
- Bueno, G.; Feiden, A.; Roubach, R.; Klein, S.; Boscolo, W.; Tavares, F. Different sources of phosphorus supple-mentation and its excretion by Nile tilapia juveniles (Oreochromis niloticus). Panam. J. Aquat. Sci. 2016, 11, 151–158. [Google Scholar]
- Prabhu, P.A.J. Minerals in Fish: Does the Source Matter? Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Davis, D.; Arnold, C. Estimation of apparent phosphorus availability from inorganic phosphorus sources for Penaeus vannamei. Aquaculture 1994, 127, 245–254. [Google Scholar] [CrossRef]
- Peruzzi, S.; Puvanendran, V.; Riesen, G.; Seim, R.R.; Hagen, Ø.; Martínez-Llorens, S.; Falk-Petersen, I.-B.; Fernandes, J.M.O.; Jobling, M. Growth and development of skeletal anomalies in diploid and triploid Atlantic salmon (Salmo salar) fed phosphorus-rich diets with fish meal and hydrolyzed fish protein. PLoS ONE 2018, 13, e0194340. [Google Scholar] [CrossRef]
- Blank, R.; Mosenthin, R.; Sauer, W.C.; Huang, S. Effect of fumaric acid and dietary buffering capacity on ileal and fecal amino acid digestibilities in early-weaned pigs. J. Anim. Sci. 1999, 77, 2974–2984. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B.; Caffrey, P.J.; O’Reilly, J.J.; O’Connell, M.K. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir. Vet. J. 2005, 58, 447–452. [Google Scholar] [CrossRef]
- Parma, L.; Yúfera, M.; Navarro-Guillén, C.; Moyano, F.J.; Soverini, M.; D’Amico, F.; Candela, M.; Fontanillas, R.; Gatta, P.P.; Bonaldo, A. Effects of calcium carbonate inclusion in low fishmeal diets on growth, gastrointestinal pH, digestive enzyme activity and gut bacterial community of European sea bass (Dicentrarchus labrax L.) juveniles. Aquaculture 2019, 510, 283–292. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Peruzzi, S.; Falk-Petersen, I.-B.; Godoy-Olmos, S.; Ulleberg, L.O.; Tomás-Vidal, A.; Puvanendran, V.; Odei, D.K.; Hagen, Ø.; Fernandes, J.M.O.; et al. Digestive tract morphology and enzyme activities of juvenile diploid and triploid Atlantic salmon (Salmo salar) fed fishmeal-based diets with or without fish protein hydrolysates. PLoS ONE 2021, 16, e0245216. [Google Scholar] [CrossRef] [PubMed]
- Monge-Ortiz, R.; Martínez-Llorens, S.; Márquez, L.; Moyano, F.J.; Jover-Cerdá, M.; Tomás-Vidal, A. Potential use of high levels of vegetal proteins in diets for market-sized gilthead sea bream (Sparus aurata). Arch. Anim. Nutr. 2016, 70, 155–172. [Google Scholar] [CrossRef]
- Márquez, L.; Robles, R.; Morales, G.A.; Moyano, F.J. Gut pH as a limiting factor for digestive proteolysis in cultured juveniles of the gilthead sea bream (Sparus aurata). Fish Physiol. Biochem. 2011, 38, 859–869. [Google Scholar] [CrossRef]
- Bucking, C.; Wood, C. The effect of postprandial changes in pH along the gastrointestinal tract on the distribution of ions between the solid and fluid phases of chyme in rainbow trout. Aquac. Nutr. 2009, 15, 282–296. [Google Scholar] [CrossRef]
- Coloso, R.M. Phosphorus utilization in rainbow trout (Oncorhynchus mykiss) fed practical diets and its consequences on effluent phosphorus levels. Aquaculture 2003, 220, 801–820. [Google Scholar] [CrossRef]
- Rodehutscord, M.; Gregus, Z.; Pfeffer, E. Effect of phosphorus intake on faecal and non-faecal phosphorus excretion in rainbow trout (Oncorhynchus mykiss) and the consequences for comparative phosphorus availability studies. Aquaculture 2000, 188, 383–398. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Babbitt, J.K.; Dong, F.M.; Hardy, R.W. Utilization of fish and animal by-product meals in low-pollution feeds for rainbow trout Oncorhynchus mykiss (Walbaum). Aquac. Res. 2000, 31, 585–593. [Google Scholar] [CrossRef]
- Avila, E.M.; Tu, H.; Basantes, S.; Ferraris, R.P. Dietary phosphorus regulates intestinal transport and plasma concentrations of phosphate in rainbow trout. J. Comp. Physiol. B 2000, 170, 201–209. [Google Scholar] [CrossRef]
- Coloso, R.; Basantes, S.; King, K.; Hendrix, M.; Fletcher, J.; Weis, P.; Ferraris, R. Effect of dietary phosphorus and vitamin D3 on phosphorus levels in effluent from the experimental culture of rainbow trout (Oncorhynchus mykiss). Aquaculture 2001, 202, 145–161. [Google Scholar] [CrossRef]
- Olsen, L.M.; Holmer, M.; Olsen, Y. Perspectives of Nutrient Emission from Fish Aquaculture in Coastal Waters; ResearchGate: Berlin, Germany, 2008; Volume 542014. [Google Scholar]
- del Estado, B.O. Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia. BOE 2013, 34, 11370–11421. [Google Scholar]


| Experimental Diets | MAP | SCP-2% | SCP-5% | MCP |
|---|---|---|---|---|
| Ingredients (g/kg) | ||||
| Albumin 1 | 450 | 450 | 450 | 450 |
| Fish oil 2 | 180 | 180 | 180 | 180 |
| Starch 3 | 200 | 200 | 200 | 200 |
| Maltodextrin 4 | 3 | 108.2 | 107.9 | 107.1 |
| Feeding stimulant 5 | 5 | 5 | 5 | 5 |
| Cr2O3 | 5 | 5 | 5 | 5 |
| Monoammonium phosphate 6 | 31.7 | |||
| Monosodium/monocalcium phosphate (2%) 7 | 31.8 | |||
| Monosodium/monocalcium phosphate (5%) 8 | 32.1 | |||
| Monocalcium phosphate 9 | 32.9 | |||
| Multivitamin and minerals mix 10 | 20 | 20 | 20 | 20 |
| Analyzed composition (% dry weight) | ||||
| Dry matter (% DM) | 95.86 | 96.83 | 97.50 | 96.88 |
| Crude Protein (% CP) | 45.80 | 45.41 | 45.44 | 45.75 |
| Crude Lipids (% CL) | 17.83 | 18.01 | 18.10 | 17.95 |
| Crude phosphorous (% P) | 0.84 | 0.87 | 0.85 | 0.82 |
| Ash (%) | 4.96 | 5.98 | 5.75 | 5.12 |
| Nitrogen (% N) | 7.33 | 7.27 | 7.27 | 7.32 |
| Carbon (% C) | 42.9 | 43.1 | 43.2 | 42.7 |
| Calculated values | ||||
| Gross Energy (kJ/g) 11 | 22.21 | 22.32 | 22.36 | 22.11 |
| Inorganic Source | EPCD Value (%) |
|---|---|
| MAP | 92 |
| SCP-2%(AQphos+) | 91 |
| SCP-5% | 75 |
| MCP | 78 |
| MAP | SCP-2% | SCP-5% | MCP | |
|---|---|---|---|---|
| ADC P | 92.26 a ± 2.19 | 90.08 a ± 1.26 | 75.21 b ± 2.51 | 71.11 b ± 1.89 |
| ADC protein | 88.98 b ± 2.81 | 92.80 a ± 1.56 | 87.58 b ± 5.01 | 87.91 b ± 2.81 |
| ADC energy | 84.68 b ± 6.86 | 93.07 a ± 1.45 | 78.51 c ± 5.02 | 75.99 c ± 2.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milián-Sorribes, M.C.; Tomás-Vidal, A.; Peñaranda, D.S.; Carpintero, L.; Mesa, J.S.; Dupuy, J.; Donadeu, A.; Macías-Vidal, J.; Martínez-Llorens, S. Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources. Animals 2021, 11, 1700. https://doi.org/10.3390/ani11061700
Milián-Sorribes MC, Tomás-Vidal A, Peñaranda DS, Carpintero L, Mesa JS, Dupuy J, Donadeu A, Macías-Vidal J, Martínez-Llorens S. Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources. Animals. 2021; 11(6):1700. https://doi.org/10.3390/ani11061700
Chicago/Turabian StyleMilián-Sorribes, Maria Consolación, Ana Tomás-Vidal, David S. Peñaranda, Laura Carpintero, Juan S. Mesa, Javier Dupuy, Andrés Donadeu, Judit Macías-Vidal, and Silvia Martínez-Llorens. 2021. "Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources" Animals 11, no. 6: 1700. https://doi.org/10.3390/ani11061700
APA StyleMilián-Sorribes, M. C., Tomás-Vidal, A., Peñaranda, D. S., Carpintero, L., Mesa, J. S., Dupuy, J., Donadeu, A., Macías-Vidal, J., & Martínez-Llorens, S. (2021). Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources. Animals, 11(6), 1700. https://doi.org/10.3390/ani11061700









