Growth Performance, Growth-Related Genes, Digestibility, Digestive Enzyme Activity, Immune and Stress Responses of de novo Camelina Meal in Diets of Red Seabream (Pagrus major)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Camelina Meal and Test Diets
2.2. Fish Husbandry and Sampling Methods
2.3. Determination of Antinutrients Contents in Camelina Meal
2.4. Digestive Enzyme Assay
2.5. Protease Activity
2.5.1. Pepsin
2.5.2. Lipase
2.5.3. Amylase
2.6. Blood Function Assessment
2.7. Non-Specific Immunological and Antioxidative Assays
2.8. Low Salinity Stress Evaluation
2.9. Digestibility Assessment
2.10. Real Time PCR Analysis
2.11. Statistical Analysis
3. Results
3.1. Growth and Nutrient Utilization Variables
3.2. Whole Body Proximate Evaluation
3.3. Digestive Enzyme Activity
3.4. Serum Biochemical Constituents
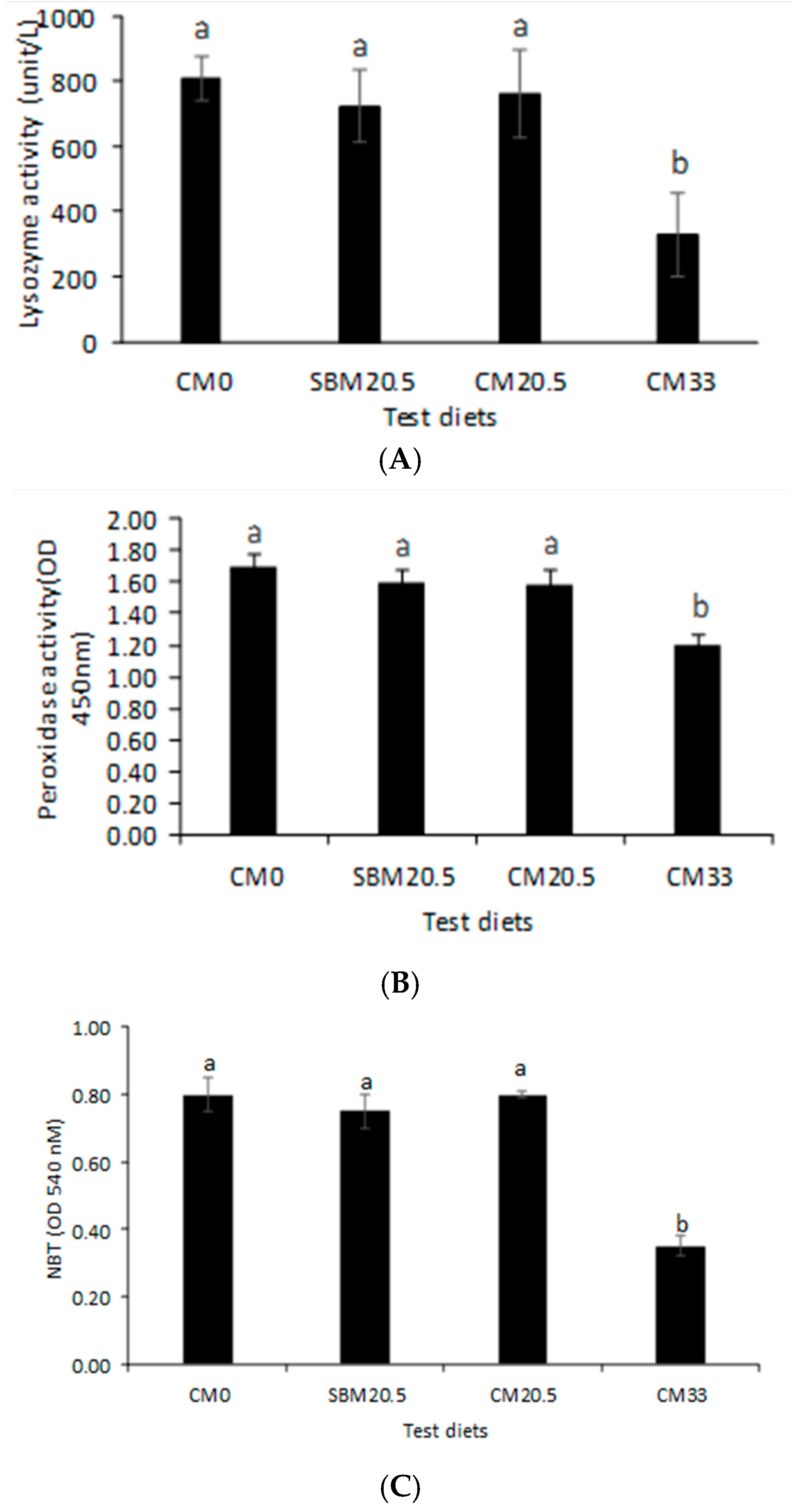
3.5. Immunological Response
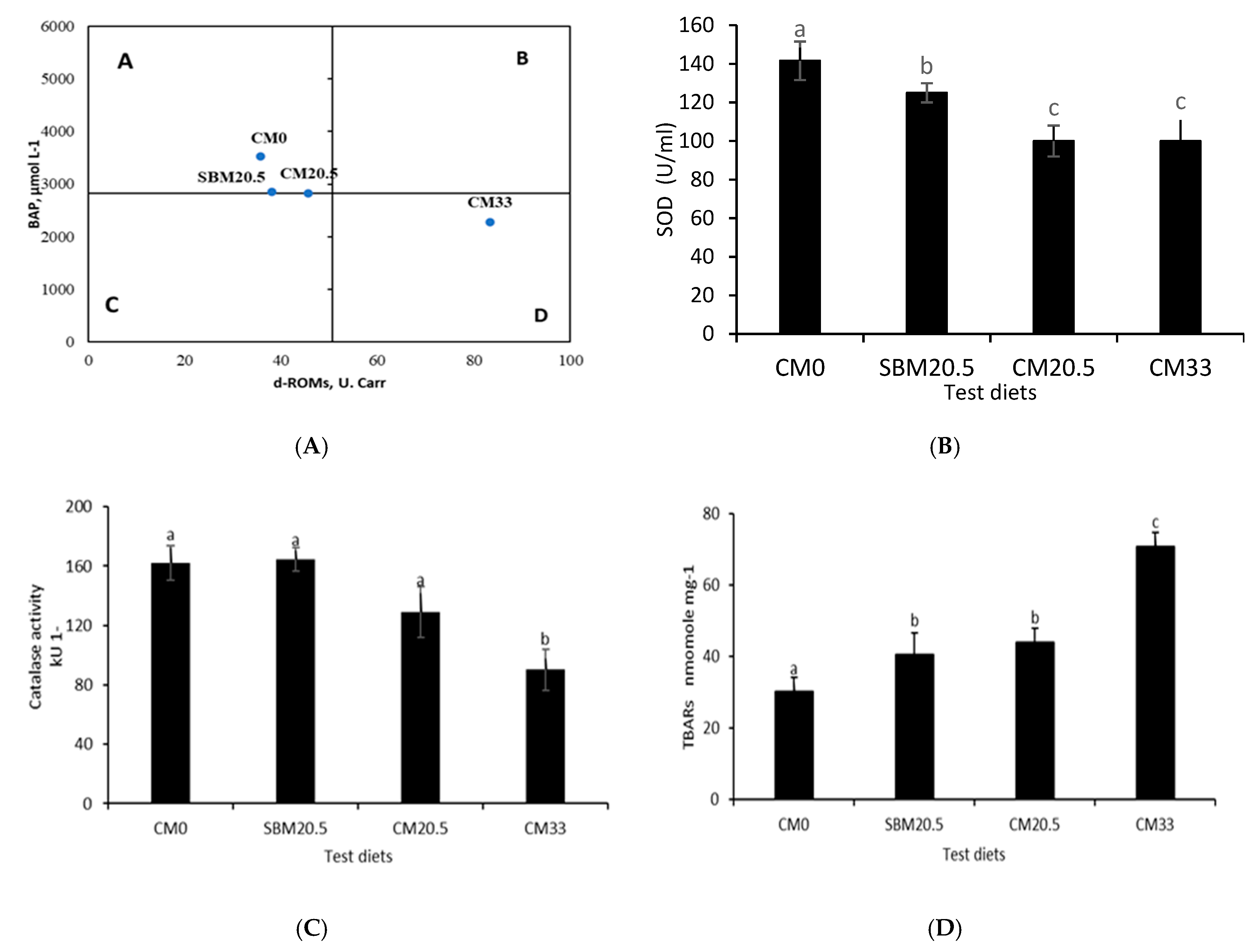
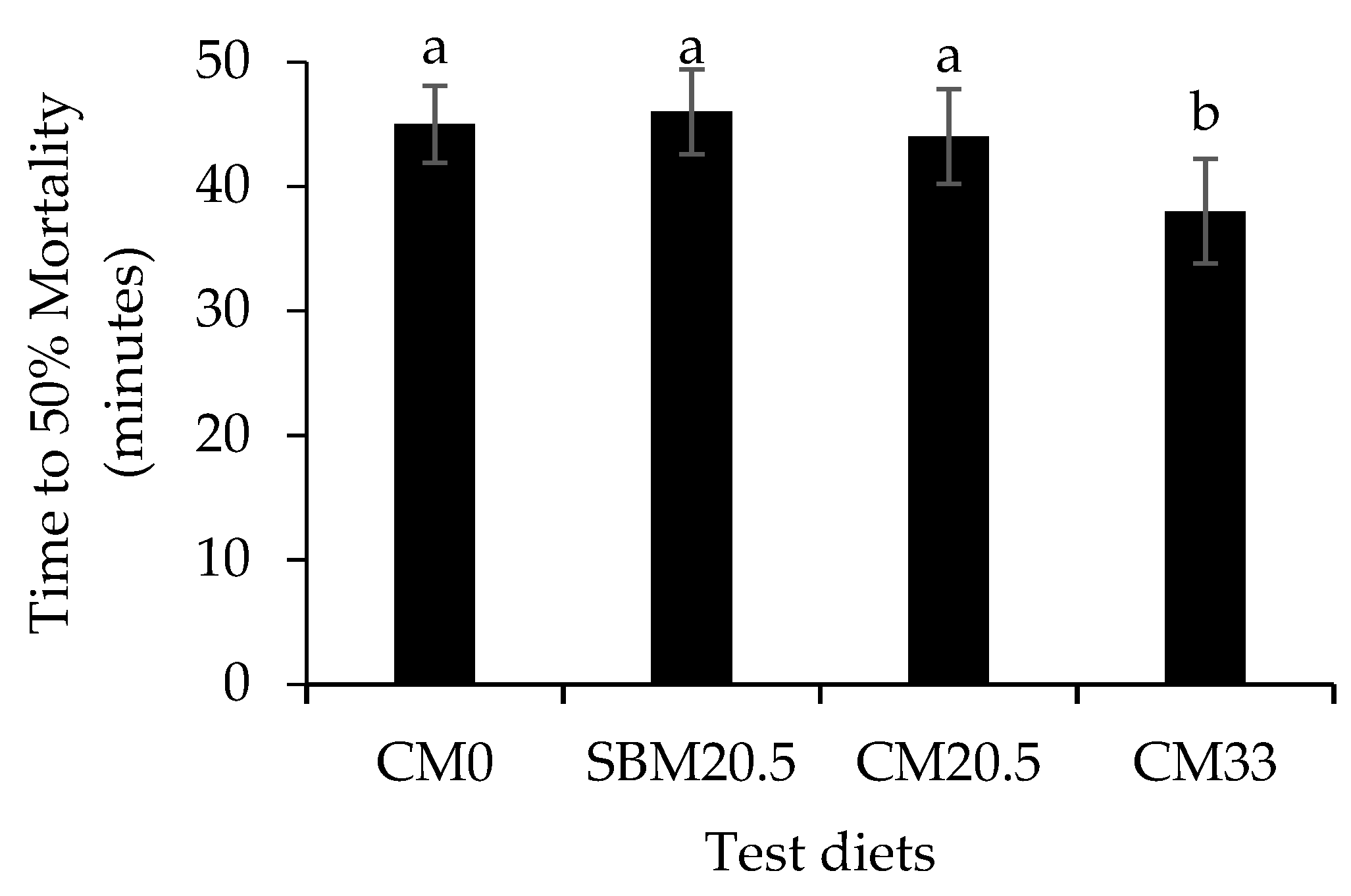
3.6. Resistance to Oxidative and Salinity Stress
3.7. Apparent Digestibility Coefficients of Nutrients
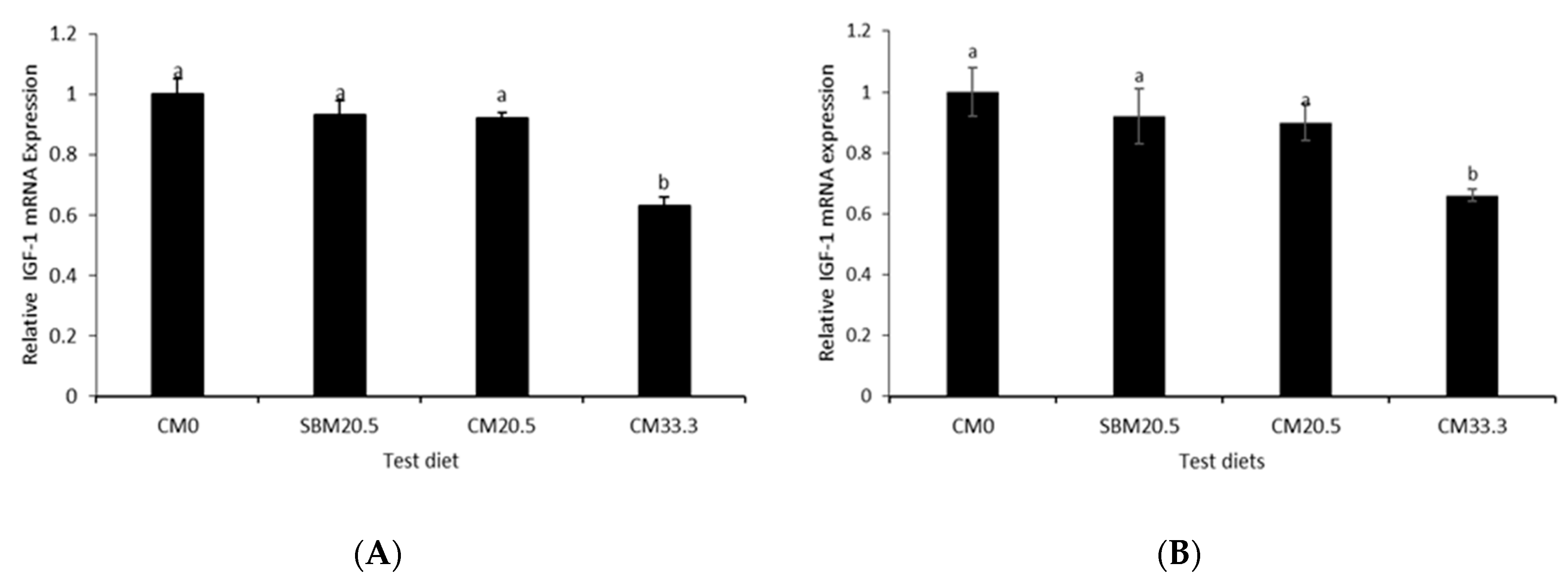
3.8. Relative Growth Gene Expression
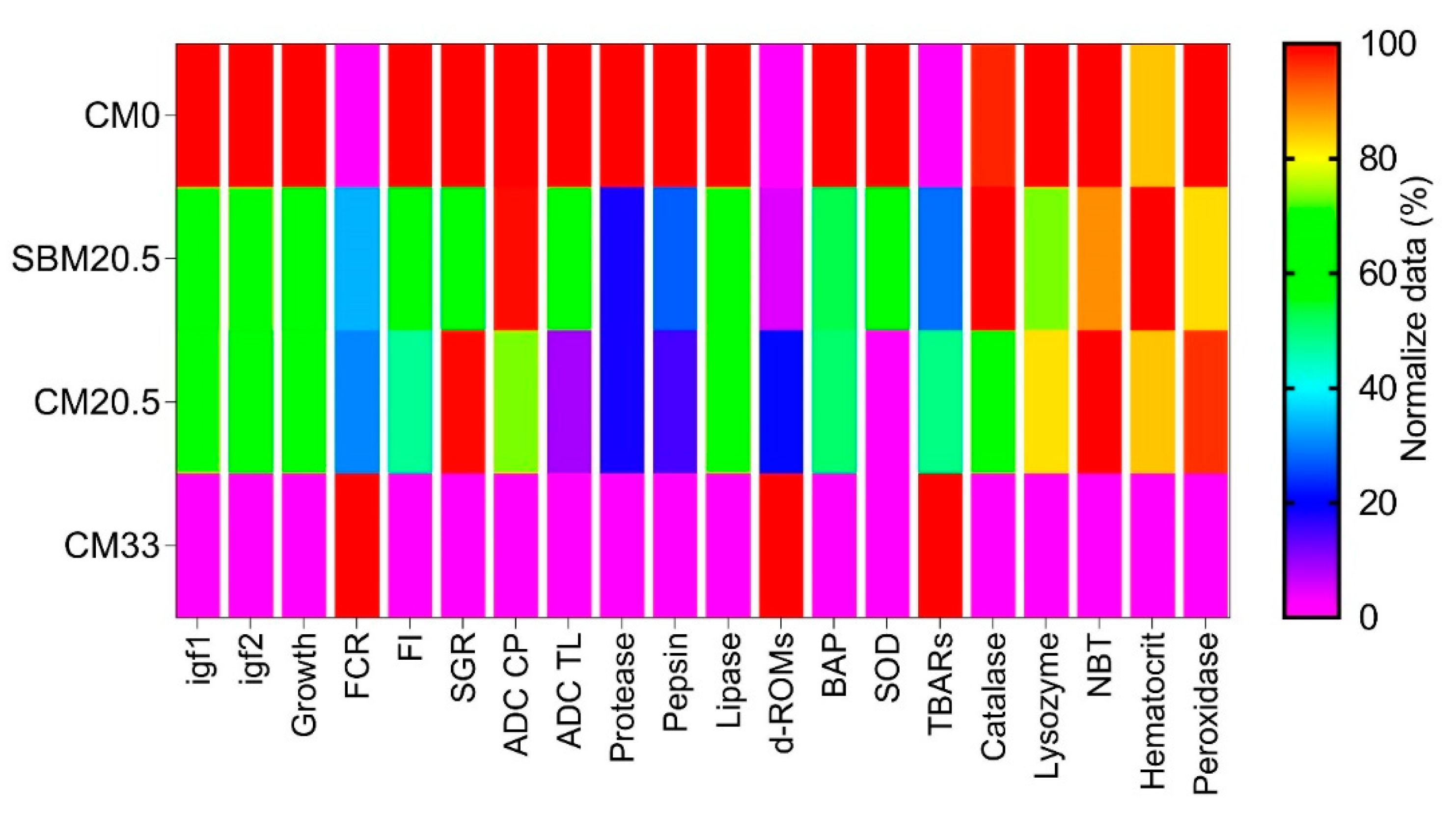
3.9. The Heatmap Analysis
3.10. Principal Components Analysis (PCA)
3.11. UPMGA Correlation Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, M.; Anderson, D.M.; Zhang, Z.; Colombo, S.M. High-oil residue camelina meal, a viable source of protein at low levels in diets for juvenile salmonids. Aquac. Nutr. 2020, 26, 558–567. [Google Scholar] [CrossRef]
- Ido, A.; Iwai, T.; Ito, K.; Ohta, T.; Mizushima, T.; Kishida, T.; Miura, C.; Miura, T. Dietary effects of housefly (Musca Domestica) (Diptera: Muscidae) pupae on the growth performance and the resistance against bacterial pathogen in red sea bream (Pagrus major) (Perciformes: Sparidae). Appl. Entomol. Zool. 2015, 50, 213–221. [Google Scholar] [CrossRef]
- Bianchi, M.C.G.; Chopin, F.; Farme, T.; Franz, N.; Fuentevilla, C.; Garibaldi, L.; Laurenti, A.L.G. FAO: The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; p. 200. [Google Scholar]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zambelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of graded dietary inclusion level of full-fat Hermetic illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, A.; Araki, H.; Sakata, T.; Nakamori, T.; Kato, K.; Takii, K. Fish meal replacement by soy protein from soymilk in the diets of red sea bream (Pagrus major). Aquac. Nutr. 2017, 23, 1379–1389. [Google Scholar] [CrossRef]
- Dossou, S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Dawood, M.A.O.; El Basuini, M.F.; El-Hais, A.M.; Olivier, A. Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 2018, 490, 228–235. [Google Scholar] [CrossRef]
- Seong, T.; Matsutani, H.; Haga, Y.; Kitajima, R.; Satoh, S. First step of non-fish meal, non-fish oil diet development for red seabream, (Pagrus major), with plant protein sources and microalgae Schizochytrium sp. Aquac. Res. 2019, 50, 2460–2468. [Google Scholar] [CrossRef]
- Seong, T.; Kitajima, R.; Haga, Y.; Satoh, S. Non-fish meal, non-fish oil diet development for red sea bream, Pagrus major, with plant protein and graded levels of Schizochytrium sp.: Effect on growth and fatty acid composition. Aquac. Nutr. 2020, 26, 1173–1185. [Google Scholar] [CrossRef]
- Espe, M.; Lemme, A.; Petri, A.; El-Mowafi, A. Can Atlantic salmon (Salmo salar) grow on diets devoid of fish meal? Aquaculture 2006, 255, 255–262. [Google Scholar] [CrossRef]
- Jiang, D.; Zheng, J.; Dan, Z.; Tang, Z.; Ai, Q.; Mai, K. Effects of five compound attractants in high plant-based diets on feed intake and growth performance of juvenile turbot (Scophthalmus maximus L.). Aquac. Res. 2019, 50, 2350–2358. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Mazumder, A.; Dwivedi, A.; Du Plessis, J. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glencross, B.D.; Baily, J.; Berntsen, M.H.G.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2020, 12, 703–758. [Google Scholar] [CrossRef] [Green Version]
- Mudalkar, S.; Golla, R.; Ghatty, S.; Reddy, A.R. De novo transcriptome analysis of an imminent biofuel crop, Camelina sativa l. Using Illumina gaiix sequencing platform and identification of SSR markers. Plant Mol. Biol. 2014, 84, 159–171. [Google Scholar] [CrossRef]
- Onyilagha, J.; Bala, A.; Hallett, R.; Gruber, M.; Soroka, J.; Westcott, N. Leaf flavonoids of the cruciferous species, Camelina sativa, Crambe spp., Thlaspi arvense and several other genera of the family Brassicaceae. Biochem. Syst. Ecol. 2003, 31, 1309–1322. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C. Substitution of fish oil with camelina oil and inclusion of camelina meal in diets fed to Atlantic cod (Gadus morhua) and their effects on growth, tissue lipid classes, and fatty acids1. J. Anim. Sci. 2014, 92, 1055–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betancor, M.B.; Li, K.; Bucerzan, V.S.; Sprague, M.; Sayanova, O.; Usher, S.; Han, L.; Norambuena, F.; Torrissen, O.; Napier, J.A.; et al. Oil from transgenic camelina sativa containing over 25 % n-3 long-chain pufa as the major lipid source in feed for Atlantic salmon (Salmo salar). Br. J. Nutr. 2018, 119, 1378–1392. [Google Scholar] [CrossRef] [Green Version]
- Toyes-Vargas, E.A.; Parrish, C.C.; Viana, M.T.; Carreón-Palau, L.; Magallón-Servín, P.; Magallón-Barajas, F.J. Replacement of fish oil with camelina (Camelina sativa) oil in diets for juvenile tilapia (var. Gift Oreochromis niloticus) and its effect on growth, feed utilization and muscle lipid composition. Aquaculture 2020, 523, 735177. [Google Scholar] [CrossRef]
- Mzengereza, K.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Yukun, Z.; Shadrack, R.S.; Seo, S.; Duy Khoa, T.N.; Moss, A.; Dossou, S.; et al. Effect of substituting fish oil with camelina oil on growth performance, fatty acid profile, digestibility, liver histology, and antioxidative status of red seabream (Pagrus major). Animals 2021, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Bullerwell, C.N.; Collins, S.A.; Lall, S.P.; Anderson, D.M. Growth performance, proximate and histological analysis of rainbow trout fed diets containing Camelina Sativa seeds, meal (high-oil and solvent-extracted) and oil. Aquaculture 2016, 452, 342–350. [Google Scholar] [CrossRef]
- Hixson, S.M.; Parrish, C.C.; Wells, J.S.; Winkowski, E.M.; Anderson, D.M.; Bullerwell, C.N. Inclusion of camelina meal as a protein source in diets for farmed salmonids. Aquac. Nutr. 2016, 22, 615–630. [Google Scholar] [CrossRef]
- Agency, C.F.I. The Biology of Aamelina sativa (L.) Crantz (Camelina). Directive 94–08 (dir94-08), Assessment Criteria for Determining Environmental Safety of Plant with Novel Traits. 2017. Available online: http://www.Inspection.Gc.Ca/plants/plants-withnovel-traits/applicants/directive-94-08/biology-documents/camelina-Sativa-l-/eng/1330971423348/1330971509470 (accessed on 1 January 2017).
- Matthäs, B. Antinutritive compounds in different oilseeds. Lipid/Fett 1997, 99, 170–174. [Google Scholar] [CrossRef]
- Dossou, S.; Dawood, M.A.O.; Zaineldin, A.I.; Abouelsaad, I.A.; Mzengereza, K.; Shadrack, R.S.; Zhang, Y.; El-Sharnouby, M.; Ahmed, H.A.; El Basuini, M.F. Dynamical hybrid system for optimizing and controlling efficacy of plant-based protein in aquafeeds. Complexity 2021, 2021, 9957723. [Google Scholar] [CrossRef]
- Olivotto, I.; Mosconi, G.; Maradonna, F.; Cardinali, M.; Carnevali, O. Diplodus sargus interrenal–pituitary response: Chemical communication in stressed fish. Gen. Comp. Endocrinol. 2002, 127, 66–70. [Google Scholar] [CrossRef]
- Panserat, S.; Kaushik, S.J. Regulation of gene expression by nutritional factors in fish. Aquac. Res. 2010, 41, 751–762. [Google Scholar] [CrossRef]
- Picha, M.E.; Turano, M.J.; Beckman, B.R.; Borski, R.J. Endocrine biomarkers of growth and applications to aquaculture: A minireview of growth hormone, insulin-like growth factor (IGF)-i, and IGF-binding proteins as potential growth indicators in fish. N. Am. J. Aquac. 2008, 70, 196–211. [Google Scholar] [CrossRef]
- Duan, C. Nutritional and developmental regulation of insulin-like growth factors in fish. J. Nutr. 1998, 128, 306S–314S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Ai, Q.; Mai, K.; Zhang, W.; Xu, W.; Zhang, Y. Effects of dietary rapeseed meal on growth performance, digestion and protein metabolism in relation to gene expression of juvenile cobia (Rachycentron canadum). Aquaculture 2012, 368–369, 109–116. [Google Scholar] [CrossRef]
- NRC. Nutrient requirements of fish and shrimp. In Animal Nutrition Series, National Research Council of the National Academies; The National Academies Press: Washington, DC, USA, 2011; p. 376. [Google Scholar]
- AOAC. Official Methods of Analysis of Aoac International, 19th ed.; Aoac International: Gaithersburg, MD, USA, 2012; Available online: Www.Eoma.Aoac.Org (accessed on 1 January 2021).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Teshima, S.-I.; Kanazawa, A.; Yamashita, M. Dietary value of several proteins and supplemental amino acids for larvae of the prawn Penaeus japonicus. Aquaculture 1986, 51, 225–235. [Google Scholar] [CrossRef]
- Kader, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M. Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture 2010, 308, 136–144. [Google Scholar] [CrossRef]
- Qing, P.L.; Xing, W.K. The experimental studies on activities of digestive enzyme in the larvae Penaeus chinensis. J. Fish. China 1997, 1. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-SCKX701.004.htm (accessed on 3 August 2021).
- Natalia, Y.; Hashim, R.; Ali, A.; Chong, A. Characterization of digestive enzymes in a carnivorous ornamental fish, the Asian bony tongue Scleropages formosus (osteoglossidae). Aquaculture 2004, 233, 305–320. [Google Scholar] [CrossRef]
- Roberts, I.M. Hydrolysis of 4-methylumbelliferyl butyrate: A convenient and sensitive fluorescent assay for lipase activity. Lipids 1985, 20, 243–247. [Google Scholar] [CrossRef]
- Morishita, K.; Matsunari, H.; Furuta, H.; Rønnestad, I.; Oku, H.; Yamamoto, T. Effects of dietary soybean meal on the digestive physiology of red sea bream Pagrus major. Aquaculture 2018, 493, 219–228. [Google Scholar] [CrossRef]
- Salinas, I.; Abelli, L.; Bertoni, F.; Picchietti, S.; Roque, A.; Furones, D.; Cuesta, A.; Meseguer, J.; Esteban, M.Á. Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata l.). Fish Shellfish Immunol. 2008, 25, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lygren, B.; Sevier, H.; Hjeltness, B.; Waagbø, R. Examination of the immunomodulatory properties and the effect on disease resistance of dietary bovine lactoferrin and vitamin c fed to Atlantic salmon (Salmo salar) for a short-term period. Fish Shellfish Immunol. 1999, 9, 95–107. [Google Scholar] [CrossRef]
- Anderson, D.; Siwicki, A. Basic Hematology and Serology for Fish Health Programs; Fish Health Section; Asian Fisheries Society: Manila, PA, USA, 1995; p. 18. [Google Scholar]
- Moe, Y.Y.; Koshio, S.; Teshima, S.I.; Ishikawa, M.; Matsunaga, Y.; Panganiban, A. Effect of vitamin c derivatives on the performance of larval kuruma shrimp, Marsupenaeus japonicus. Aquaculture 2004, 242, 501–512. [Google Scholar] [CrossRef]
- Furukawa, A. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Nippon. Suisan Gakkaishi 1966, 32, 502–506. [Google Scholar] [CrossRef]
- Hossain, M.S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M.; Dawood, M.A.O.; Kader, M.A.; Bulbul, M.; Fujieda, T. Efficacy of nucleotide related products on growth, blood chemistry, oxidative stress and growth factor gene expression of juvenile red sea bream, Pagrus major. Aquaculture 2016, 464, 8–16. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P. Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Dawood, M.A.O.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A.; Astley, A.E.; Fadl, S.E.; Amin, A.A.; Paray, B.A.; Ahmed, H.A. Saccharomyces cerevisiae increases the acceptability of Nile tilapia (Oreochromis niloticus) to date palm seed meal. Aquac. Rep. 2020, 17, 100314. [Google Scholar] [CrossRef]
- Brown, T.D.; Hori, T.S.; Xue, X.; Ye, C.L.; Anderson, D.M.; Rise, M.L. Functional genomic analysis of the impact of camelina (camelina sativa) meal on Atlantic salmon (Salmo salar) distal intestine gene expression and physiology. Mar. Biotechnol. 2016, 18, 418–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Xie, W.; Caldwel, C.; Anderson, D. Growth performance and carcass composition of rainbow trout (Oncorhynchus mykiss) fed practical diets containing graded levels of high fat residue camelina meal. Can. J. Anim. Sci. 2011, 91, 484. [Google Scholar]
- Matthäus, B.; Zubr, J. Variability of specific components in Camelina sativa oilseed cakes. Ind. Crop. Prod. 2000, 12, 9–18. [Google Scholar] [CrossRef]
- Adeoye, A.A.; Jaramillo-Torres, A.; Fox, S.W.; Merrifield, D.L.; Davies, S.J. Supplementation of formulated diets for tilapia (Oreochromis niloticus) with selected exogenous enzymes: Overall performance and effects on intestinal histology and microbiota. Anim. Feed. Sci. Technol. 2016, 215, 133–143. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Goda, A.M.A.S.; Kumar, V. Evaluation of nutritive value of fermented de-oiled physic nut, jatropha curcas, seed meal for Nile tilapia Oreochromis niloticus fingerlings. Aquac. Nutr. 2017, 23, 571–584. [Google Scholar] [CrossRef]
- Refstie, S.; Storebakken, T.; Roem, A.J. Feed consumption and conversion in Atlantic salmon (Salmo salar) fed diets with fish meal, extracted soybean meal or soybean meal with reduced content of oligosaccharides, trypsin inhibitors, lectins and soya antigens. Aquaculture 1998, 162, 301–312. [Google Scholar] [CrossRef]
- Nagel, F.; Appel, T.; Rohde, C.; Kroeckel, S.; Schulz, C. Blue mussel protein concentrate versus prime fish meal protein as a dietary attractant for turbot (Psetta maxima L.) given rapeseed protein-based diets. Aquac. Res. Dev. S 2017, 2, 2. [Google Scholar]
- Kokou, F.; Fountoulaki, E. Aquaculture waste production associated with antinutrient presence in common fish feed plant ingredients. Aquaculture 2018, 495, 295–310. [Google Scholar] [CrossRef]
- Eppler, E.; Berishvili, G.; Mazel, P.; Callers, A.; Hwang, G.; Maclean, N.; Reinecke, M. Distinct organ-specific up-and down-regulation of igf-i and igf-ii mRNA in various organs of a gh-overexpressing transgenic Nile tilapia. Transgenic Res. 2010, 19, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Amilburu, V.; Salmerón, C.; Codina, M.; Navarro, I.; Capilla, E.; Gutiérrez, J. Insulin-like growth factors effects on the expression of myogenic regulatory factors in gilthead sea bream muscle cells. Gen. Comp. Endocrinol. 2013, 188, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-Y.; Wang, Y.-Y.; Chen, F.-Y.; Tang, B.-B.; Luo, C.-Z.; Wang, Y.; Ge, X.-P.; Yang, Y.-H. An evaluation of replacing fishmeal with rapeseed meal in the diet of Pseudobagrus ussuriensis: Growth, feed utilization, non-specific immunity, and growth-related gene expression. J. World Aquac. Soc. 2018, 49, 1068–1080. [Google Scholar] [CrossRef]
- Bu, X.; Lian, X.; Zhang, Y.; Chen, F.; Tang, B.; Ge, X.; Yang, Y. Effects of replacing fish meal with corn gluten meal on growth, feed utilization, nitrogen and phosphorus excretion and igf-i gene expression of juvenile Pseudobagrus ussuriensis. Aquac. Res. 2018, 49, 977–987. [Google Scholar] [CrossRef]
- Hevrøy, E.M.; Ezpeleta, C.; Shimizu, M.; Lanzén, A.; Kaiya, H.; Espe, M.; Olsvik, P.A. Effects of short-term starvation on ghrelin, gh-igf system, and IGF-binding proteins in Atlantic salmon. Fish Physiol. Biochem. 2011, 37, 217–232. [Google Scholar] [CrossRef]
- Kumar, V.; Makkar, H.P.S.; Becker, K. Detoxified Jatropha curcas kernel meal as a dietary protein source: Growth performance, nutrient utilization and digestive enzymes in common carp (Cyprinus Carpio L.) fingerlings. Aquac. Nutr. 2011, 17, 313–326. [Google Scholar] [CrossRef]
- Gómez-Requeni, P.; Calduch-Giner, J.; Vega-Rubín de Celis, S.; Médale, F.; Kaushik, S.J.; Pérez-Sánchez, J. Regulation of the somatotropic axis by dietary factors in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2005, 94, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslamloo, K.; Falahatkar, B.; Yokoyama, S. Effects of dietary bovine lactoferrin on growth, physiological performance, iron metabolism and non-specific immune responses of Siberian sturgeon Acipenser baeri. Fish Shellfish Immunol. 2012, 32, 976–985. [Google Scholar] [CrossRef]
- Lin, S.; Luo, L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. Anim. Feed. Sci. Technol. 2011, 168, 80–87. [Google Scholar] [CrossRef]
- Shen, J.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Effects of replacement of fishmeal with cottonseed protein concentrate on the growth, intestinal microflora, haematological and antioxidant indices of juvenile golden pompano (Trachinotus ovatus). Aquac. Nutr. 2020, 26, 1119–1130. [Google Scholar] [CrossRef]
- Piccinetti, C.C.; Grasso, L.; Maradonna, F.; Radaelli, G.; Ballarin, C.; Chemello, G.; Evjemo, J.O.; Carnevali, O.; Olivotto, I. Growth and stress factors in Ballan wrasse (Labrus bergylta) larval development. Aquac. Res. 2017, 48, 2567–2580. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Fadl, S.E.; Ahmed, H.A.; El Astley, A.; Abdel-Daim, M.M.; Alkahtani, S. The modulatory effect of mannan oligosaccharide on oxidative status, selected immune parameters and tolerance against low salinity stress in red sea bream (Pagrus major). Aquac. Rep. 2020, 16, 100278. [Google Scholar] [CrossRef]
- KUBİLAY, A.; ULUKÖY, G. The effects of acute stress on rainbow trout (Oncorhynchus mykiss). Turk. J. Zool. 2002, 26, 249–254. [Google Scholar]
- Tovar-Ramírez, D.; Mazurais, D.; Gatesoupe, J.F.; Quazuguel, P.; Cahu, C.L.; Zambonino-Infante, J.L. Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 2010, 300, 142–147. [Google Scholar] [CrossRef] [Green Version]


| Ingredient (g/kg DM) | CM0 | SBM20.5 | CM20.5 | CM33 |
|---|---|---|---|---|
| Brown fish meal a | 59.0 | 45.0 | 45.0 | 31.0 |
| Soybean meal b | - | 20.5 | - | - |
| Camelina meal c | - | - | 20.5 | 33.0 |
| Casein d | 1.0 | 1.0 | 1.0 | 6.0 |
| Squid meal e | 5.0 | 5.0 | 5.0 | 5.0 |
| Krill meal f | 4.0 | 4.0 | 4.0 | 4.0 |
| Soybean lecithin g | 3.0 | 3.0 | 3.0 | 3.0 |
| Pollack liver oil h | 2.0 | 2.0 | 2.0 | 2.0 |
| Wheat flour i | 1.4 | 1.4 | 1.4 | 1.4 |
| Activated gluten j | 5.0 | 5.0 | 5.0 | 5.0 |
| Mineral mix k | 3.0 | 3.0 | 3.0 | 3.0 |
| Vitamin mix l | 3.0 | 3.0 | 3.0 | 3.0 |
| Stay C m | 0.08 | 0.08 | 0.08 | 0.08 |
| Alpha-cellulose n | 13.52 | 7.02 | 7.2 | 4.00 |
| Methionine | 0 | 0.1 | 0.2 | 0.5 |
| Lysine | 0 | 0.09 | 0.29 | 0.65 |
| Taurine | 0 | 0.17 | 0.18 | 0.29 |
| Total | 100 | 100 | 100 | 100 |
| Proximate constituents | ||||
| Crude protein | 49.6 ± 0.4 | 49.9 ± 0.2 | 48.2 ± 0.9 | 48.6 ± 0.1 |
| Total lipid | 9.5 ± 0.1 | 9.4 ± 0.0 | 9.0 ± 0.0 | 8.7 ± 0.0 |
| Moisture | 11.2 ± 1.0 | 10.86 ± 0.3 | 10.95 ± 0.6 | 11.26 ± 0.5 |
| Ash | 12.4 ± 0.3 | 12.5 ± 0.3 | 11.6 ± 0.3 | 9.5 ± 0.1 |
| Amino Acids | CM | CM0 | SBM20.5 | CM20.5 | CM33 |
|---|---|---|---|---|---|
| Essential | |||||
| Arginine | 3.24 | 2.53 | 2.57 | 2.54 | 2.85 |
| Histidine | 0.91 | 2.02 | 2.12 | 2.37 | 2.21 |
| Isoleucine | 1.64 | 1.89 | 1.95 | 1.87 | 1.96 |
| Leucine | 2.67 | 3.64 | 3.18 | 3.61 | 3.52 |
| Lysine | 1.83 | 4.08 | 4.14 | 3.67 | 3.75 |
| Methionine | 0.64 | 1.38 | 1.28 | 1.38 | 1.49 |
| Phenylalanine | 1.76 | 2.45 | 2.32 | 2.53 | 2.65 |
| Threonine | 1.68 | 2.12 | 2.12 | 2.3 | 2.2 |
| Tryptophan | 0.42 | 0.25 | 0.32 | 0.28 | 0.33 |
| Valine | 1.98 | 2.17 | 2.14 | 2.23 | 1.94 |
| Non-essential | |||||
| Taurine | nd | 0.77 | 0.75 | 0.8 | 0.9 |
| Aspartic acid | 3.24 | 4.65 | 4.35 | 4.26 | 4.25 |
| Glutamic acid | 6.56 | 8.03 | 8.12 | 8.22 | 8.08 |
| Serine | 2.01 | 2.04 | 2.25 | 2.3 | 2.2 |
| Proline | 2.21 | 3.40 | 2.93 | 3.39 | 3.38 |
| Glycine | 2.01 | 2.38 | 2.28 | 2.51 | 2.47 |
| Alanine | 1.09 | 2.70 | 2.37 | 2.56 | 2.24 |
| Tyrosine | 1.23 | 2.67 | 2.68 | 2.61 | 2.65 |
| Anti-Nutrient Factor | g/kg |
|---|---|
| Tannin | 34.2 ± 0.2 |
| Phytic acid | 40.7± 0.0 |
| Trypsin inhibitor | 20.3 ± 0.0 |
| Protease inhibitor | 12.0 ± 0.1 |
| Name | Primer Sequence (5′–3′) | Accession Number |
|---|---|---|
| β-actin-F | TCTGTCTGGATCGGAGGTC | JN226150.1 |
| β-actin-R | AAGCATTTGCGGTGGACG | |
| IGF-1-F | TAAACCCACACCGAGTGACA | AB050670.1 |
| IGF-1-R | GCGATGSSGAAAAGCTACGG | |
| IGF-2-F | CGGCAAACTAGTGATGAGCA | AB360966.1 |
| IGF-2-R | CAGTGTCAAGGGGGAAGTGT |
| Parameter | CM0 | SBM20.5 | CM20.5 | CM33 |
|---|---|---|---|---|
| Initial weight(g/fish) | 6.5 | 6.5 | 6.4 | 6.4 |
| Final weight(g/fish) | 29.9 ± 0.7 a | 28.1 ± 0.9 a | 28.3 ± 0.8 a | 24.9 ± 0.3 b |
| SGR 1 | 2.52 ± 0.0 a | 2.42 ± 0.1 a | 2.45 ± 0.1 a | 2.2 ± 0.0 b |
| FI(g/fish/56days) 2 | 27.6 ± 0.8 a | 24.7 ± 1.1 ab | 23.6 ± 1.5 ab | 20.6 ± 0.9 bc |
| FCR 3 | 1.0 ± 0.0 b | 1.1 ± 0.1 b | 1.1 ± 0.0 b | 1.2 ± 0.1 a |
| BWG 4 | 353.5 ± 4.9 a | 327.6 ± 18.5 a | 334.1 ± 14.3 a | 275.09 ± 5.8 b |
| HIS 5 | 1.8 ± 0.1 | 1.6 ± 0.1 | 2.0 ± 0.2 | 1.9 ± 0.1 |
| SR 6 | 100± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| CF 7 | 1.9 ± 0.1 | 1.9 ± 0.0 | 1.8 ± 0.1 | 1.8 ± 0.2 |
| Parameter | CM0 | SBM20.5 | CM20.5 | CM33 |
|---|---|---|---|---|
| Crude protein | 21.9 ± 0.8 a | 20.9 ± 0.1 a | 20.3 ± 1.7 a | 18.3 ± 0.5 b |
| Total Lipid | 16.6 ± 0.4 | 17.8 ± 2.4 | 18.1 ± 0.1 | 18.2 ± 0.2 |
| Moisture | 69.6 ± 0.5 | 68.0 ± 1.2 | 69.4 ± 0.3 | 69.1 ± 0.5 |
| Ash | 4.2 ± 0.1 | 5.7 ± 0.1 | 4.6 ± 0.1 | 3.2 ± 0.05 |
| Parameters | CM0 | SBM20.5 | CM20.5 | CM33 |
|---|---|---|---|---|
| protease | 3.0 ± 0.0 a | 1.2 ± 0.1 b | 1.2 ± 0.1 b | 0. 8 ± 0.0 c |
| Pepsin | 1.05 ± 0.2 a | 0.69 ± 0.0 a,b | 0.63 ± 0.0 a,b | 0.55 ± 0.0 b |
| Lipase | 0.33 ± 0.1 | 0.28 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 |
| Amylase | 0.6 ± 0.1 a | 0.14 ±0.4 b | 0.37 ± 0.0 b | 0.28 ± 0.3 b |
| Parameter | Control | SBM 20.5 | CM 20.5 | CM 33 |
|---|---|---|---|---|
| Hematocrit (%) | 35.5 ± 0.5 a | 37.5 ± 1.5 a | 35.5 ± 2.5 a | 24 ± 1 b |
| Glucose (mg/dL) | 70.3 ± 5.7 a | 76.7 ± 4.9 a | 78 ± 16.7 a | 108 ± 20.4 b |
| Total bilirubin (mg/dL) | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a | 0.3 ± 0.0 a |
| Total protein (mg/dL) | 3.2 ± 0.1 a | 3.4 ± 0.7 a | 3.2 ± 0.1 a | 3.7 ± 0.0 a |
| T-Cho g/dL) 1 | 178 ± 58.2 a | 184.3 ± 28.9 a | 188.3 ± 14 a | 225 ± 2.6 a |
| TG (g/dL) 2 | 181 ± 9.7 a | 173.6 ± 8.8 a | 164.3 ± 10.7 a | 170.6 ± 12.6 a |
| AST (IU/L) 3 | 35 ± 3.4 a | 36.5 ± 6.4 a | 31.7 ± 3.7 a | 26.5 ± 11.5 a |
| ALT (IU/L) 4 | 33 ± 2.3 a | 18 ± 3.1 a | 28 ± 1.8 a | 10.7 ± 0.6 a |
| Parameter | CM0 | SBM20.5 | CM20.5 | CM33 |
|---|---|---|---|---|
| Total lipid | 87.8 ± 0.7 | 86.8 ± 0.9 | 84.9 ± 0.1 | 84.6 ± 0.6 |
| Dry matter | 72.9 ± 2.5 | 72.4 ± 1.4 | 70.4 ± 2.5 | 72.6 ± 0.4 |
| Crude protein | 91.9 ± 0.5 a | 91.2 ± 0.3 a | 90.8 ± 0.2 a | 87.6 ± 0.3 b |
| Parameter | PC1 | PC2 | PC3 |
|---|---|---|---|
| igf1 | 0.24 | −0.05 | 0.08 |
| igf2 | 0.24 | -0.01 | 0.02 |
| Growth | 0.24 | −0.02 | 0.13 |
| FCR | −0.24 | 0.04 | −0.12 |
| FI | 0.23 | 0.14 | 0.06 |
| SGR | 0.21 | −0.21 | 0.44 |
| ADC CP | 0.23 | −0.12 | −0.27 |
| ADC TL | 0.20 | 0.35 | −0.35 |
| Protease | 0.20 | 0.4 | 0.34 |
| Pepsin | 0.20 | 0.41 | 0.20 |
| Lipase | 0.24 | −0.05 | 0.07 |
| d-ROMs | −0.23 | 0.16 | 0.20 |
| BAP | 0.23 | 0.13 | 0.15 |
| SOD | 0.20 | 0.40 | −0.33 |
| TBARs | −0.24 | −0.08 | 0.11 |
| Catalase | 0.23 | −0.11 | −0.35 |
| Lysozyme | 0.23 | −0.14 | 0.12 |
| NBT | 0.22 | −0.27 | 0.05 |
| Hematocrit | 0.21 | −0.30 | −0.28 |
| Peroxidase | 0.23 | −0.24 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mzengereza, K.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Yukun, Z.; Shadrack, R.S.; Seo, S.; Kotani, T.; Dossou, S.; Basuini, M.F.E.; et al. Growth Performance, Growth-Related Genes, Digestibility, Digestive Enzyme Activity, Immune and Stress Responses of de novo Camelina Meal in Diets of Red Seabream (Pagrus major). Animals 2021, 11, 3118. https://doi.org/10.3390/ani11113118
Mzengereza K, Ishikawa M, Koshio S, Yokoyama S, Yukun Z, Shadrack RS, Seo S, Kotani T, Dossou S, Basuini MFE, et al. Growth Performance, Growth-Related Genes, Digestibility, Digestive Enzyme Activity, Immune and Stress Responses of de novo Camelina Meal in Diets of Red Seabream (Pagrus major). Animals. 2021; 11(11):3118. https://doi.org/10.3390/ani11113118
Chicago/Turabian StyleMzengereza, Kumbukani, Manabu Ishikawa, Shunsuke Koshio, Saichiro Yokoyama, Zhang Yukun, Ronick S. Shadrack, Seok Seo, Tomonari Kotani, Serge Dossou, Mohammed F. El Basuini, and et al. 2021. "Growth Performance, Growth-Related Genes, Digestibility, Digestive Enzyme Activity, Immune and Stress Responses of de novo Camelina Meal in Diets of Red Seabream (Pagrus major)" Animals 11, no. 11: 3118. https://doi.org/10.3390/ani11113118
APA StyleMzengereza, K., Ishikawa, M., Koshio, S., Yokoyama, S., Yukun, Z., Shadrack, R. S., Seo, S., Kotani, T., Dossou, S., Basuini, M. F. E., & Dawood, M. A. O. (2021). Growth Performance, Growth-Related Genes, Digestibility, Digestive Enzyme Activity, Immune and Stress Responses of de novo Camelina Meal in Diets of Red Seabream (Pagrus major). Animals, 11(11), 3118. https://doi.org/10.3390/ani11113118









