SARS-Cov-2 Natural Infection in a Symptomatic Cat: Diagnostic, Clinical and Medical Management in a One Health Vision
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
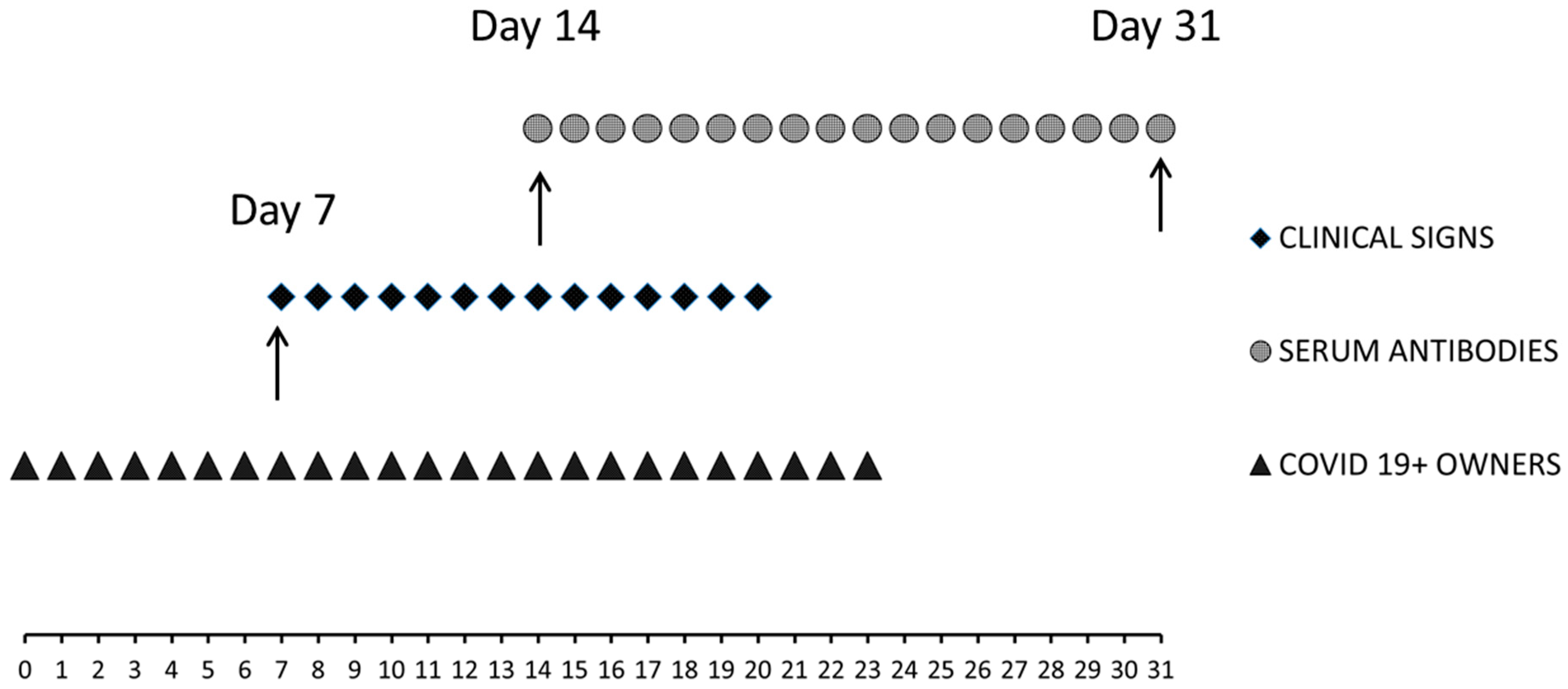
2.2. Time of the Study
2.3. Molecular Investigation: Nucleic Acid Extraction and Qualitative Real-Time Rt-Pcr Analyses
2.4. Serological Investigation
2.5. Electrochemiluminescence Immunoassay (ECLIA)
2.6. Elisa
2.7. Plaque Reduction Neutralization Test (Prnt)
2.8. Sequencing Analysis
2.9. Clinical Procedures
2.10. Haematology and Biochemistry
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.; Abdelwhab, E.M. Evidence for SARS-CoV-2 Infection of Animal Hosts. Pathogens 2020, 9, 529. [Google Scholar] [CrossRef]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Li, J.-L.; Yang, X.-L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef]
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.-J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nat. Cell Biol. 2020, 583, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Vale, B.D.; Lopes, A.P.; Fontes, M.D.C.; Silvestre, M.; Cardoso, L.; Coelho, A.C. Bats, pangolins, minks and other animals— villains or victims of SARS-CoV-2? Veter. Res. Commun. 2021, 45, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.-S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Reid, T.J. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound. Emerg. Dis. 2020, 13885. [Google Scholar] [CrossRef]
- Kumar, R.; Harilal, S.; Al-Sehemi, A.G.; Pannipara, M.; Behl, T.; Mathew, G.E.; Mathew, B. COVID-19 and Domestic Animals: Exploring the Species Barrier Crossing, Zoonotic and Reverse Zoonotic Transmission of SARS-CoV-2. Curr. Pharm. Des. 2021, 27, 1194–1201. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Kim, S.-G.; Kim, S.-M.; Kim, E.-H.; Park, S.-J.; Yu, K.-M.; Chang, J.-H.; Lee, S.; Casel, M.A.B.; Um, J.; et al. Infection and Rapid Transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe 2020, 27, 704–709.e2. [Google Scholar] [CrossRef]
- Hossain, G.; Javed, A.; Akter, S.; Saha, S. SARS-CoV-2 host diversity: An update of natural infections and experimental evidence. J. Microbiol. Immunol. Infect. 2021, 54, 175–181. [Google Scholar] [CrossRef]
- Gibbons, A. Captive gorillas test positive for coronavirus. Science 2021. [Google Scholar] [CrossRef]
- Patterson, E.I.; Elia, G.; Grassi, A.; Giordano, A.; Desario, C.; Medardo, M.; Smith, S.L.; Anderson, E.R.; Prince, T.; Patterson, G.T.; et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; Libanori Artiaga, B.; et al. SARS-CoV-2 Infection, Disease and Transmission in Domestic Cats Running Title: SARS-CoV-2 in Domestic Cats. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.08.04.235002v1 (accessed on 6 May 2021).
- Bosco-Lauth, A.M.; Jeffrey Root, J.; Porter, S.M.; Walker, A.E.; Guilbert, L.; Hawvermale, D.; Pepper, A.; Maison, R.M.; Hartwig, A.E.; Bielefeldt-Ohmann, H.; et al. Survey of Peridomestic Mammal Susceptibility to SARS-CoV-2 Infection. BioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.01.21.427629v1 (accessed on 6 May 2021). [CrossRef]
- Palmer, M.V.; Martins, M.; Falkenberg, S.; Buckley, A.; Caserta, L.C.; Mitchell, P.K.; Cassmann, E.D.; Rollins, A.; Zylich, N.C.; Renshaw, R.W.; et al. Susceptibility of White-Tailed Deer (Odocoileus Virginianus) to SARS-CoV-2. BioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.01.13.426628v1 (accessed on 6 May 2021). [CrossRef]
- McAloose, D.; Laverack, M.; Wang, L.; Killian, M.L.; Caserta, L.C.; Yuan, F.; Mitchell, P.K.; Queen, K.; Mauldin, M.R.; Cronk, B.D.; et al. From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio 2020, 11. [Google Scholar] [CrossRef]
- Gryseels, S.; De Bruyn, L.; Gyselings, R.; Calvignac-Spencer, S.; Leendertz, F.H.; Leirs, H. Risk of human-to-wildlife transmission of SARS-CoV-2. Mammal Rev. 2021, 51, 272–292. [Google Scholar] [CrossRef]
- Munnink, B.B.O.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2021, 371, 172–177. [Google Scholar] [CrossRef]
- Mykytyn, A.Z.; Lamers, M.M.; Okba, N.M.A.; Breugem, T.I.; Schipper, D.; Doel, P.B.V.D.; van Run, P.; van Amerongen, G.; de Waal, L.; Koopmans, M.P.G.; et al. Susceptibility of rabbits to SARS-CoV-2. Emerg. Microbes Infect. 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Leroy, E.M.; Gouilh, M.A.; Brugère-Picoux, J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health 2020, 10, 100133. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Smith, D.; Ghai, R.R.; Wallace, R.M.; Torchetti, M.K.; LoIacono, C.; Murrell, L.S.; Carpenter, A.; Moroff, S.; Rooney, J.A.; et al. First Reported Cases of SARS-CoV-2 Infection in Companion Animals—New York, March–April 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Abad, D.; Eiros, J.M.; Rodríguez-Lázaro, D. Are Animals a Neglected Transmission Route of SARS-CoV-2? Pathogens 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; De Oliveira-Filho, E.F.; Rasche, A.; Greenwood, A.D.; Osterrieder, K.; Drexler, J.F. Potential zoonotic sources of SARS-CoV-2 infections. Transbound. Emerg. Dis. 2020, 13872. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; Cardona-Trujillo, M.C.; García-Barco, A.; Holguin-Rivera, Y.; Cortes-Bonilla, I.; Bedoya-Arias, H.A.; Patiño-Cadavid, L.J.; Tamayo-Orozco, J.D.; Paniz-Mondolfi, A.; Zambrano, L.I.; et al. MERS-CoV and SARS-CoV Infections in Animals: A Systematic Review and Meta-Analysis of Prevalence Studies. Preprints 2020, 28 (Suppl. 1), 71–83. Available online: https://www.preprints.org/manuscript/202003.0103/v2 (accessed on 6 May 2021). [CrossRef]
- Stout, A.E.; André, N.M.; Jaimes, J.A.; Millet, J.K.; Whittaker, G.R. Coronaviruses in cats and other companion animals: Where does SARS-CoV-2/COVID-19 fit? Vet. Microbiol. 2020, 247, 108777. [Google Scholar] [CrossRef]
- Costagliola, A.; Liguori, G.; D’Angelo, D.; Costa, C.; Ciani, F.; Giordano, A. Do Animals Play a Role in the Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)? A Commentary. Animals 2020, 11, 16. [Google Scholar] [CrossRef]
- Hamer, S.A.; Pauvolid-Corrêa, A.; Zecca, I.B.; Davila, E.; Auckland, L.D.; Roundy, C.M.; Tang, W.; Torchetti, M.; Killian, M.L.; Jenkins-Moore, M.; et al. Natural SARS-CoV-2 Infections, Including Virus Isolation, among Serially Tested Cats and Dogs in Households with Con-firmed Human COVID-19 Cases in Texas, USA. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.12.08.416339v1 (accessed on 6 May 2021). [CrossRef]
- Sailleau, C.; Dumarest, M.; Vanhomwegen, J.; Delaplace, M.; Caro, V.; Kwasiborski, A.; Hourdel, V.; Chevaillier, P.; Barbarino, A.; Comtet, L.; et al. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound. Emerg. Dis. 2020, 67, 2324–2328. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Hatta, M.; Chiba, S.; Maemura, T.; Fan, S.; Takeda, M.; Kinoshita, N.; Hattori, S.-I.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; et al. Transmission of SARS-CoV-2 in Domestic Cats. N. Engl. J. Med. 2020, 383, 592–594. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef]
- Chiba, S.; Halfmann, P.J.; Hatta, M.; Maemura, T.; Fan, S.; Armbrust, T.; Swartley, O.M.; Crawford, L.K.; Kawaoka, Y. Protective Immunity and Persistent Lung Sequelae in Domestic Cats after SARS-CoV-2 Infection. Emerg. Infect. Dis. 2021, 27, 660–663. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Gao, J.; Huang, K.; Yang, Y.; Hui, X.; He, X.; Li, C.; Gong, W.; Zhang, Y.; et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg. Microbes Infect. 2020, 9, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, Y.; Sun, C.; Bai, J.; Sun, J.; Hao, L.; Li, X.; Tian, K. SARS-CoV-2 Serological Survey of Cats in China before and after the Pandemic. Virol. Sin. 2020, 35, 846–848. [Google Scholar] [CrossRef]
- Stevanovic, V.; Vilibic-Cavlek, T.; Tabain, I.; Benvin, I.; Kovac, S.; Hruskar, Z.; Mauric, M.; Milasincic, L.; Antolasic, L.; Skrinjaric, A.; et al. Seroprevalence of SARS-CoV-2 infection among pet animals in Croatia and potential public health impact. Transbound. Emerg. Dis. 2020, 13924. [Google Scholar] [CrossRef]
- Fritz, M.; Rosolen, B.; Krafft, E.; Becquart, P.; Elguero, E.; Vratskikh, O.; Denolly, S.; Boson, B.; Vanhomwegen, J.; Gouilh, M.A.; et al. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health 2020, 11, 100192. [Google Scholar] [CrossRef] [PubMed]
- Michelitsch, A.; Hoffmann, D.; Wernike, K.; Beer, M. Occurrence of Antibodies against SARS-CoV-2 in the Domestic Cat Population of Germany. Vaccines 2020, 8, 772. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Morozov, I.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020, 9, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Garigliany, M.; Van Laere, A.-S.; Clercx, C.; Giet, D.; Escriou, N.; Huon, C.; Van Der Werf, S.; Eloit, M.; Desmecht, D. SARS-CoV-2 Natural Transmission from Human to Cat, Belgium, March 2020. Emerg. Infect. Dis. 2020, 26, 3069–3071. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R.; Peiris, M.; Tam, K.W.; Law, P.Y.; Brackman, C.J.; To, E.M.; Yu, V.Y.; Chu, D.K.; Perera, R.A.; Sit, T.H. SARS-CoV-2 in Quarantined Domestic Cats from COVID-19 Households or Close Contacts, Hong Kong, China. Emerg. Infect. Dis. 2020, 26, 3071–3074. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Hofmann-Lehmann, R.; Hartmann, K.; Egberink, H.; Truyen, U.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Anthropogenic Infection of Cats during the 2020 COVID-19 Pandemic. Viruses 2021, 13, 185. [Google Scholar] [CrossRef]
- Klaus, J.; Meli, M.; Willi, B.; Nadeau, S.; Beisel, C.; Stadler, T.; Egberink, H.; Zhao, S.; Lutz, H.; Riond, B.; et al. Detection and Genome Sequencing of SARS-CoV-2 in a Domestic Cat with Respiratory Signs in Switzerland. Viruses 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Patients Infected with SARS-CoV-2: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Segalés, J.; Puig, M.; Rodon, J.; Avila-Nieto, C.; Carrillo, J.; Cantero, G.; Terrón, M.T.; Cruz, S.; Parera, M.; Noguera-Julián, M.; et al. Detection of SARS-CoV-2 in a Cat Owned by a COVID-19−affected Patient in Spain. Proc. Natl. Acad. Sci. USA 2020, 117, 24790–24793. [Google Scholar] [CrossRef]
- Klaus, J.; Palizzotto, C.; Zini, E.; Meli, M.L.; Leo, C.; Egberink, H.; Zhao, S.; Hofmann-Lehmann, R. SARS-CoV-2 Infection and Antibody Response in a Symptomatic Cat from Italy with Intestinal B-Cell Lymphoma. Viruses 2021, 13, 527. [Google Scholar] [CrossRef]
- De Morais, H.A.; dos Santos, A.P.; do Nascimento, N.C.; Kmetiuk, L.B.; Barbosa, D.S.; Brandão, P.E.; Guimarães, A.M.S.; Pettan-Brewer, C.; Biondo, A.W. Natural Infection by SARS-CoV-2 in Companion Animals: A Review of Case Reports and Current Evidence of Their Role in the Epidemiology of COVID-19. Front. Vet. Sci. 2020, 7, 591216. [Google Scholar] [CrossRef]
- World Health Organization. Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19) and Considerations during Severe Shortages; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Home Care for Patients with Suspected Novel Coronavirus (NCoV) Infection Presenting with Mild Symptoms and Management of Contacts; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- European Centre for Disease Prevention and control. Cloth Masks and Mask Sterilisation as Options in Case of Shortage of Surgical Masks and Respirators Use of Cloth Face Masks for Protection against COVID-19 in Clinical Settings. 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Cloth-face-masks-in-case-shortage-surgical-masks-respirators2020-03-26.pdf (accessed on 6 May 2021).
- European Centre for Disease Prevention and control. Infection Prevention and Control for COVID-19 in Healthcare Settings. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings (accessed on 6 May 2021).
- Hofmann-Lehmann, R.; Huder, J.B.; Gruber, S.; Boretti, F.; Sigrist, B.; Lutz, H. Feline Leukaemia Provirus Load during the Course of Experimental Infection and in Naturally Infected Cats. J. Gen. Virol. 2001, 82. [Google Scholar] [CrossRef]
- Meli, M.L.; Berger, A.; Willi, B.; Spiri, A.M.; Riond, B.; Hofmann-Lehmann, R. Molecular Detection of Feline Calicivirus in Clinical Samples: A Study Comparing Its Detection by RT-QPCR Directly from Swabs and after Virus Isolation. J. Virol. Methods 2018, 251. [Google Scholar] [CrossRef]
- Hussein, I.T.M.; Field, H.J. Development of a Quantitative Real-Time TaqMan PCR Assay for Testing the Susceptibility of Feline Herpesvirus-1 to Antiviral Compounds. J. Virol. Methods 2008, 152. [Google Scholar] [CrossRef]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Di Trani, L.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A Real-Time PCR Assay for Rapid Detection and Quantitation of Canine Parvovirus Type 2 in the Feces of Dogs. Vet. Microbiol. 2005, 105. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A Universal Heterologous Internal Control System for Duplex Real-Time RT-PCR Assays Used in a Detection System for Pestiviruses. J. Virol. Methods 2006, 136. [Google Scholar] [CrossRef]
- Neil Carey, R.; Paul Durham, A.; Hauck, W.W.; Kallner, A.; Kondratovich, M.V.; Guy Middle, J.; Pierson-Perry, J.F.; Smith, M.B.; Srinivasan, A. EP15-A3 User Verification of Precision and Estimation of Bias. In Approved Guideline, 3rd ed.; Clinical and Laboratory Standards Institute: Pittsburg, PA, USA, 2014; Volume 34. [Google Scholar]
- Padoan, A.; Bonfante, F.; Pagliari, M.; Bortolami, A.; Negrini, D.; Zuin, S.; Bozzato, D.; Cosma, C.; Sciacovelli, L.; Plebani, M. Analytical and Clinical Performances of Five Immunoassays for the Detection of SARS-CoV-2 Antibodies in Comparison with Neutralization Activity. EBioMedicine 2020, 62, 103101. [Google Scholar] [CrossRef]
- Houtgast, E.J.; Sima, V.M.; Bertels, K.; Al-Ars, Z. Hardware Acceleration of BWA-MEM Genomic Short Read Mapping for Longer Read Lengths. Comput. Biol. Chem. 2018, 75, 54–64. [Google Scholar] [CrossRef]
- Houtgast, E.J.; Sima, V.M.; Bertels, K.; Al-Ars, Z. Comparative Analysis of System-Level Acceleration Techniques in Bioinformatics: A Case Study of Accelerating the Smith-Waterman Algorithm for BWA-MEM. In Proceedings of the 2018 IEEE 18th International Conference on Bioinformatics and Bioengineering, BIBE 2018, Taichung, Taiwan, 29–31 October 2018. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Hopkins, S.; Gandy, A.; Rambaut, A.; Ferguson, N.M. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from Linking Epidemiological and Genetic Data. medRxiv 2021. [Google Scholar] [CrossRef]
- O’Toole, Á.; Hill, V.; McCrone, J.T.; Scher, E.; Rambaut, A. Pangolin COVID-19 Lineage Assigner Phylogenetic Assignment of Named Global Outbreak LINeages. Available online: https://pangolin.cog-uk.io/ (accessed on 23 December 2020).
- Trzil, J.E. Feline Asthma. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 375–391. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 6 May 2021).
- OIE. World Organization for Animal Health. Available online: https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/ (accessed on 6 May 2021).
- Promed International Society for Infectious Disease Homepage. Available online: https://promedmail.org/ (accessed on 6 May 2021).
- Wu, L.; Chen, Q.; Liu, K.; Wang, J.; Han, P.; Zhang, Y.; Hu, Y.; Meng, Y.; Pan, X.; Qiao, C.; et al. Broad Host Range of SARS-CoV-2 and the Molecular Basis for SARS-CoV-2 Binding to Cat ACE2. Cell Discov. 2020, 6, 68. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, D.; Szabla, R.; Zheng, M.; Li, G.; Du, P.; Zheng, S.; Li, X.; Song, C.; Li, R.; et al. Broad and Differential Animal Angiotensin-Converting Enzyme 2 Receptor Usage by SARS-CoV-2. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Von Roedern, M.; Buriko, Y.; Prittie, J.; Lamb, K. Investigation of Iron Status and Markers of Inflammation in Anaemic and Non-Anaemic Hospitalised Cats. J. Small Anim. Pract. 2017, 58. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.S.; Kinnaird, E.; Baglioni, A.; Blackshaw, J.; Priest, J. Acute Stress Hyperglycemia in Cats Is Associated with Struggling and Increased Concentrations of Lactate and Norepinephrine. J. Vet. Intern. Med. 2002, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, J.; Duke, T.; Focken, A.P.; Snead, E.C.; Cosford, K.L. Effects of Dexmedetomidine on Glucose Homeostasis in Healthy Cats. J. Feline Med. Surg. 2020, 22, 344–349. [Google Scholar] [CrossRef]
- Paltrinieri, S. The Feline Acute Phase Reaction. Vet. J. 2008, 177, 26–35. [Google Scholar] [CrossRef]
- Willard, M.D.; Tvedten, H. Small Animal Clinical Diagnosis by Laboratory Methods, 5th ed.; Saunders: St. Louis, MO, USA, 2011. [Google Scholar]
- Fernandez, N.J.; Kidney, B.A. Alkaline Phosphatase: Beyond the Liver. Vet. Clin. Pathol. 2007, 36, 223–233. [Google Scholar] [CrossRef]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef]
- Kajikawa, T.; Furuta, A.; Onishi, T.; Tajima, T.; Sugii, S. Changes in Concentrations of Serum Amyloid A Protein, A1-Acid Glycoprotein, Haptoglobin, and C-Reactive Protein in Feline Sera Due to Induced Inflammation and Surgery. Vet. Immunol. Immunopathol. 1999, 68, 91–98. [Google Scholar] [CrossRef]
- Marly-Voquer, C.; Riond, B.; Jud Schefer, R.; Kutter, A.P.N. Reference Values for Rotational Thromboelastometry (ROTEM) in Clinically Healthy Cats. J. Vet. Emerg. Crit. Care 2017, 27, 185–192. [Google Scholar] [CrossRef]
- Solbak, S.; Epstein, S.E.; Hopper, K. Influence of Needle Gauge Used for Venipuncture on Measures of Hemostasis in Cats. J. Feline Med. Surg. 2019, 21, 143–147. [Google Scholar] [CrossRef]
- Khelik, I.A.; Berger, D.J.; Mochel, J.P.; Seo, Y.-J.; Palerme, J.-S.; Ware, W.A.; Ward, J.L. Clinicopathologic, Hemodynamic, and Echocardiographic Effects of Short-Term Oral Administration of Anti-Inflammatory Doses of Prednisolone to Systemically Normal Cats. Am. J. Vet. Res. 2019, 80, 743–755. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Huang, K.; Yang, Y.; Hui, X.; Gao, J.; He, X.; Li, C.; Gong, W.; Zhang, Y.; et al. SARS-CoV.2 Neutralizing Serum Antibodies in Cats: A Serological Investigation. bioRxiv Prepr. Serv. Biol. 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.04.01.021196v1 (accessed on 6 May 2021). [CrossRef]
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the Effects of SARS-CoV-2 Spike Mutation D614G on Transmissibility and Pathogenicity. Cell 2021, 184, 64–75.e11. [Google Scholar] [CrossRef]
- Davis, M.F.; Innes, G.K. The Cat’s in the Bag: Despite Limited Cat-to-Cat SARS-CoV-2 Transmission, One Health Surveillance Efforts Are Needed. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Ferasin, L.; Fritz, M.; Ferasin, H.; Legros, V.; Leroy, E.M. Myocarditis in Naturally Infected Pets with the British Variant of COVID-19. bioRxiv 2021, 2021.03.18.435945. Available online: https://www.biorxiv.org/content/10.1101/2021.03.18.435945v1 (accessed on 6 May 2021). [CrossRef]
- Centers for Disease Control and Prevention. Interim Guidance for Public Health Professionals Managing People with COVID-19 in Home Care and Isolation Who Have Pets or Other Animals. Available online: https://www.cdc.gov/coronavirus/2019-ncov/animals/interimguidance-%0Amanaging-people-in-home-care-and-isolation-who-have-pets.html (accessed on 6 May 2021).
| Haematology | IDEXX VetConnect® PLUS | Laboratory Medicine (IZSVE) |
|---|---|---|
| Day 14 | Day 31 | |
| RBC (M/μL) | 6.9 (7.1–11.5) | 7.69 (5.1–10) |
| Hgb (g/dL) | 9.4 (10.3–16.2) | 11.1 (8–15) |
| Hct (%) | 32.6 (28.2–52.7) | 31.4 (30.0–45.0) |
| MCV (fL) | 47.2 (39–56) | 40.8 (39–55) |
| MCH (pg) | 13.6 (12.6–16.5 pg) | 14.4 (13.0–17.0) |
| MCHC (g/dL) | 28.8 (28.5–37.8) | 35.4 (30.0–36.0) |
| Reticulocytes (K/μL) | 8.3 * | - |
| RDW (%) | - | 17.3 (16.4–21.7) |
| PLT (K/μL) | 133(155–641) | 430 (100–400) |
| MPV (fL) | - | 13.3 (8.1–15.4) |
| WBC (K/μL) | 7.1 (3.9–19) | 7.24 K/μL (5–19) |
| NEUT (K/μL) | 4.52 (2.62–15.17) | 3.79 (1.80–14.80) |
| LYMPH (K/μL) | 1.98 (0.85–5.85) | 3.29 (1.10–8.60) |
| MONO (K/μL) | 0.28 (0.04–0.53) | 0.09 (0.05–0.80) |
| EO (K/μL) | 0.28 (0.09–2.18) | 0.06 (0.05–2.30) |
| BASO (K/μL) | 0 (0.09–2.18) | 0.01 (0.00–0.80) |
| BIOCHEMISTRY | Day 14 | Day 31 | Reference Values |
|---|---|---|---|
| Haptoglobin 1 | 99 | 18 | 18–74 mg/dL |
| Serum Amyloid A 2 | <5.0 | <5.0 | 0–9 μg/mL |
| Total Proteins | 68 g/L | 72 g/L | 62–80 g/L |
| Albumin | 38 g/L | 40 g/L | 30–47 g/L |
| Globuline | 30 g/L | 32 g/L | 22–47 g/L |
| Ratio A/G | 1.28 | 1.40 | 1.07–1.87 |
| Urea Nitrogen | 10.0 | 7.3 | 4.8–12.6 mmol/L |
| Creatinine | 150 | 122 | 66–178 μmol/L |
| Glucose | 13.9 | 11.7 | 3.2–8.9 mmol/L |
| Cholesterol | 4.37 | 4.79 | 1.35–6.09 mmol/L |
| Triglycerides | 0.58 | 4.21 | 0–2.48 mmol/L |
| Total Bilirubine | <2.5 | <2.5 | 0–8.55 μmol/L |
| Direct Birubine | <1.5 | <1.5 | 0–2.56 μmol/L |
| Unconj Bilirubine | 0 | 0 | 0–6.5 μmol/L |
| AST | 15 | 29 | 0–61 U/L |
| ALT | 27 | 77 | 19–71 U/L |
| ALP | <5 | 17 | 6–46 U/L |
| GGT | <3 | <3 | 1–5 U/L |
| Cholinesterase | 1245 | 1781 | 1749–2905 U/L |
| CK | 11 | 141 | 0–305 U/L |
| Calcium | 2.90 | 2.38 | 2.26–2.73 mmol/L |
| Phosphorus | 1.43 | 1.03 | 0.94–1.98 mmol/L |
| Magnesium | 0.92 | 0.88 | 0.79–1.07 mmol/L |
| Sodium | 152 | 151 | 141–168 mmol/L |
| Potassium | 4.37 | 4.29 | 3.55–5.15 mmol/L |
| Chlorine | 114 | 112 | 103–126 mmol/L |
| Iron | 74 μg/dL | 82 | 68–215 μg/dL |
| Uibc | 168 μg/dL | * | 105–205 μg/dL |
| Tibc | 242 μg/dL | * | 222–423 μg/dL |
| Saturated Transferrin | 30.6% | * | 20–56% |
| Serum Protein Electrophoresis | Day 14 | Day 31 | Reference Values |
|---|---|---|---|
| Albumin (%) | 56.2 | 58.4 | 52.4–66.2 |
| Alpha 1 (%) | 1.3 | 1.9 | 0.8–1.9 |
| Alpha 2 (%) | 15.6 | 18.6 | 7.4–15.4 |
| Beta 1 (%) | 9.0 | 5.7 | 4.5–6.2 |
| Beta 2 (%) | 5.5 | 5.8 | 4.5–8 |
| Gamma (%) | 12.4 | 9.6 | 8.5–24.2 |
| Albumin (g/L) | 38.2 | 42.0 | 35.7–48.7 |
| Alpha 1 (g/L) | 0.9 | 1.4 | 0.6–1.3 |
| Alpha 2 (g/L) | 10.6 | 13.4 | 5.6–10.6 |
| Beta 1 (g/L) | 6.1 | 4.1 | 3–4.7 |
| Beta 2 (g/L) | 3.7 | 4.2 | 3.2–5.8 |
| Gamma (g/L) | 8.4 | 6.9 | 5.1–18.3 |
| A/G Ratio | 1.28 | 1.40 | 1.07–1.87 |
| Day 14 | Day 31 | |||||||
|---|---|---|---|---|---|---|---|---|
| - | Ct Values | Conclusive Laboratory Diagnosis | Ct Values | Conclusive Laboratory Diagnosis | ||||
| Swab | E Gene | N Gene | RdRp Gene | - | E Gene | N Gene | RdRp Gene | - |
| OP | 30.14 | 36.38 | 39.60 | Positive | n.d. | n.d, | n.d, | Negative |
| N | 27.83 | 34.47 | 36.00 | Positive | 36.00 | n.d. | n.d. | Positive |
| R | n.d. | n.d. | n.d. | Negative | n.d. | n.d. | n.d. | Negative |
| SEROLOGY SARS-CoV-2 | Day 14 | Day 31 | Reference Ranges |
|---|---|---|---|
| ELISA KIT 1 | NEGATIVE | POSITIVE (68%) | Cut-off ≥ 60% |
| ELISA KIT 2 | POSITIVE (33.6%) | POSITIVE (20.8%) | Cut-off ≥ 20% |
| ECLIA | 47.20 U/mL | 1598 U/mL | POSITIVE ≥ 0.8 U/mL |
| PNRT | 1:5120 | 1:2560 | <1:10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natale, A.; Mazzotta, E.; Mason, N.; Ceglie, L.; Mion, M.; Stefani, A.; Fincato, A.; Bonfante, F.; Bortolami, A.; Monne, I.; et al. SARS-Cov-2 Natural Infection in a Symptomatic Cat: Diagnostic, Clinical and Medical Management in a One Health Vision. Animals 2021, 11, 1640. https://doi.org/10.3390/ani11061640
Natale A, Mazzotta E, Mason N, Ceglie L, Mion M, Stefani A, Fincato A, Bonfante F, Bortolami A, Monne I, et al. SARS-Cov-2 Natural Infection in a Symptomatic Cat: Diagnostic, Clinical and Medical Management in a One Health Vision. Animals. 2021; 11(6):1640. https://doi.org/10.3390/ani11061640
Chicago/Turabian StyleNatale, Alda, Elisa Mazzotta, Nicoletta Mason, Letizia Ceglie, Monica Mion, Annalisa Stefani, Alice Fincato, Francesco Bonfante, Alessio Bortolami, Isabella Monne, and et al. 2021. "SARS-Cov-2 Natural Infection in a Symptomatic Cat: Diagnostic, Clinical and Medical Management in a One Health Vision" Animals 11, no. 6: 1640. https://doi.org/10.3390/ani11061640
APA StyleNatale, A., Mazzotta, E., Mason, N., Ceglie, L., Mion, M., Stefani, A., Fincato, A., Bonfante, F., Bortolami, A., Monne, I., Bellinati, L., Guadagno, C., Quaranta, E., Pastori, A., & Terregino, C. (2021). SARS-Cov-2 Natural Infection in a Symptomatic Cat: Diagnostic, Clinical and Medical Management in a One Health Vision. Animals, 11(6), 1640. https://doi.org/10.3390/ani11061640










