Expression Characteristics of microRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Treatment
2.3. Analysis of Sequencing Data
2.4. Target Prediction and Functional Annotation of Target Genes
2.5. Real-Time Quantitative PCR
2.6. Blood Routine Examination
2.7. Statistical Analysis
3. Results
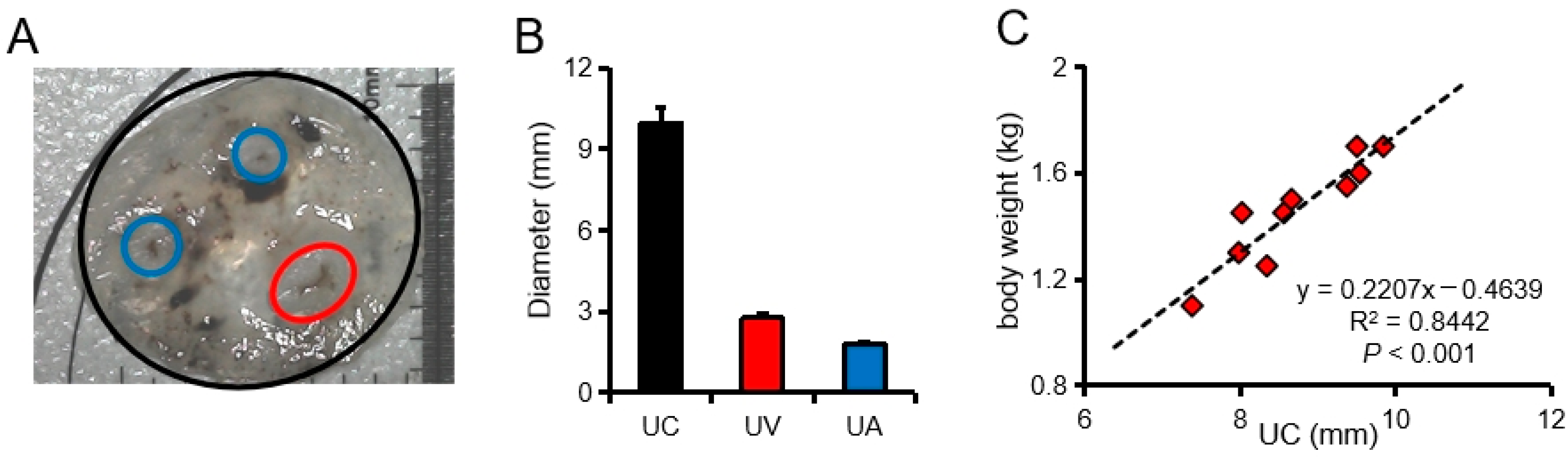
3.1. Characteristics of Pig Umbilical Cord
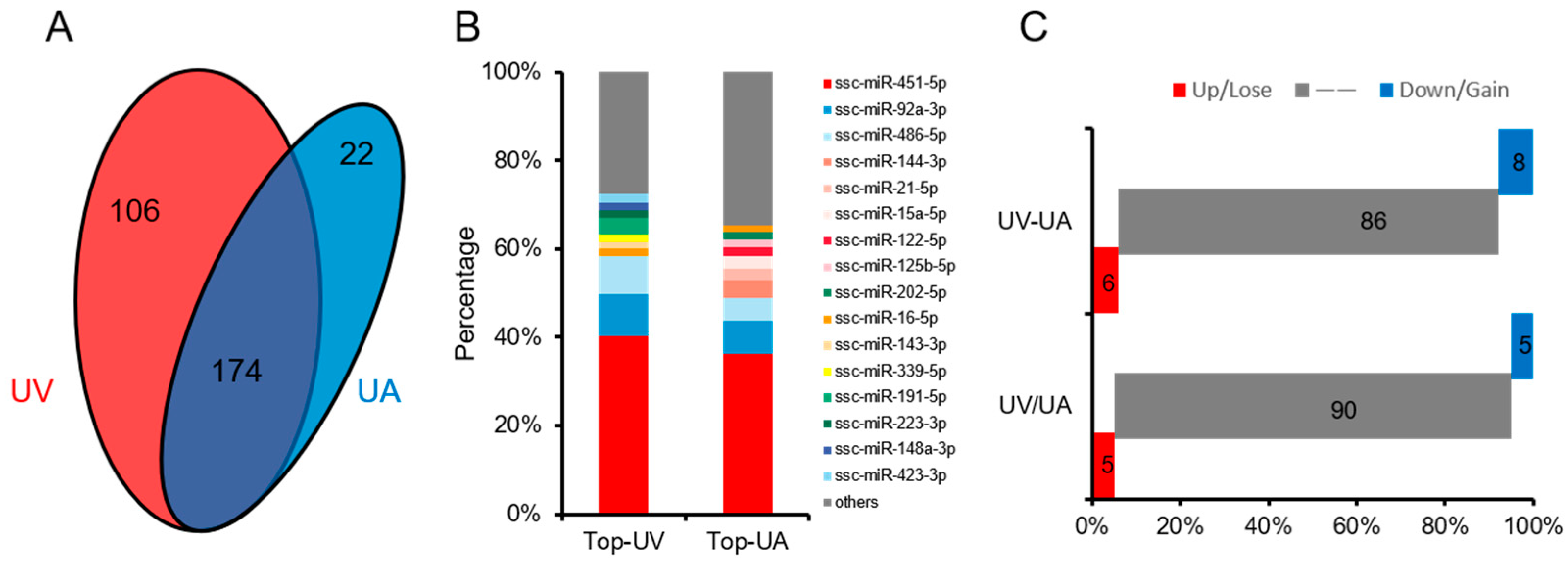
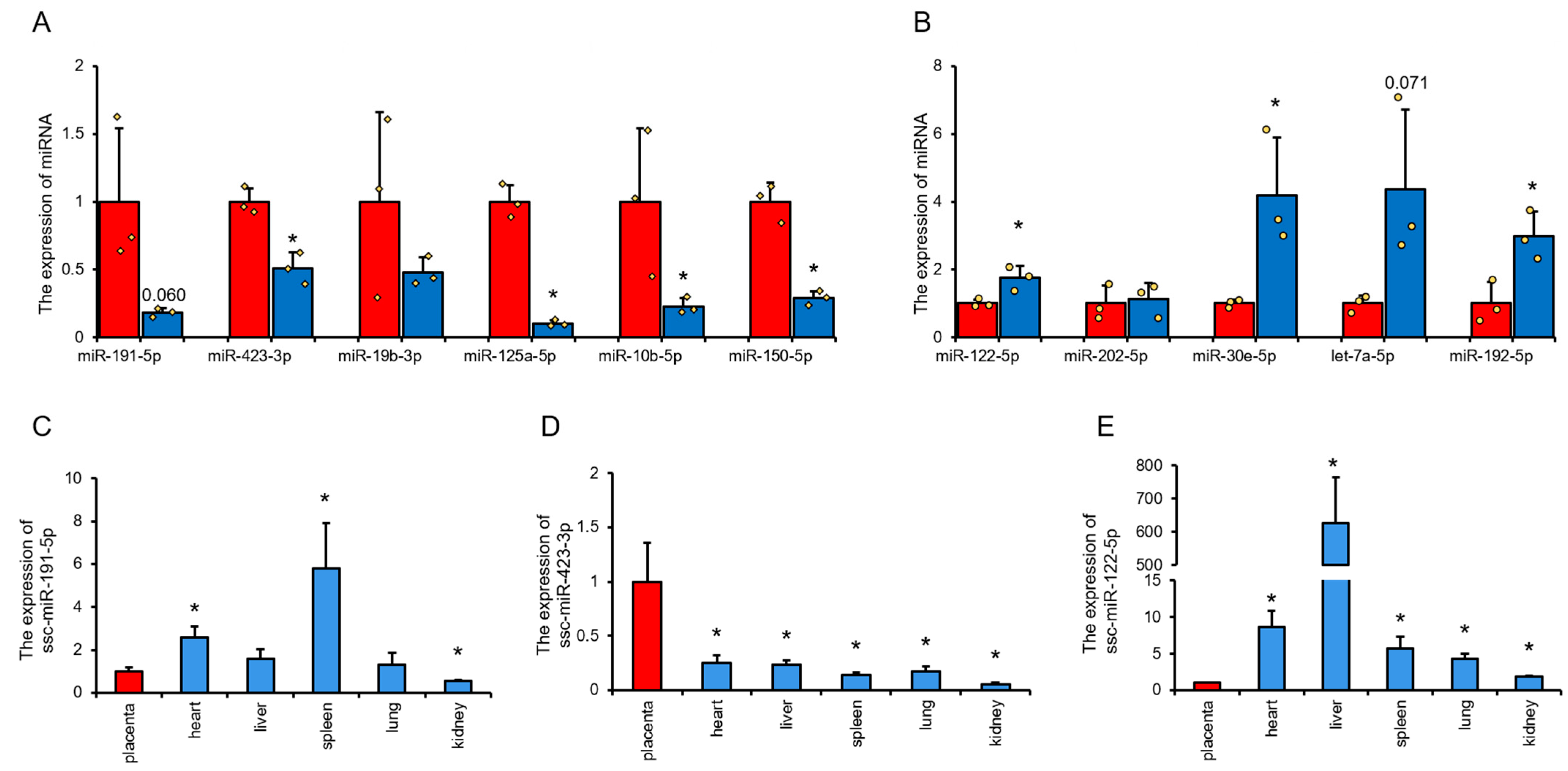
3.2. Expression Analysis of miRNAs in Pig UVB and UAB
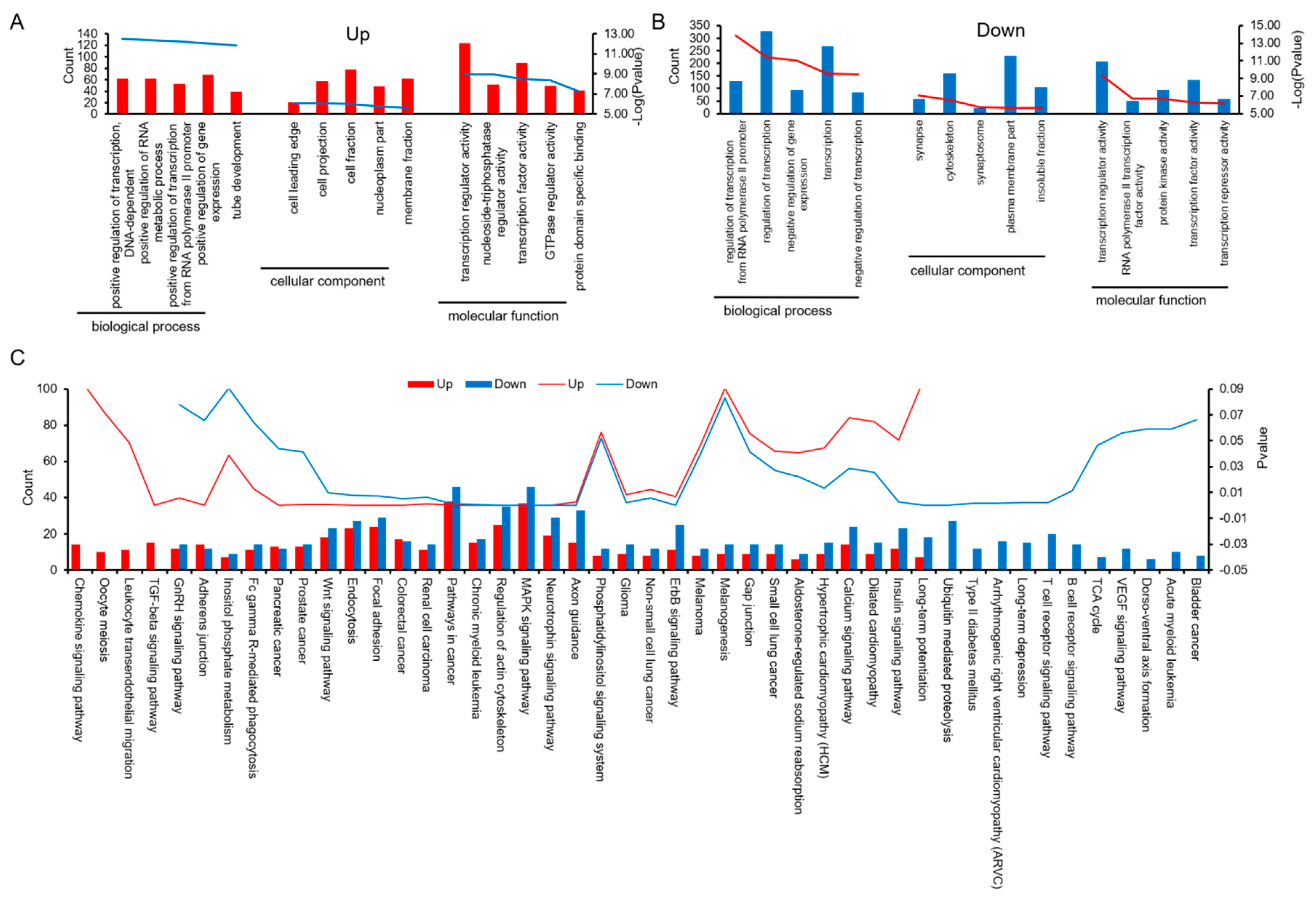
3.3. Prediction of miRNA Target Genes, and Analysis of GO and KEGG Pathways
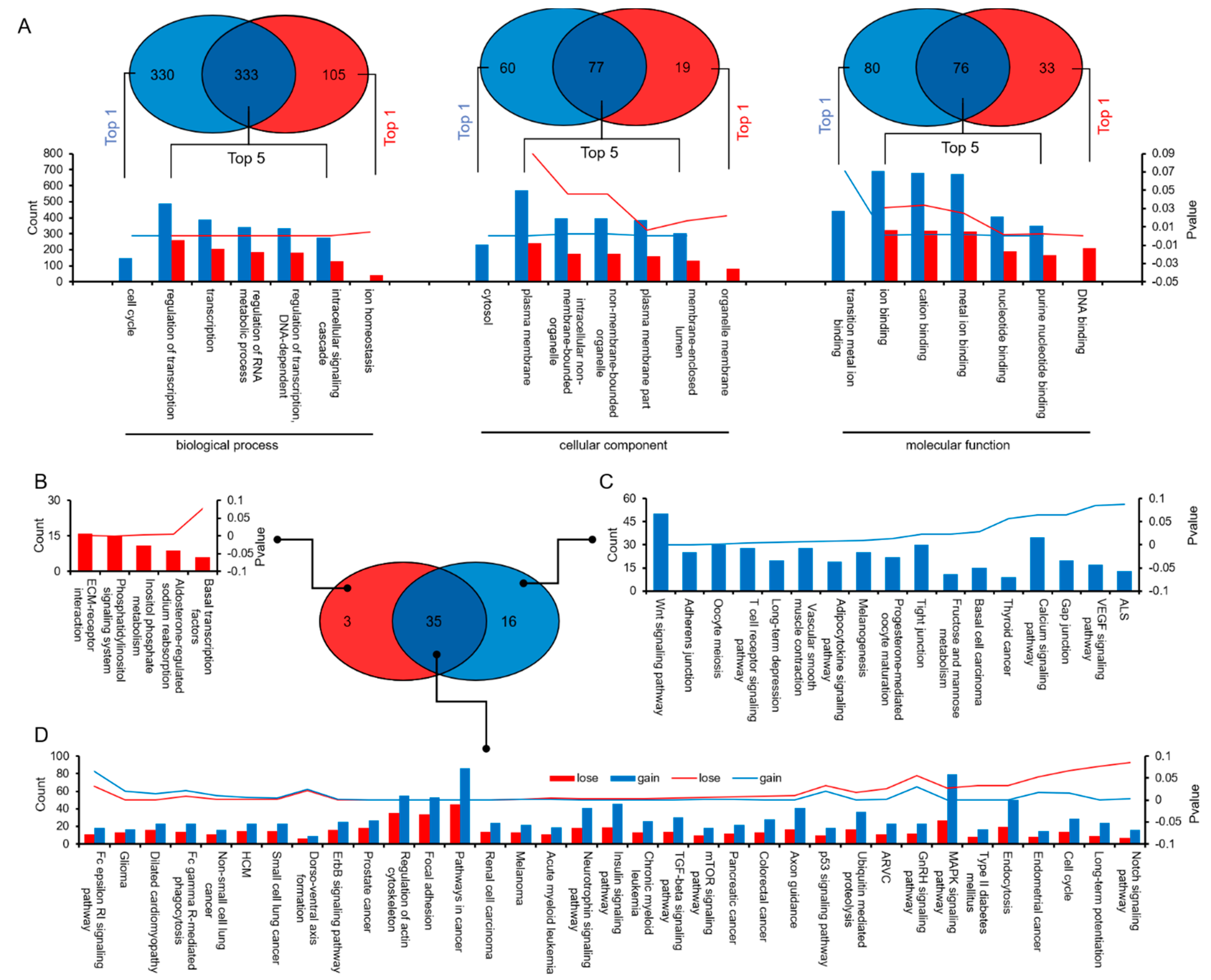
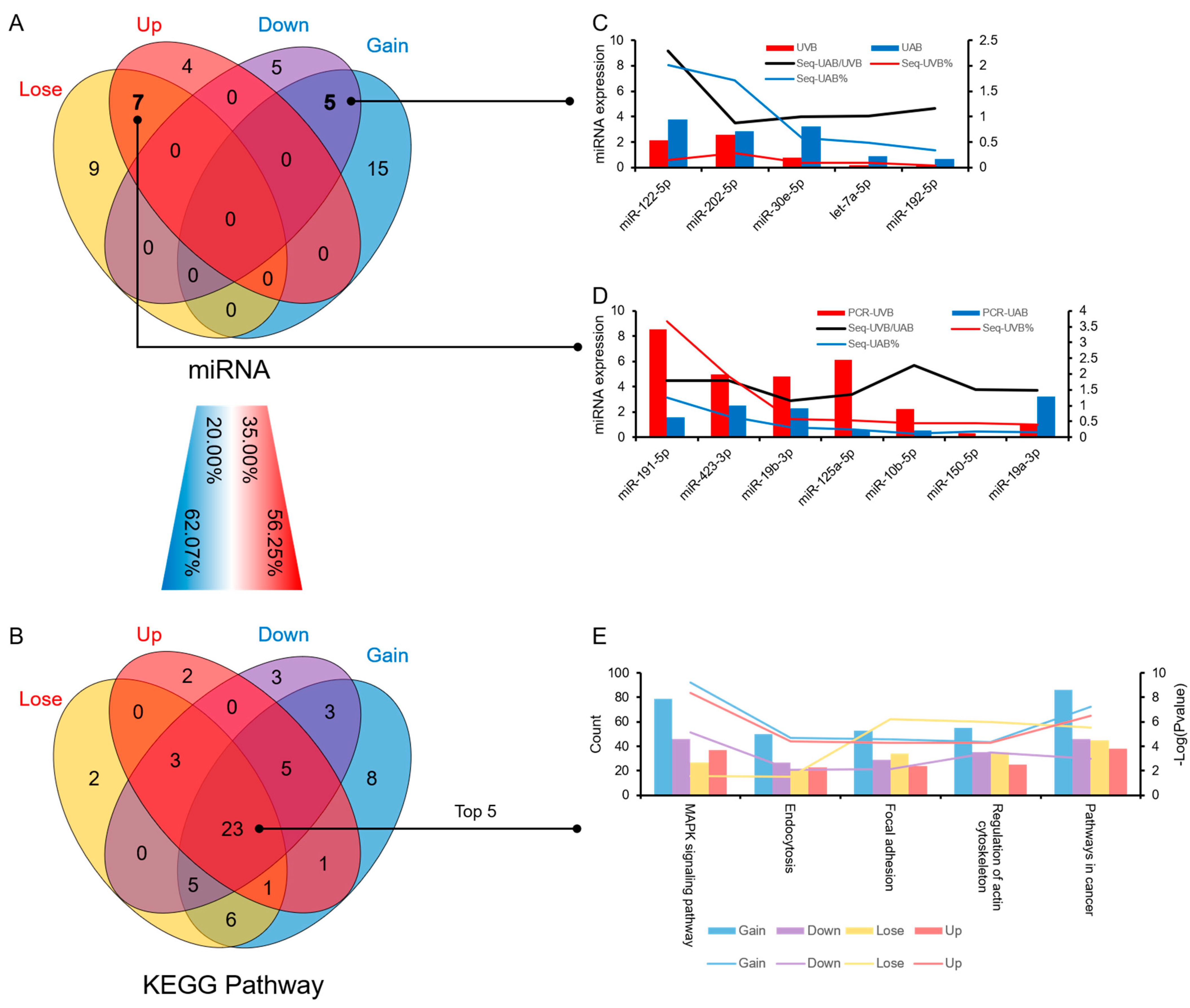
3.4. Similarities and Differences between the Two Differential Expression Analysis Modes
3.5. The Influence of Typical Differential miRNAs in Umbilical Cord Blood on Disease Occurrence and Its Potential Application Value
4. Discussion
4.1. Characteristics of miRNA Expression in Swine Cord Venous Blood and Umbilical Artery Blood
4.2. Potential Application of miRNAs from Porcine Cord Venous Blood and Cord Arterial Blood
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figueras, F.; Fernandez, S.; Hernandez Andrade, E.; Gratacós, E. Umbilical venous blood flow measurement: Accuracy and reproducibility. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 32, 587–591. [Google Scholar] [CrossRef]
- Lazarus, H.; Pavletic, S. Umbilical cord blood-derived mesenchymal stromal cells for reducing chronic graft-versus-host disease after haploidentical transplantation: Just another labor-intensive strategy, or showing the way? J. Clin. Oncol. 2016, 34, 2812–2813. [Google Scholar] [CrossRef]
- Forraz, N.; McGuckin, C. The umbilical cord: A rich and ethical stem cell source to advance regenerative medicine. Cell Prolif. 2011, 44 (Suppl. 1), 60–69. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Y.; Liu, X.; Ye, S.; Yu, K.; Huang, Z.; Yu, J.; Zhou, X.; Chen, H.; Mo, D. Integrative analysis of porcine micrornaome during skeletal muscle development. PLoS ONE 2013, 8, e72418. [Google Scholar] [CrossRef]
- Ørom, U.; Nielsen, F.; Lund, A. Microrna-10a binds the 5′utr of ribosomal protein mrnas and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xiao, L.; Zhang, Y.; Li, P.; Wu, Y.; Lin, Y. Mir-26b-3p regulates osteoblast differentiation via targeting estrogen receptor α. Genomics 2019, 111, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Rayner, K. Macrophage mirnas in atherosclerosis. Biochim. Biophys. Acta 2016, 1861, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, Q.; Jia, Y.; Cong, R.; Ni, Y.; Yang, X.; Jiang, Z.; Zhao, R. Coordinated mirna/mrna expression profiles for understanding breed-specific metabolic characters of liver between erhualian and large white pigs. PLoS ONE 2012, 7, e38716. [Google Scholar] [CrossRef]
- Ruvkun, G. Molecular biology. Glimpses of a tiny rna world. Science 2001, 294, 797–799. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant mir168a specifically targets mammalian ldlrap1: Evidence of cross-kingdom regulation by microrna. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.; Ahmad, H.; Shen, M.; Zhao, Y.; Gan, Z.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Dietary curcumin supplementation increases antioxidant capacity, upregulates nrf2 and hmox1 levels in the liver of piglet model with intrauterine growth retardation. Nutrients 2019, 11, 2978. [Google Scholar] [CrossRef] [PubMed]
- Furlow, B. Living pig bioreactors can repair damaged donor lungs. Lancet Respir. Med. 2020, 8, e72. [Google Scholar] [CrossRef]
- Vermeulen, L.; Van Beirendonck, S.; Bulens, A.; Thielen, J.; Driessen, B. The perception about batch management production systems among pig producers. Can. J. Anim. Sci. 2016, 97, 109–117. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, S.; Wang, X.; Qazi, I.; Long, K.; Luo, Y.; Li, G.; Ning, C.; Wang, Y.; Hu, S.; et al. Exploration of exosomal microrna expression profiles in pigeon ‘milk’ during the lactation period. BMC Genom. 2018, 19, 828. [Google Scholar] [CrossRef]
- Luo, J.; Fan, Y.; Shen, L.; Niu, L.; Zhao, Y.; Jiang, D.; Zhu, L.; Jiang, A.; Tang, Q.; Ma, J.; et al. The pro-angiogenesis of exosomes derived from umbilical cord blood of intrauterine growth restriction pigs was repressed associated with mirnas. Int. J. Biol. Sci. 2018, 14, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Hu, J.; Xiao, P.; Zhan, S.; Wang, L.; Guo, J.; Li, L.; Zhang, H.; Niu, L. Capra hircusidentification and characterization of micrornas in the goat (capra hircus) rumen during embryonic development. Front. Genet. 2017, 8, 163. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. Mirbase: Tools for microrna genomics. Nucleic Acids Res 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.; Nam, J.; Bartel, D. Predicting effective microrna target sites in mammalian mrnas. Elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. Mirdb: An online database for prediction of functional microrna targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.; Lempicki, R. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Proietti, F.; De Bernardo, G.; Longini, M.; Sordino, D.; Scaramuzzini, G.; Tataranno, M.; Belvisi, E.; Bazzini, F.; Perrone, S.; Buonocore, G. Neonatal oxidative stress depends on oxygen blood pressure in umbilical artery. J. Biol. Regul. Homeost. Agents 2016, 30, 929–934. [Google Scholar]
- Saw, S.; Dawn, C.; Biswas, A.; Mattar, C.; Yap, C. Characterization of the in vivo wall shear stress environment of human fetus umbilical arteries and veins. Biomech. Modeling Mechanobiol. 2017, 16, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free micrornas in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Sun, B.; Ji, W.; Peng, Z.; Xu, Y.; Wu, M.; Su, C. An artificially designed interfering lncrna expressed by oncolytic adenovirus competitively consumes oncomirs to exert antitumor efficacy in hepatocellular carcinoma. Mol. Cancer Ther. 2016, 15, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zhang, S.; Fan, Y.; Tan, Y.; Guo, Z.; Chen, L.; Bai, L.; Jiang, D.; Hao, X.; Li, X.; et al. The expression of microrna in adult rat heart with isoproterenol-induced cardiac hypertrophy. Cells 2020, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yu, Y.; Niu, B.; Wang, D. Red blood cells as potential repositories of micrornas in the circulatory system. Front. Genet. 2020, 11, 442. [Google Scholar] [CrossRef]
- Bianchi, N.; Zuccato, C.; Finotti, A.; Lampronti, I.; Borgatti, M.; Gambari, R. Involvement of mirna in erythroid differentiation. Epigenomics 2012, 4, 51–65. [Google Scholar] [CrossRef]
- Zhang, Z.W.; An, Y.; Teng, C.B. The roles of mir-17-92 cluster in mammal development and tumorigenesis. Yi Chuan Hered. 2009, 31, 1094–1100. [Google Scholar] [CrossRef]
- Kiani, M.; Salehi, M.; Mogheiseh, A.; Mohammadi-Yeganeh, S.; Shahidi, S. The effect of increased mir-16-1 levels in mouse embryos on epigenetic modification, target gene expression, and developmental processes. Reprod. Sci. 2020, 27, 2197–2210. [Google Scholar] [CrossRef]
- Sun, T.; Li, W.; Li, T.; Ling, S. Microrna profiling of amniotic fluid: Evidence of synergy of micrornas in fetal development. PLoS ONE 2016, 11, e0153950. [Google Scholar] [CrossRef] [PubMed]
- Jakobovits, A.; Jorn, H. Fetal blood circulation in multiple pregnancy. Orv. Hetil. 1995, 136, 285–289. [Google Scholar]
- Clark, E.; Kalomoiris, S.; Nolta, J.; Fierro, F. Concise review: Microrna function in multipotent mesenchymal stromal cells. Stem Cells 2014, 32, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Shchurevska, O.; Zhuk, S. Assessment of correlation between mirnas-21-3p and -210-3p expression in maternal and umbilical cord plasma and fetal weight at birth. Wiadomosci Lekarskie 2021, 74, 236–240. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Ivankova, K.; Vedmetskaya, Y.; Krofta, L. Profiling of cardiovascular and cerebrovascular disease associated microrna expression in umbilical cord blood in gestational hypertension, preeclampsia and fetal growth restriction. Int. J. Cardiol. 2017, 249, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Liu, L.; Lei, Y.; Hu, Y. Mirna-142-3p increases radiosensitivity in human umbilical cord blood mononuclear cells by inhibiting the expression of cd133. Sci. Rep. 2018, 8, 5674. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhou, X.; Huang, X.; Xu, X.; Jia, Y.; Wu, Y.; Yao, J.; Wu, Y.; Wang, K. Maternal and umbilical cord serum-derived exosomes enhance endothelial cell proliferation and migration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4534–4543. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Chen, B.; Li, X.; Kang, J.; Fan, K.; Hu, Y.; Xu, J.; Yi, L.; Yang, J.; et al. Mammalian ncrna-disease repository: A global view of ncrna-mediated disease network. Cell Death Dis 2013, 4, e765. [Google Scholar] [CrossRef]
| Indexes | WBC (109/L) | RBC (1012/L) | HGB (g/L) | HCT (%) | MCV (fL) |
|---|---|---|---|---|---|
| UV | 19.43 ± 9.56 | 8.48 ± 1.27 | 112.67 ± 14.29 | 55.63 ± 9.65 | 65.53 ± 1.50 |
| UA | 35.7 ± 15.00 | 8.33 ± 0.98 | 115.33 ± 12.74 | 57.13 ± 9.58 | 68.40 ± 3.72 |
| miRNA | Disease | Pattern | Tissue |
|---|---|---|---|
| miR-122-5p | AIDS dementia complex | Down | Brain |
| Myasthenia gravis, autoimmune, experimental | Down | Blood | |
| Stroke, lacunar | Down | Blood | |
| Huntington’s disease | Down | Cell line | |
| Cardiovascular diseases | Down | Cell line | |
| Hepatitis C | Down | Liver | |
| Hepatitis B | Down | Hepatocytes | |
| Malignant glioma | Down | Cell line | |
| Amyotrophic lateral sclerosis | Down | Spinal cord | |
| Liver cancer | Down | Serum | |
| Cervical squamous cell carcinoma | Down | Tissue | |
| Hepatocellular carcinoma | Down | Serum | |
| Type 2 diabetes mellitus | Down | Cell line | |
| Fatty liver disease | Down | Cell line | |
| HCV | Down | Liver | |
| miR-423-3p | Dermatomyositis | Down | Muscle |
| Duchenne muscular dystrophy | Down | Muscle | |
| Temporal lobe epilepsy | Down | Brain | |
| Inclusion body myositis | Down | Muscle |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, M.; Liu, L.; Zhang, S.; Guo, Z.; Tan, Y.; Luo, J.; Yang, Q.; Pan, H.; Li, X.; Wang, J.; et al. Expression Characteristics of microRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood. Animals 2021, 11, 1563. https://doi.org/10.3390/ani11061563
Gan M, Liu L, Zhang S, Guo Z, Tan Y, Luo J, Yang Q, Pan H, Li X, Wang J, et al. Expression Characteristics of microRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood. Animals. 2021; 11(6):1563. https://doi.org/10.3390/ani11061563
Chicago/Turabian StyleGan, Mailin, Lin Liu, Shunhua Zhang, Zongyi Guo, Ya Tan, Jia Luo, Qiong Yang, Hongmei Pan, Xuewei Li, Jinyong Wang, and et al. 2021. "Expression Characteristics of microRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood" Animals 11, no. 6: 1563. https://doi.org/10.3390/ani11061563
APA StyleGan, M., Liu, L., Zhang, S., Guo, Z., Tan, Y., Luo, J., Yang, Q., Pan, H., Li, X., Wang, J., Shen, L., & Zhu, L. (2021). Expression Characteristics of microRNA in Pig Umbilical Venous Blood and Umbilical Arterial Blood. Animals, 11(6), 1563. https://doi.org/10.3390/ani11061563









