Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Horses
2.3. Human Subjects
2.4. Study Design
- An equine-assisted session with the horse ridden by a child with ASD (ASD session).
- Control session with the horse ridden by a TD child (TD session).
2.5. Setting
2.6. Experimental Procedure
2.6.1. Blood Sampling
2.6.2. Behavioural Analysis
2.6.3. Infrared Thermography (IRT)
2.6.4. Heart Rate and Heart Rate Variability
2.7. Statistical Analysis
3. Results
3.1. ACTH, Cortisol, and Catecholamine
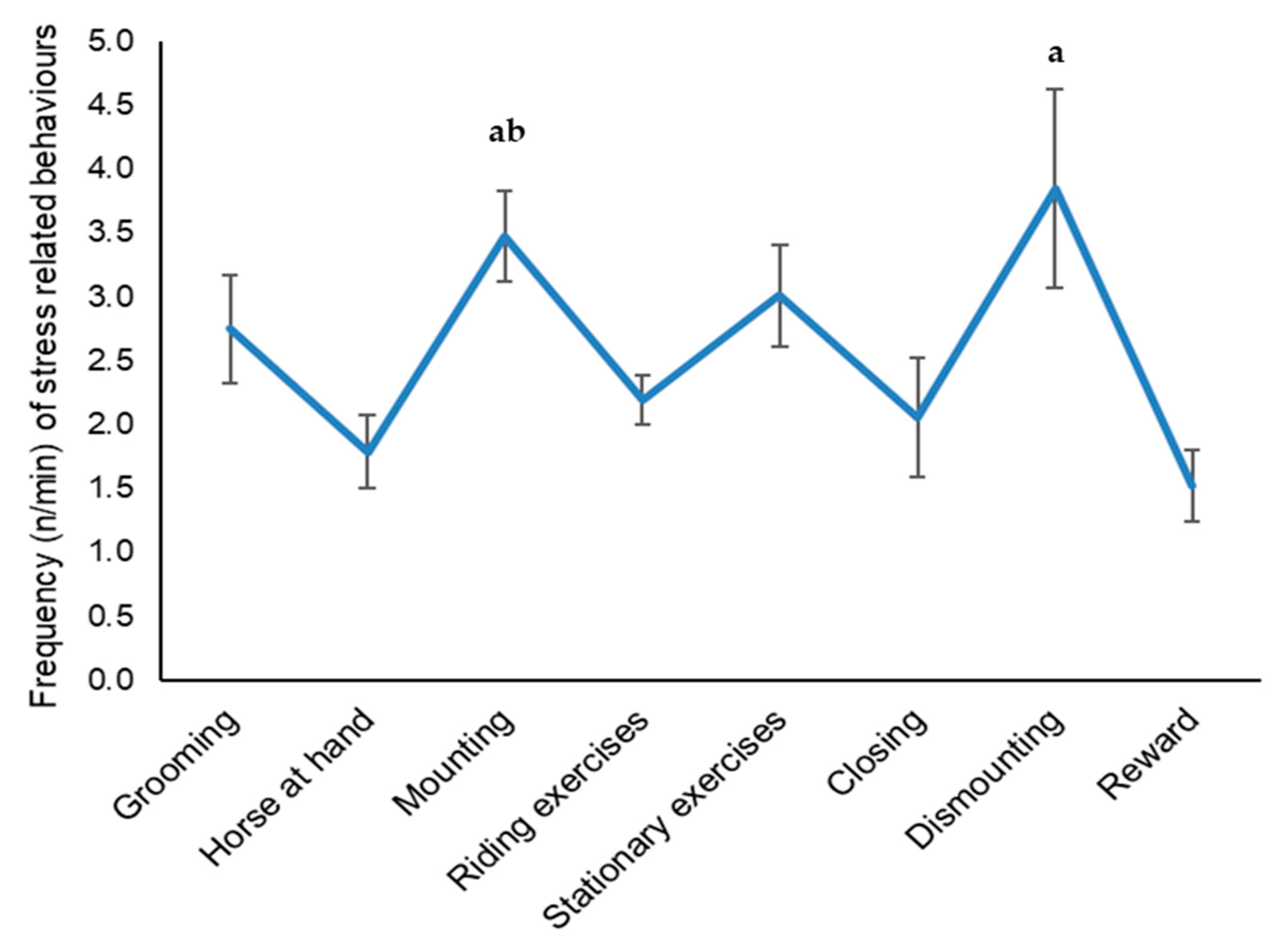
3.2. Behaviour Analysis
3.3. Eye Temperature
3.4. Heart Rate and Heart Rate Variability (a Subset of Horses)
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendall, E.; Maujean, A.; Pepping, C.A.; Downes, M.; Lakhani, A.; Byrne, J.; Macfarlane, K. A systematic review of the efficacy of equine-assisted interventions on psychological outcomes. Eur. J. Psychother. Couns. 2015, 17, 57–79. [Google Scholar] [CrossRef]
- Rigby, B.R.; Grandjean, P.W. The Efficacy of Equine-Assisted Activities and Therapies on Improving Physical Function. J. Altern. Complem. Med. 2016, 22, 9–24. [Google Scholar] [CrossRef]
- Borgi, M.; Loliva, D.; Cerino, S.; Chiarotti, F.; Venerosi, A.; Bramini, M.; Nonnis, E.; Marcelli, M.; Vinti, C.; De Santis, C.; et al. Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. J. Autism. Dev. Disord. 2016, 46, 1–9. [Google Scholar] [CrossRef]
- Stergiou, A.; Tzoufi, M.; Ntzani, E.; Varvarousis, D.; Beris, A.; Ploumis, A. Therapeutic Effects of Horseback Riding Interventions: A Systematic Review and Meta-analysis. Am. J. Phys. Med. Rehabil. 2017, 96, 717–725. [Google Scholar] [CrossRef]
- Trzmiel, T.; Purandare, B.; Michalak, M.; Zasadzka, E.; Pawlaczyk, M. Equine assisted activities and therapies in children with autism spectrum disorder: A systematic review and a meta-analysis. Complem. Ther. Med. 2019, 42, 104–113. [Google Scholar] [CrossRef]
- Winchester, P.; Kendall, K.; Peters, H.; Sears, N.; Winkley, T. The Effect of Therapeutic Horseback Riding on Gross Motor Function and Gait Speed in Children Who Are Developmentally Delayed. Phys. Occup. Ther. Pediatr. 2002, 22, 37–50. [Google Scholar] [CrossRef]
- Bass, M.M.; Duchowny, C.A.; Llabre, M.M. The effect of therapeutic horseback riding on social functioning in children with autism. J. Autism. Dev. Disord. 2009, 39, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Gabriels, R.L.; Agnew, J.A.; Holt, K.D.; Shoffner, A.; Zhaoxing, P.; Ruzzano, S.; Clayton, G.H.; Mesibov, G. Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Res. Autism. Spectr. Disord. 2012, 6, 578–588. [Google Scholar] [CrossRef]
- Keino, H.; Funahashi, A.; Keino, H.; Miwa, C.; Hosokawa, M.; Hayashi, Y.; Kawakita, K. Psycho-educational Horseback Riding to Facilitate Communication Ability of Children with Pervasive Developmental Disorders. J. Equine Sci. 2009, 20, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Garver, C.R.; Mehta, J.; Trivedi, M.H. Prospective Trial of Equine-Assisted Activities in Autism Spectrum Disorder Chronic Mercury Toxicity: Comprehensive Review View Project Brain Network Analysis in Depression View Project. Available online: https://www.researchgate.net/publication/51874213 (accessed on 11 September 2020).
- Lanning, B.A.; Baier, M.E.M.; Ivey-Hatz, J.; Krenek, N.; Tubbs, J.D. Effects of equine assisted activities on autism spectrum disorder. J. Autism. Dev. Disord. 2014, 44, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.C.; Whalon, K.; Rusnak, K.; Wendell, K.; Paschall, N. The association between therapeutic horseback riding and the social communication and sensory reactions of children with autism. J. Autism Dev. Disord. 2013, 43, 2190–2198. [Google Scholar] [CrossRef]
- Christon, L.M.; Mackintosh, V.H.; Myers, B.J. Use of complementary and alternative medicine (CAM) treatments by parents of children with autism spectrum disorders. Res. Autism. Spectr. Disord. 2010, 4, 249–259. [Google Scholar] [CrossRef]
- De Santis, M.; Contalbrigo, L.; Borgi, M.; Cirulli, F.; Luzi, F.; Redaelli, V.; Stefani, A.; Toson, M.; Odore, R.; Vercelli, C.; et al. Equine Assisted Interventions (EAIs): Methodological Considerations for Stress Assessment in Horses. Vet. Sci. 2017, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Gautier, E.; Biquand, V.; Lunel, C.; Jégo, P. Could Work Be a Source of Behavioural Disorders? A Study in Horses. PLoS ONE 2009, 4, e7625. [Google Scholar] [CrossRef]
- Munsters, C.C.B.M.; Visser, K.E.K.; van den Broek, J.; Sloet van Oldruitenborgh-Oosterbaan, M.M. The influence of challenging objects and horse-rider matching on heart rate, heart rate variability and behavioural score in riding horses. Vet. J. 2012. [Google Scholar] [CrossRef]
- Bartolomé, E.; Cockram, M.S. Potential Effects of Stress on the Performance of Sport Horses. J. Equine Vet. Sci. 2016, 40, 84–93. [Google Scholar] [CrossRef]
- Waran, N.; Randle, H. What we can measure, we can manage: The importance of using robust welfare indicators in Equitation Science. Appl. Anim. Behav. Sci. 2017, 190, 74–81. [Google Scholar] [CrossRef]
- Hausberger, M.; Stomp, M.; Sankey, C.; Brajon, S.; Lunel, C.; Henry, S. Mutual interactions between cognition and welfare: The horse as an animal model. Neurosci. Biobehav. Rev. 2019. [Google Scholar] [CrossRef]
- Serpell, J.A.; Coppinger, R.; Fine, A.H.; Peralta, J.M. Welfare Considerations in Therapy and Assistance Animals. In Handbook on Animal-Assisted Therapy; Elsevier Inc.: San Diego, CA, USA, 2010; pp. 481–503. [Google Scholar] [CrossRef]
- Hartley, S.L.; Sikora, D.M.; McCoy, R. Prevalence and risk factors of maladaptive behaviour in young children with autistic disorder. J. Intellect. Disabil. Res. 2008, 52, 819–829. [Google Scholar] [CrossRef]
- Hirota, T.; Deserno, M.; McElroy, E. The Network Structure of Irritability and Aggression in Individuals with Autism Spectrum Disorder. J. Autism. Dev. Disord. 2020, 50, 1210–1220. [Google Scholar] [CrossRef]
- Fenner, K.; Yoon, S.; White, P.; Starling, M.; McGreevy, P. The Effect of Noseband Tightening on Horses’ Behavior, Eye Temperature, and Cardiac Responses. PLoS ONE 2016, 11, e0154179. [Google Scholar] [CrossRef]
- Bartolomé, E.; Sanchez, M.; Molina, E.; Schaefer, A.; Cervantes, I.; Valera, M. Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible influence on sport performance. Animal 2013, 7, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Rietmann, T.R.; Stauffacher, M.; Bernasconi, P.; Auer, J.A.; Weishaupt, M.A. The Association between Heart Rate, Heart Rate Variability, Endocrine and Behavioural Pain Measures in Horses Suffering from Laminitis. J. Vet. Med. Ser. A. 2004, 51, 218–225. [Google Scholar] [CrossRef] [PubMed]
- McConachie, E.L.; Giguère, S.; Rapoport, G.; Barton, M.H. Heart rate variability in horses with acute gastrointestinal disease requiring exploratory laparotomy. J. Vet. Emerg. Crit. Care 2016, 26, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; De Santis, M.; Contalbrigo, L.; Benedetti, D.; Finocchi Mahne, E.; Santucci, V.U.; Borrello, S.; Farina, L. The Italian Agreement between the Government and the Regional Authorities: National Guidelines for AAI and Institutional Context. People Anim. Int. J. Res. Pract. 2018, 1, 1. [Google Scholar]
- May, M.L.; Nolen-Walston, R.D.; Utter, M.E.; Boston, R.C. Comparison of Hematologic and Biochemical Results on Blood Obtained by Jugular Venipuncture as Compared with Intravenous Catheter in Adult Horses. J. Vet. Intern. Med. 2010, 24, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Reimers, T.J.; Salerno, V.J.; Lamb, S.V. Validation and application of solid-phase chemiluminescent immunoassays for diagnosis of endocrine diseases in animals. Comp. Haematol. Int. 1996, 6, 170–175. [Google Scholar] [CrossRef]
- Singh, A.K.; Jiang, Y.; White, T.; Spassova, D. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J. Vet. Diagn. Investig. 1997, 9, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Froin, H.R. Diagnosis of Equine Cushing’s Syndrome by ACTH Measurement; Diagnostic Products Corporation Technical Report; Diagnostic Products Corporation: Los Angeles, CA, USA, 1998. [Google Scholar]
- Perkins, G.A.; Lamb, S.; Erb, H.N.; Schanbacher, B.; Nydam, D.V.; Divers, T.J. Plasma adrenocorticotropin (ACTH) concentrations and clinical response in horses treated for equine Cushing’s disease with cyproheptadine or pergolide. Equine Vet. J. 2010, 34, 679–685. [Google Scholar] [CrossRef]
- Westermann, J.; Hubl, W.; Kaiser, N.; Salewski, L. Simple, rapid and sensitive determination of epinephrine and norepinephrine in urine and plasma by non-competitive enzyme immunoassay, compared with HPLC method. Clin. Lab. 2002, 48, 61–71. [Google Scholar] [CrossRef]
- Bachmann, I.; Bernasconi, P.; Herrmann, R.; Weishaupt, M.A.; Stauffacher, M. Behavioural and physiological responses to an acute stressor in crib-biting and control horses. Appl. Anim. Behav. Sci. 2003, 82, 297–311. [Google Scholar] [CrossRef]
- Kaiser, L.; Heleski, C.R.; Siegford, J.; Smith, K.A. Stress-related behaviors among horses used in a therapeutic riding program. J. Am. Vet. Med. Assoc. 2006, 228, 39–45. [Google Scholar] [CrossRef]
- Rietmann, T.R.; Stuart, A.E.A.; Bernasconi, P.; Stauffacher, M.; Auer, J.A.; Weishaupt, M.A. Assessment of mental stress in warmblood horses: Heart rate variability in comparison to heart rate and selected behavioural parameters. Appl. Anim. Behav. Sci. 2004. [Google Scholar] [CrossRef]
- Visser, E.K.; Van Reenen, C.G.; Hopster, H.; Schilder, M.B.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Quantifying aspects of young horses’ temperament: Consistency of behavioural variables. Appl. Anim. Behav. Sci. 2001, 74, 241–258. [Google Scholar] [CrossRef]
- Fureix, C.; Gorecka-Bruzda, A.; Gautier, E.; Hausberger, M. Cooccurrence of Yawning and Stereotypic Behaviour in Horses (Equus caballus). ISRN Zool. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Fureix, C.; Beaulieu, C.; Argaud, S.; Rochais, C.; Quinton, M.; Henry, S.; Hausberger, M.; Mason, G. Investigating anhedonia in a non-conventional species: Do some riding horses Equus caballus display symptoms of depression? Appl. Anim. Behav. Sci. 2015, 162, 26–36. [Google Scholar] [CrossRef]
- Lesimple, C.; Hausberger, M. How accurate are we at assessing others’ well-being? The example of welfare assessment in horses. Front. Psychol. 2014, 5, 21. [Google Scholar] [CrossRef]
- Hall, C.; Kay, R.; Yarnell, K. Assessing ridden horse behavior: Professional judgment and physiological measures. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 22–29. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef]
- Redaelli, V.; Bergero, D.; Zucca, E.; Ferrucci, F.; Costa, L.N.; Crosta, L.; Luzi, F. Use of Thermography Techniques in Equines: Principles and Applications. J. Equine Vet. Sci. 2014, 34, 345–350. [Google Scholar] [CrossRef]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007. [Google Scholar] [CrossRef]
- West, B.; Welch, K.; Galecki, A. Linear Mixed Models: A Practical Guide Using Statistical Software. 2014. Available online: https://books.google.com/books?hl=it&lr=&id=hjT6AwAAQBAJ&oi=fnd&pg=PP1&dq=West+BT,+Welch+KB,+Galecki+AT.+Linear+mixed+models:+a+practical+guide+using+statistical+software.+Boca+Raton,+FL,+USA:+Chapman+%26+Hall/CRC%3B+2006.&ots=QyE9sQCvZg&sig=UQW-OadoHV33nEzHvmKvleCtIx8 (accessed on 14 September 2020).
- Dunnett, C.W. Pairwise multiple comparisons in the unequal variance case. J. Am. Stat. Assoc. 1980, 75, 796–800. [Google Scholar] [CrossRef]
- Ayala, I.; Martos, N.F.; Silvan, G.; Gutierrez-Panizo, C.; Clavel, J.G.; Carlos Illera, J. Cortisol, adrenocorticotropic hormone, serotonin, adrenaline and noradrenaline serum concentrations in relation to disease and stress in the horse. Res. Vet. Sci. 2012, 93, 103–107. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Donaldson, M.T.; Beech, J.; Lamb, S.V.; Schanbacher, B.J.; McFarlane, D. Variation in Plasma Adrenocorticotropic Hormone Concentration and Dexamethasone Suppression Test Results with Season, Age, and Sex in Healthy Ponies and Horses. J. Vet. Intern. Med. 2010. [Google Scholar] [CrossRef]
- Kurosawa, M.; Nagata, S.; Takeda, F.; Mima, K.; Hiraga, A.; Kai, M.; Taya, K. Plasma catecholamine, adrenocorticotropin and cortisol responses to exhaustive incremental treadmill exercise of the thoroughbred horse. J Equine Sci. 1998, 9, 9–18. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Cravana, C.; Fazio, E.; Medica, P. The different hormonal system during exercise stress coping in horses. Vet. World 2020, 13, 847–859. [Google Scholar] [CrossRef]
- Kazmi, S.Z.; Zhang, H.; Aziz, W.; Monfredi, O.; Abbas, S.A.; Shah, S.A.; Kazmi, S.S.; Butt, W.H. Inverse Correlation between Heart Rate Variability and Heart Rate Demonstrated by Linear and Nonlinear Analysis. PLoS ONE 2016, 11, e0157557. [Google Scholar] [CrossRef] [PubMed]
- König Borstel, U.; Visser, E.; Hall, C. Indicators of stress in equitation. Appl. Anim. Behav. Sci. 2017, 190, 43–56. [Google Scholar] [CrossRef]
- van der Kolk, J.H.; Fouché, N.; Gross, J.J.; Gerber, V.; Bruckmaier, R.M. A comparison between the equine and bovine hypothalamus-pituitary-adrenocortical axis. Domest. Anim. Endocrinol. 2016, 56, S101–S111. [Google Scholar] [CrossRef]
- Terada, M.; Momozawa, Y.; Komano, M.; Kusunose, R.; Sato, F.; Saito, T.R. Changes in the heart rate and plasma epinephrine and norepinephrine concentrations of the stallion during copulation. Reprod. Med. Biol. 2005, 4, 143–147. [Google Scholar] [CrossRef]
- Hyyppä, S. Endocrinal responses in exercising horses. Livest. Prod. Sci. 2005, 92, 113–121. [Google Scholar] [CrossRef]
- Martín-Valero, R.; Vega-Ballón, J.; Perez-Cabezas, V. Benefits of hippotherapy in children with cerebral palsy: A narrative review. Eur. J. Paediatr. Neurol. 2018. [Google Scholar] [CrossRef]
- Pritchard, J.C.; Burn, C.C.; Barr, A.R.S.; Whay, H.R. Haematological and serum biochemical reference values for apparently healthy working horses in Pakistan. Res. Vet. Sci. 2009, 87, 389–395. [Google Scholar] [CrossRef]
- Sample, S.H.; Fox, K.M.; Wunn, D.; Roth, E.; Friedrichs, K.R. Hematologic and biochemical reference intervals for adult Friesian horses from North America. Vet. Clin. Pathol. 2015, 44, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Albright, D.L.; Marzolf, J.R.; Bibbo, J.L.; Yaglom, H.D.; Crowder, S.M.; Carlisle, G.K.; Willard, A.; Russell, C.L.; Grindler, K.; et al. Effects of therapeutic horseback riding on post-traumatic stress disorder in military veterans. Mil. Med. Res. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, K.; Yee, C.; Tevlin, J.M.; Birks, E.K.; Durando, M.M.; Pournajafi-Nazarloo, H.; Cavaiola, A.A.; McKeever, K.H. The Effects of Equine-Assisted Activities Therapy on Plasma Cortisol and Oxytocin Concentrations and Heart Rate Variability in Horses and Measures of Symptoms of Posttraumatic Stress Disorder in Veterans. J. Equine Vet. Sci. 2018. [Google Scholar] [CrossRef]
- Mckinney, C.; Mueller, M.K.; Frank, N. Effects of Therapeutic Riding on Measures of Stress in Horses. J. Equine Vet. Sci. 2015, 35, 922–928. [Google Scholar] [CrossRef]
- Chamove, A.S.; Crawley-Hartrick, O.J.E.; Stafford, K.J. Horse reactions to human attitudes and behavior. Anthrozoös 2002, 15, 323–331. [Google Scholar] [CrossRef]
- Janczarek, I.; Kędzierski, W. Emotional Response to Novelty and to Expectation of Novelty in Young Race Horses. J. Equine Vet. Sci. 2011, 31, 549–554. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Cravana, C.; Ferlazzo, A. Hypothalamic-pituitary-adrenal axis responses of horses to therapeutic riding program: Effects of different riders. Physiol. Behav. 2013, 118, 138–143. [Google Scholar] [CrossRef]
- Lorello, O.; Ramseyer, A.; Burger, D.; Gerber, V.; Bruckmaier, R.M.; van der Kolk, J.H.; de Solis, C.N. Repeated Measurements of Markers of Autonomic Tone Over a Training Season in Eventing Horses. J. Equine Vet. Sci. 2017, 53, 38–44. [Google Scholar] [CrossRef]
- Hall, C.; Randle, H.; Pearson, G.; Preshaw, L.; Waran, N. Assessing equine emotional state. Appl. Anim. Behav. Sci. 2018, 205, 183–193. [Google Scholar] [CrossRef]
- Proops, L.; Walton, M.; McComb, K. The use of human-given cues by domestic horses, Equus caballus, during an object choice task. Anim. Behav. 2010, 79, 1205–1209. [Google Scholar] [CrossRef]
- Yarnell, K.; Hall, C.; Billett, E. An assessment of the aversive nature of an animal management procedure (clipping) using behavioral and physiological measures. Physiol. Behav. 2013, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.; Warren-Smith, A.; Guisard, Y. The effect of double bridles and jaw-clamping crank nosebands on temperature of eyes and facial skin of horses. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 142–148. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Grandi, L.C.; Heinzl, E.; Pelosi, A.; Previde, E.P.; Valsecchi, P. How good is this food? A study on dogs’ emotional responses to a potentially pleasant event using infrared thermography. Physiol. Behav. 2016, 159, 80–87. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, E.; Grant, C.C.; Fletcher, L.; Ganswindt, A.; Page, P.C. Repeatability and Reliability of Heart Rate Variability in Healthy, Adult Pony Mares. J. Equine Vet. Sci. 2016, 46, 73–81. [Google Scholar] [CrossRef]
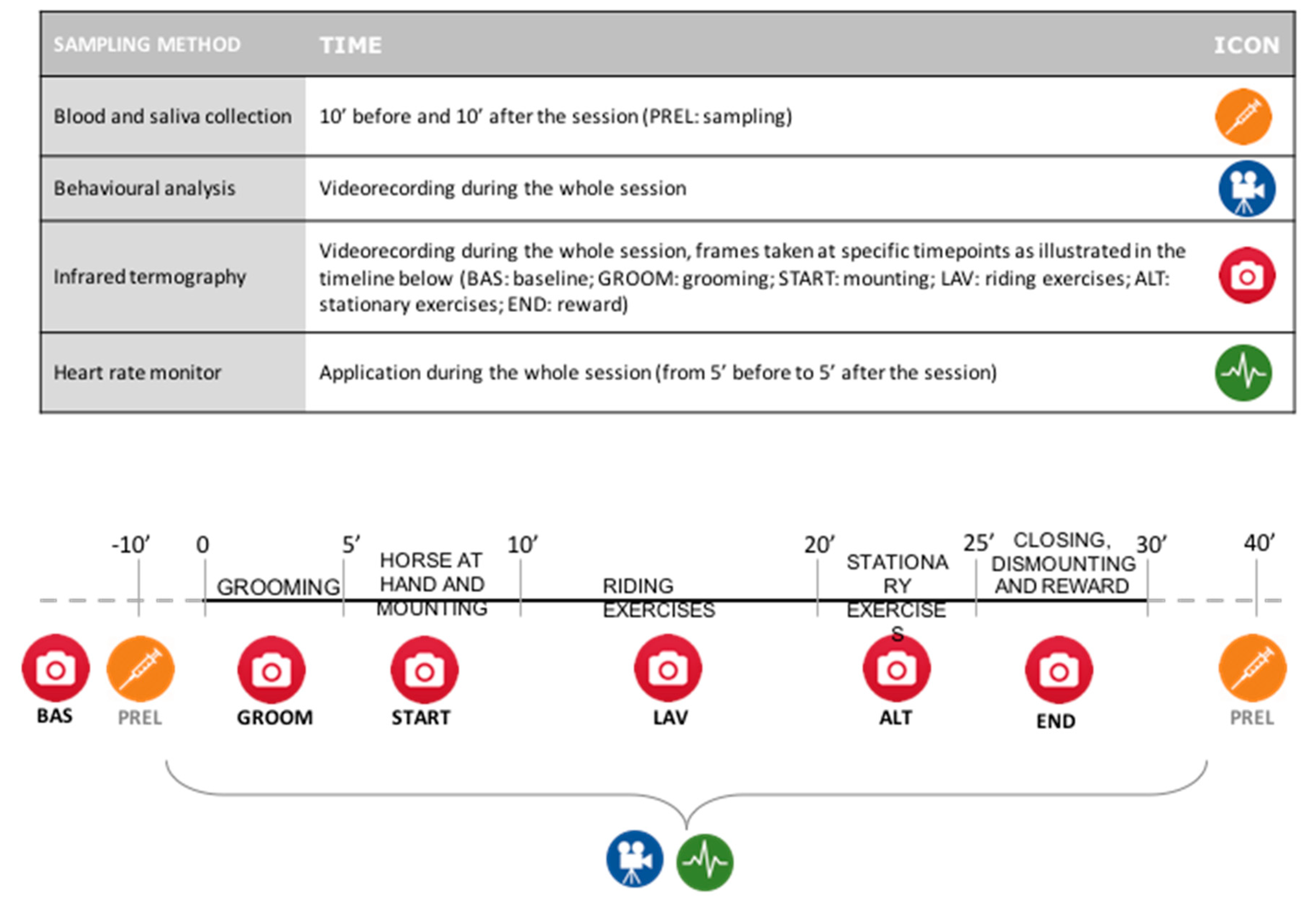

| Phase | Duration (min) | Description of the Activity and Main Goals |
|---|---|---|
| Grooming | 5’ | Child’s approach to the horse and first contact; child’s knowledge of the horse (morphology, behaviour); main security rules; grooming techniques (brushing the body of the horse) and saddling |
| Horse at hand | 5’ | The child leads the horse with a lead rope around the arena |
| Mounting | - | The horse stops and the child mounts the horse |
| Riding exercises | 10’ | Learning riding basic elements (walk). Performing exercises while riding the horse (rotating/bending, outstretching upper arms and trunk) |
| Stationary exercises | 5’ | Performing exercises on the horse (horse halted). Games such as rods, cones, or balls are used |
| Closing | 5’ | Riding the horse around the arena |
| Dismounting | - | The horse stops and the child dismounts |
| Reward | - | The child rewards the horse (e.g., carrot, sugar, hay, etc.) |
| Behaviour | Description |
|---|---|
| Head nodding | The horse repetitively moves its head vertically (>3 movements up and down) |
| Head shaking | The horse tosses its head in sudden bouts |
| Head tossing | Head lowered with the ears pinned back interrupted with momentary sharp tossing or rotating gestures of the head |
| Head raised/high | Head held higher than the normal carriage with nose extended upward and with a slight extension of the neck |
| Head down | The horse held its nose below its belly-line; neck may be stretched out with nose pushed forward |
| Ears pinned back | Ears pressed caudally against the head and neck |
| Snorting | Forceful expulsion of air through the nostril incidentally preceded by a raspy inhalation sound |
| Lip play | The horse moves its upper lip up and down without making contact with an object, or the horse smacks its lips together |
| Tongue play | The horse sticks out its tongue and twists it in the air |
| Chomping the bit | Any mouth or tongue manipulation of the bit independent of the rider’s use of the reins |
| Lip/Teeth rubbing | The horse rubs its upper lip or its upper teeth repetitively against the arena wall |
| Head bumping | The horse bumps or attempts to bump its head against the side walker or the instructor |
| Biting leads | The horse bites or attempts to bite the side walker/instructor the lead rope |
| Avoidance/Halt | The horse stops walking; cessation of movement of all feet, or backward movement |
| Pawing | The horse hits the ground with the paws |
| Tail swishing | Any exaggerated movement of the tail, usually more of a wringing motion than a rhythmic or directed swishing (no insect present) |
| Effect | DFnum | DFden | F | Pr > F |
|---|---|---|---|---|
| Phase | 7 | 284 | 6.43 | <0.001 |
| Group (ASD vs. TD) | 1 | 284 | 0.52 | 0.4733 |
| Group*phase | 7 | 284 | 1.61 | 0.1327 |
| Age | 1 | 18.8 | 0.38 | 0.5434 |
| Experience (exp) | 1 | 18.8 | 1.40 | 0.2509 |
| Sex (M/F) | 1 | 18.8 | 0.10 | 0.7592 |
| Phase*age | 7 | 284 | 2.07 | 0.0468 |
| Phase*exp | 7 | 284 | 2.00 | 0.0552 |
| Phase*exp*M | 8 | 96.8 | 2.06 | 0.0475 |
| Phase*age*exp | 8 | 96.8 | 2.06 | 0.0475 |
| Effect | HR | LF/HF | RMMSD | |||
|---|---|---|---|---|---|---|
| F | Pr > F | F | Pr > F | F | Pr > F | |
| Phase | 48.29 | <0.0001 | 6.76 | <0.0001 | 4.53 | 0.0002 |
| Group (ASD vs. TD) | 1.52 | 0.2201 | 1.49 | 0.2243 | 13.42 | 0.0004 |
| Group*Phase | 3.85 | 0.0008 | 0.56 | 0.7909 | 1.01 | 0.4297 |
| Age | 2.76 | 0.1298 | 0.37 | 0.5566 | 7.91 | 0.0187 |
| Phase*age | 4.04 | 0.0005 | 1.39 | 0.2149 | 6.38 | <0.0001 |
| Group*age | 3.48 | 0.0643 | 1.86 | 0.1751 | 0.27 | 0.6058 |
| Group*phase*age | 0.93 | 0.4836 | 0.34 | 0.9348 | 1.02 | 0.4177 |
| Age*exp | 0.07 | 0.7997 | 0.01 | 0.9275 | 1.17 | 0.3055 |
| Phase*age*exp | 2.58 | 0.0163 | 1.23 | 0.2894 | 0.71 | 0.6658 |
| Group*age*exp | 0.10 | 0.7539 | 0.61 | 0.4370 | 0.02 | 0.8764 |
| Group*phase*age*exp | 2.62 | 0.0147 | 0.56 | 0.7878 | 1.04 | 0.4053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contalbrigo, L.; Borgi, M.; De Santis, M.; Collacchi, B.; Tuozzi, A.; Toson, M.; Redaelli, V.; Odore, R.; Vercelli, C.; Stefani, A.; et al. Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions. Animals 2021, 11, 1562. https://doi.org/10.3390/ani11061562
Contalbrigo L, Borgi M, De Santis M, Collacchi B, Tuozzi A, Toson M, Redaelli V, Odore R, Vercelli C, Stefani A, et al. Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions. Animals. 2021; 11(6):1562. https://doi.org/10.3390/ani11061562
Chicago/Turabian StyleContalbrigo, Laura, Marta Borgi, Marta De Santis, Barbara Collacchi, Adele Tuozzi, Marica Toson, Veronica Redaelli, Rosangela Odore, Cristina Vercelli, Annalisa Stefani, and et al. 2021. "Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions" Animals 11, no. 6: 1562. https://doi.org/10.3390/ani11061562
APA StyleContalbrigo, L., Borgi, M., De Santis, M., Collacchi, B., Tuozzi, A., Toson, M., Redaelli, V., Odore, R., Vercelli, C., Stefani, A., Luzi, F., Valle, E., & Cirulli, F. (2021). Equine-Assisted Interventions (EAIs) for Children with Autism Spectrum Disorders (ASD): Behavioural and Physiological Indices of Stress in Domestic Horses (Equus caballus) during Riding Sessions. Animals, 11(6), 1562. https://doi.org/10.3390/ani11061562










