Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Preparation
2.2. Laying Hens and Farm Facilities
3. Experimental Procedure
3.1. Ethics Statement
3.2. Hens Performance
3.3. Intestinal Morphology
3.4. Quantitative Real-Time PCR
3.5. Cecal Microbiota Analysis by 16S rRNA High-Throughput Sequencing
3.6. Sequencing Data Analysis
3.7. Statistical Analysis
4. Results
4.1. Effects of B. subtilis Supplementation on Overall Performance and Morphology of the Jejunum of Laying Hens
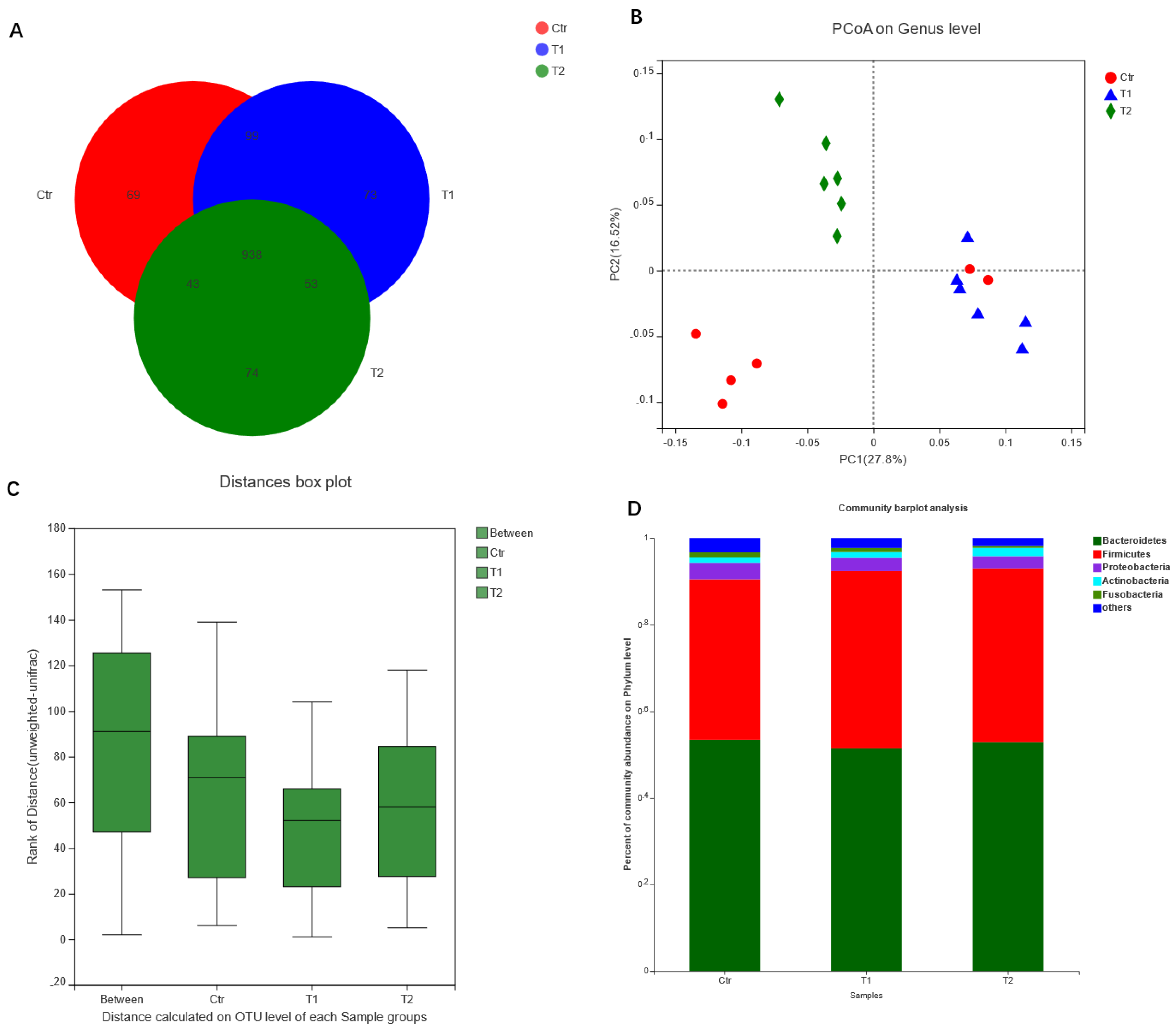
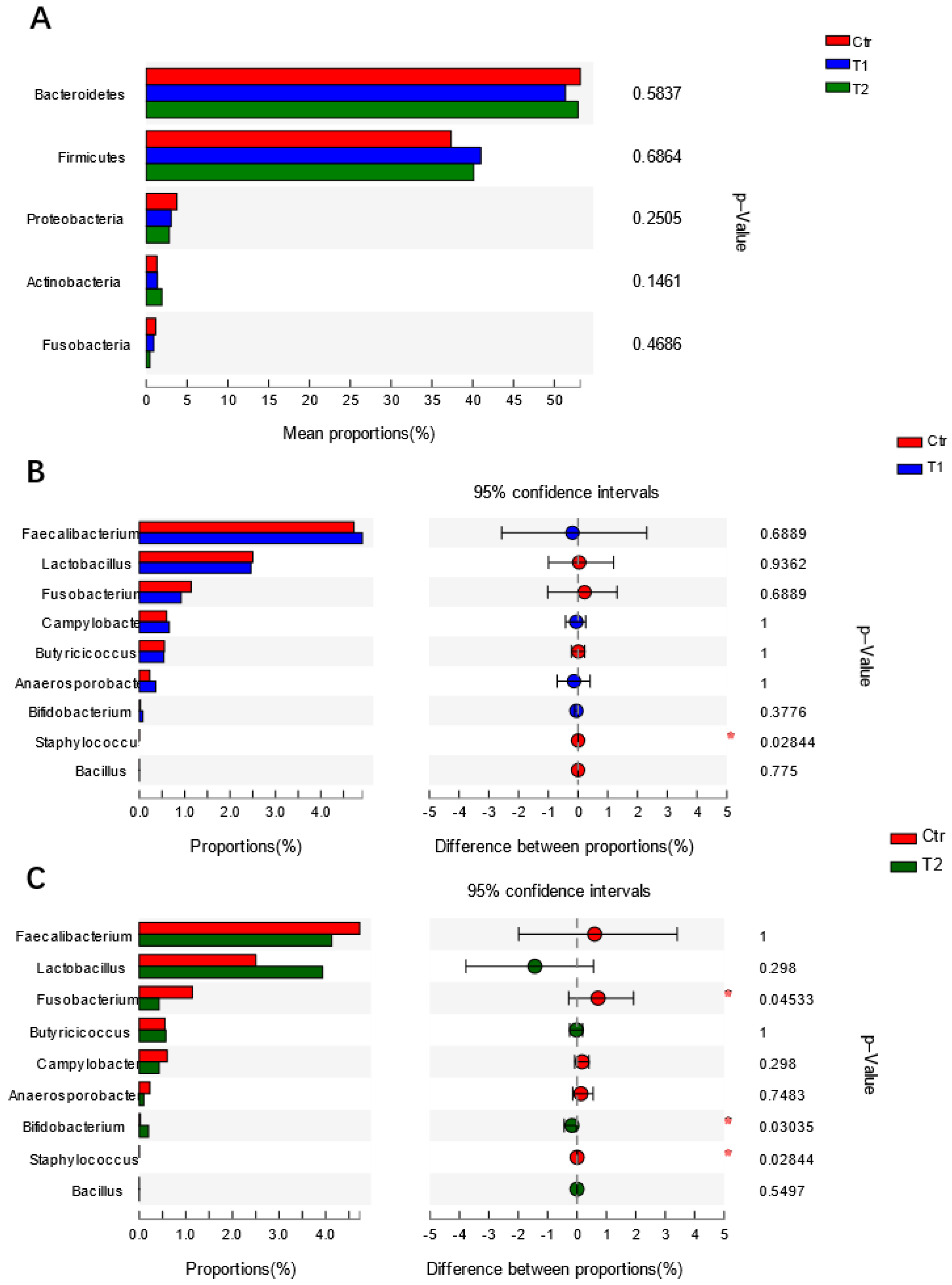
4.2. Effects of B. subtilis Supplementation on Gut Microbiota of Laying Hens
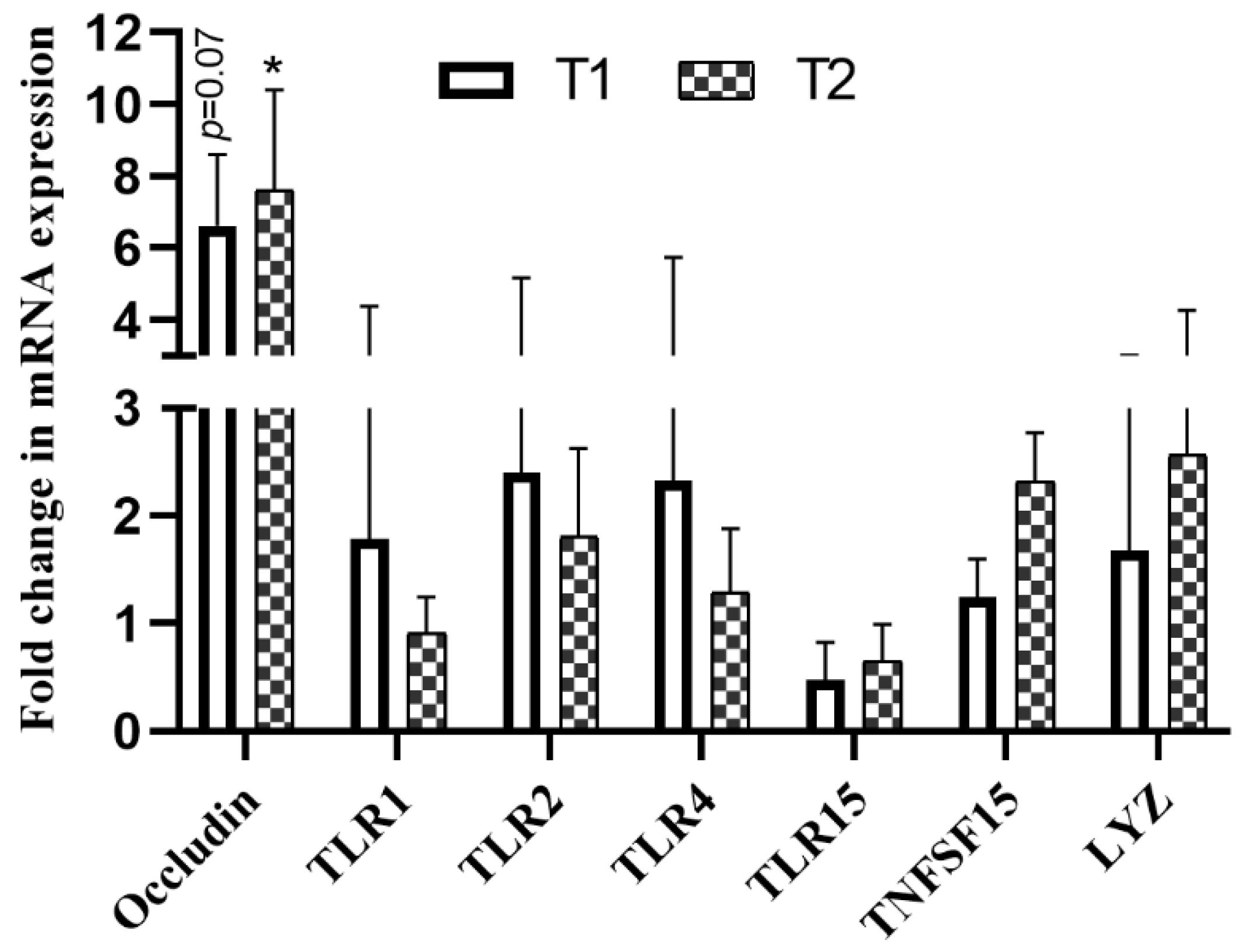
4.3. Changes in Gene mRNA Expression Level in Response to B. subtilis Administration
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaskins, H.R.; Collier, C.T.; Anderson, D.B. Antibiotics as growth promotants: Mode of action. Anim. Biotechnol. 2002, 13, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hwang, B.K.; Choi, H.; Wang, Y.; Choi, S.H.; Ryu, S.; Jeon, B. Characterization of mcr-1-Harboring Plasmids from Pan Drug-Resistant Escherichia coli Strains Isolated from Retail Raw Chicken in South Korea. Microorganisms 2019, 7, 344. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, E.; Van Coillie, E.; Van Meervenne, E.; Boon, N.; Heyndrickx, M.; Van de Wiele, T. Commensal, E. coli rapidly transfer antibiotic resistance genes to human intestinal microbiota in the Mucosal Simulator of the Human Intestinal Microbial Ecosystem (M-SHIME). Int. J. Food Microbiol. 2019, 311, 108357. [Google Scholar] [CrossRef] [PubMed]
- Vinueza-Burgos, C.; Ortega-Paredes, D.; Narváez, C.; De Zutter, L.; Zurita, J. Characterization of cefotaxime resistant Escherichia coli isolated from broiler farms in Ecuador. PLoS ONE 2019, 14, e0207567. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifah, H.S. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Calvert, A.J.; Noll, S.L.; McComb, B.; Sherwood, J.S.; Logue, C.M.; Johnson, T.J. Succession of the turkey gastrointestinal bacterial microbiome related to weight gain. Peerj 2013, 1, e237. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 1 May 2002).
- Seal, B.S.; Drider, D.; Oakley, B.B.; Brüssow, H.; Bikard, D.; Rich, J.O.; Miller, S.; Devillard, E.; Kwan, J.; Bertin, G.; et al. Microbial-derived products as potential new antimicrobials. Vet. Res. 2018, 49, 66. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Guyard-Nicodème, M.; Messaoudi, S.; Chemaly, M.; Cappelier, J.M.; Dousset, X.; Haddad, N. Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry. Front. Microbiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.L.; Weber, B.P.; Mendoza, K.M.; Danzeisen, J.L.; Llop, K.; Lang, K.; Clayton, J.B.; Grace, E.; Brannon, J.; Radovic, I.; et al. Antibiotics and Host-Tailored Probiotics Similarly Modulate Effects on the Developing Avian Microbiome, Mycobiome, and Host Gene Expression. Mbio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ramasamy, K.; Sieo, C.C.; Ho, Y.W. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE 2017, 12, e0175959. [Google Scholar] [CrossRef]
- Peng, Q.; Zeng, X.F.; Zhu, J.L.; Wang, S.; Liu, X.T.; Hou, C.L.; Thacker, P.A.; Qiao, S.Y. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Feng, C.; Jiang, L.; Zhang, L.; Zhang, J.; Zhang, L.; Wang, T. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult. Sci. 2018, 97, 2312–2321. [Google Scholar] [CrossRef]
- Peralta-Sánchez, J.M.; Martín-Platero, A.M.; Ariza-Romero, J.J.; Rabelo-Ruiz, M.; Zurita-González, M.J.; Baños, A.; Rodríguez-Ruano, S.M.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M. Egg Production in Poultry Farming Is Improved by Probiotic Bacteria. Front. Microbiol. 2019, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ducatelle, R.; De Bruyne, E.; Joosten, M.; Bosschem, I.; Smet, A.; Haesebrouck, F.; Flahou, B. Role of gamma-glutamyltranspeptidase in the pathogenesis of Helicobacter suis and Helicobacter pylori infections. Vet. Res. 2015, 46, 31. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, Y.; Wang, Z. beta-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef]
- Borowska, D.; Rothwell, L.; Bailey, R.A.; Watson, K.; Kaiser, P. Identification of stable reference genes for quantitative PCR in cells derived from chicken lymphoid organs. Vet. Immunol. Immunopathol. 2016, 170, 20–24. [Google Scholar] [CrossRef]
- Rajput, I.R.; Ying, H.; Yajing, S.; Arain, M.A.; Weifen, L.; Ping, L.; Bloch, D.M.; Wenhua, L. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs and cytokines expression patterns in jejunum and ileum of broilers. PLoS ONE 2017, 12, e0173917. [Google Scholar]
- Zhen, W.; Shao, Y.; Gong, X.; Wu, Y.; Geng, Y.; Wang, Z.; Guo, Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult. Sci. 2018, 97, 2654–2666. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, Q.; Shi, G.; Liu, R.; Zhang, W.; Zhao, X.; Li, G.; Ge, M. chTLR4 pathway activation by Astragalus polysaccharide in bursa of Fabricius. BMC Vet. Res. 2017, 13, 119. [Google Scholar] [CrossRef]
- Gao, P.F.; Hou, Q.C.; Kwok, L.Y.; Huo, D.X.; Feng, S.Z.; Zhang, H.P. Effect of feeding Lactobacillus plantarum P-8 on the faecal microbiota of broiler chickens exposed to lincomycin. Sci. Bull. 2017, 62, 105–113. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Huang, W.Q.; Hou, Q.C.; Kwok, L.Y.; Sun, Z.H.; Ma, H.M.; Zhao, F.Y.; Lee, Y.K.; Zhang, H.P. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci. Bull. 2017, 62, 767–774. [Google Scholar] [CrossRef]
- Jaramillo-Torres, A.; Rawling, M.D.; Rodiles, A.; Mikalsen, H.E.; Johansen, L.H.; Tinsley, J.; Forberg, T.; Aasum, E.; Castex, M.; Merrifield, D.L. Influence of Dietary Supplementation of Probiotic Pediococcus acidilactici MA18/5M during the Transition from Freshwater to Seawater on Intestinal Health and Microbiota of Atlantic Salmon (Salmo salar L.). Front. Microbiol. 2019, 10, 2243. [Google Scholar] [CrossRef]
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Xu, M.M.; Kang, K.L.; Tang, S.G.; He, C.Q.; Qu, X.Y.; Guo, S.C. The effects and combinational effects of Bacillus subtilis and montmorillonite on the intestinal health status in laying hens. Poult. Sci. 2020, 99, 1311–1319. [Google Scholar] [CrossRef]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 2017, 96, 1280–1289. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Mazanko, M.S.; Chistyakov, V.A.; Denisenko, Y.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Tutelyan, A.V.; Komarova, Z.B.; Gorlov, I.F.; et al. Effect of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 on the productivity, reproductive aging, and physiological characteristics of hens and roosters. Benef. Microbes 2019, 10, 395–412. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, C.; Zhang, H.; Lai, W.; Wei, H.; Peng, J. Effects of Different Probiotics on Laying Performance, Egg Quality, Oxidative Status, and Gut Health in Laying Hens. Animals 2019, 9, 1110. [Google Scholar] [CrossRef]
- Zhan, H.Q.; Dong, X.Y.; Li, L.L.; Zheng, Y.X.; Gong, Y.J.; Zou, X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019, 98, 896–903. [Google Scholar] [CrossRef]
- Neijat, M.; Shirley, R.B.; Barton, J.; Thiery, P.; Welsher, A.; Kiarie, E. Effect of dietary supplementation of Bacillus subtilis DSM29784 on hen performance, egg quality indices, and apparent retention of dietary components in laying hens from 19 to 48 weeks of age. Poult. Sci. 2019, 98, 5622–5635. [Google Scholar] [CrossRef]
- Forte, C.; Moscati, L.; Acuti, G.; Mugnai, C.; Franciosini, M.P.; Costarelli, S.; Cobellis, G.; Trabalza-Marinucci, M. Effects of dietary Lactobacillus acidophilus and Bacillus subtilis on laying performance, egg quality, blood biochemistry and immune response of organic laying hens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, J.; Velkers, F.C.; Bouwstra, R.J.; Beerens, N.; Stegeman, J.A.; de Boer, W.F.; Elbers, A.R.W.; van Hooft, P.; Feberwee, A.; Bossers, A.; et al. Limited changes in the fecal microbiome composition of laying hens after oral inoculation with wild duck feces. Poult. Sci. 2019, 98, 6542–6551. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2015, 360, 100–112. [Google Scholar] [CrossRef]
- Elokil, A.A.; Magdy, M.; Melak, S.; Ishfaq, H.; Bhuiyan, A.; Cui, L.; Jamil, M.; Zhao, S.; Li, S. Faecal microbiome sequences in relation to the egg-laying performance of hens using amplicon-based metagenomic association analysis. Animal 2020, 14, 706–715. [Google Scholar]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult. Sci. 2018, 97, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Neijat, M.; Habtewold, J.; Shirley, R.B.; Welsher, A.; Barton, J.; Thiery, P.; Kiarie, E. Bacillus subtilis Strain DSM 29784 Modulates the Cecal Microbiome, Concentration of Short-Chain Fatty Acids, and Apparent Retention of Dietary Components in Shaver White Chickens during Grower, Developer, and Laying Phases. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, S.; Li, X.; Yan, T.; Duan, Y.; Yang, X.; Duan, Y.; Sun, Q.; Yang, X. Simultaneous Supplementation of Bacillus subtilis and Antibiotic Growth Promoters by Stages Improved Intestinal Function of Pullets by Altering Gut Microbiota. Front. Microbiol. 2018, 9, 2328. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Horie, T.; Fujiwara, T.; Fukata, T.; Sasai, K.; Baba, E. Lactobacillus flora in the cloaca and vagina of hens and its inhibitory activity against Salmonella enteritidis in vitro. Poult. Sci. 2000, 79, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. Gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- Markazi, A.; Luoma, A.; Shanmugasundaram, R.; Mohnl, M.; Raj Murugesan, G.; Selvaraj, R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult. Sci. 2018, 97, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, H.; Shi, L.; Zhang, X.; Sheng, R.; Yin, F.; Gooneratne, R. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens. Anim. Nutr. 2017, 3, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Kong, J.; Zhang, L.; Gu, X.; Wang, M.; Guo, T. New crosstalk between probiotics Lactobacillus plantarum and Bacillus subtilis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yan, T.; Li, X.Y.; Duan, Y.L.; Yang, X.; Yang, X.J. Effects of Bacillus subtilis and antibiotic growth promoters on the growth performance, intestinal function and gut microbiota of pullets from 0 to 6 weeks. Animal 2020, 14, 1619–1628. [Google Scholar] [CrossRef]
- Forte, C.; Acuti, G.; Manuali, E.; Casagrande Proietti, P.; Pavone, S.; Trabalza-Marinucci, M.; Moscati, L.; Onofri, A.; Lorenzetti, C.; Franciosini, M.P. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016, 95, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Sodhi, N.; Chenu, J.W.; Cox, J.M.; Riordan, S.M.; Mitchell, H.M. The Interplay between Helicobacter and Campylobacter Species and Other Gastrointestinal Microbiota of Commercial Broiler Chickens. Helicobacter 2014, 19, 158. [Google Scholar] [CrossRef]
- Kobierecka, P.A.; Wyszyńska, A.K.; Aleksandrzak-Piekarczyk, T.; Kuczkowski, M.; Tuzimek, A.; Piotrowska, W.; Górecki, A.; Adamska, I.; Wieliczko, A.; Bardowski, J.; et al. In vitro characteristics of Lactobacillus spp. strains isolated from the chicken digestive tract and their role in the inhibition of Campylobacter colonization. Microbiologyopen 2017, 6, e00512. [Google Scholar] [CrossRef] [PubMed]
- Cartman, S.T.; La Ragione, R.M.; Woodward, M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 2008, 74, 5254–5258. [Google Scholar] [CrossRef]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Gueye, S.A.; Lupetti, A.; Senesi, S. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 2015, 119, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.P.; Moore, R.J.; Allison, G.E. Lactobacillus Strain Ecology and Persistence within Broiler Chickens Fed Different Diets: Identification of Persistent Strains. Appl. Environ. Microbiol. 2010, 76, 6494–6503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Cesare, A.; Caselli, E.; Lucchi, A.; Sala, C.; Parisi, A.; Manfreda, G.; Mazzacane, S. Impact of a probiotic-based cleaning product on the microbiological profile of broiler litters and chicken caeca microbiota. Poult. Sci. 2019, 98, 3602–3610. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Peitler, D.; Karakulska, J. Staphylococci isolated from ready-to-eat meat—Identification, antibiotic resistance and toxin gene profile. Int. J. Food Microbiol. 2016, 238, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Ziegler, D.; Pflüger, V.; Vogel, G.; Zweifel, C.; Stephan, R. Prevalence and characteristics of methicillin-resistant coagulase-negative staphylococci from livestock, chicken carcasses, bulk tank milk, minced meat, and contact persons. BMC Vet. Res. 2011, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef] [PubMed]
- York, A. Silencing Staphylococcus aureus with probiotics. Nat. Rev. Microbiol. 2018, 16, 715. [Google Scholar] [CrossRef] [PubMed]
- Kollarcikova, M.; Kubasova, T.; Karasova, D.; Crhanova, M.; Cejkova, D.; Sisak, F.; Rychlik, I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019, 98, 2347–2353. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Afra, K.; Laupland, K.; Leal, J.; Lloyd, T.; Gregson, D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect. Dis. 2013, 13, 264. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, Y.; Goo, D.; Zimmerman, N.P.; Smith, A.H.; Rehberger, T.; Lillehoj, H.S. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020, 99, 725–733. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Li, Y. Effects of Bacillus subtilis on epithelial tight junctions of mice with inflammatory bowel disease. J. Interferon Cytokine Res. 2016, 36, 75–85. [Google Scholar] [CrossRef]
- Selvam, R.; Maheswari, P.; Kavitha, P.; Ravichandran, M.; Sas, B.; Ramchand, C.N. Effect of Bacillus subtilis PB6, a natural probiotic on colon mucosal inflammation and plasma cytokines levels in inflammatory bowel disease. Indian J. Biochem. Biophys. 2009, 46, 79–85. [Google Scholar] [PubMed]
- Yi, H.; Wang, L.; Xiong, Y.; Wang, Z.; Qiu, Y.; Wen, X.; Jiang, Z.; Yang, X.; Ma, X. Lactobacillus reuteri LR1 Improved Expression of Genes of Tight Junction Proteins via the MLCK Pathway in IPEC-1 Cells during Infection with Enterotoxigenic Escherichia coli K88. Mediat. Inflamm. 2018, 2018, 6434910. [Google Scholar] [CrossRef]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, A.; Wang, M. Innate sensing of viruses by pattern recognition receptors in birds. Vet. Res. 2013, 44, 82. [Google Scholar] [CrossRef] [PubMed]
- Asgari, F.; Falak, R.; Teimourian, S.; Pourakbari, B.; Ebrahimnezhad, S.; Shekarabi, M. Effects of Oral Probiotic Feeding on Toll-Like Receptor Gene Expression of the Chicken’s Cecal Tonsil. Rep. Biochem. Mol. Biol. 2018, 6, 151–157. [Google Scholar] [PubMed]
- Rajput, I.R.; Hussain, A.; Li, Y.L.; Zhang, X.; Xu, X.; Long, M.Y.; You, D.Y.; Li, W.F. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. J. Cell Biochem. 2014, 115, 189–198. [Google Scholar] [CrossRef]
- Dong, Y.; Li, R.; Liu, Y.; Ma, L.; Zha, J.; Qiao, X.; Chai, T.; Wu, B. Benefit of Dietary Supplementation with Bacillus subtilis BYS2 on Growth Performance, Immune Response, and Disease Resistance of Broilers. Probiotics Antimicrob. Proteins 2020, 12, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, A.; Echeverry, H.; Munyaka, P.; Rodriguez-Lecompte, J.C. Innate immune response of pullets fed diets supplemented with prebiotics and synbiotics. Poult. Sci. 2015, 94, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Nutrient Level 2 | ||
|---|---|---|---|
| Corn (%) | 62.85 | CP (%) | 16.51 |
| Soybean meal (%) | 26.35 | ME (MJ/kg) | 11.16 |
| Limestone (%) | 9.00 | Calcium (%) | 3.4 |
| CaHPO4 (%) | 1.00 | AP (%) | 0.34 |
| Premix 1 (%) | 0.65 | TP (%) | 0.66 |
| DL-Met (%) | 0.15 | Met | 0.42 |
| Total | 100.00 | Lys | 0.86 |
| Met + Cys | 0.72 | ||
| Lys/Met | 2.06 | ||
| Genes | GenBank Number | Forward Sequence (5′ to 3′) | Reverse Sequence (5′ to 3′) | Reference |
|---|---|---|---|---|
| β-Actin | NM_205518.1 | ACTCTGGTGATGGTGTTAC | GGCTGTGATCTCCTTCTG | [19] |
| GAPDH | AF047874 | GAAGGCTGGGGCTCATCTG | CAGTTGGTGGTGCACGATG | [20] |
| Occludin | NM_205128.1 | TCATCGCCTCCATCGTCTAC | TCTTACTGCGCGTCTTCTGG | [19] |
| TLR1 | AB109401.1 | GGCAGTGGACGCAGACAAA | GTAGGAAATGAAGGCGTGGAA | [21] |
| TLR2 | NM_204.278 | CTGGGAAGTGGATTGTGGA | AAGGCGAAAGTGCGAGAAA | [22] |
| TLR4 | NM_001030693.1 | TTCCAAGCACCAGATAGCAACATC | ACGGGTCACAGAAGAACTTAGGG | [23] |
| TLR15 | JN112029.1 | ATCCTTGTCGTTCTGGTGCTAA | TCAGTAGATGCTCCTTCGTCCA | [21] |
| TNFSF15 | NM010245578 | CCTGAGTATTCCAGCAACGCA | ATCCACCAGCTTGATGTCACTAAC | [22] |
| LYZ | NM 205.281 | CTGGGAAACTGGGTGTGTGT | AGCGGCTGTTGATCTGTAGG | [22] |
| Parameters | Ctr | T1 | T2 |
|---|---|---|---|
| Egg production (%), before treatment | 92.64 ± 1.64 | 92.56 ± 3.35 | 93.60 ± 2.63 |
| Egg production (%), after treatment | 93.11 ± 1.44 | 95.99 ± 1.28 | 95.19 ± 1.58 |
| Egg weight (g), before treatment | 62.09 ± 0.72 | 62.71 ± 0.76 | 62.66 ± 1.03 |
| Egg weight (g), after treatment | 64.63 ± 0.96 | 63.99 ± 1.69 | 64.65 ± 0.50 |
| Parameters | Ctr | T1 | T2 |
|---|---|---|---|
| Villus height (μm) | 876.96 ± 232.38 | 869.42 ± 183.76 | 898 ± 248.37 |
| Crypt depth (μm) | 76.71 ± 26.73 | 76.02 ± 22.21 | 75.45 ± 21.05 |
| Villus height/crypt depth ratio | 11.43 ± 1.15 | 11.43 ± 3.91 | 11.93 ± 1.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, H.; Zhang, J.; Tang, X.; Raheem, A.; Wang, M.; Lin, W.; Liang, L.; Qi, Y.; Zhu, Y.; et al. Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens. Animals 2021, 11, 1523. https://doi.org/10.3390/ani11061523
Zhang G, Wang H, Zhang J, Tang X, Raheem A, Wang M, Lin W, Liang L, Qi Y, Zhu Y, et al. Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens. Animals. 2021; 11(6):1523. https://doi.org/10.3390/ani11061523
Chicago/Turabian StyleZhang, Guangzhi, Hao Wang, Jianwei Zhang, Xinming Tang, Abdul Raheem, Mingyan Wang, Weidong Lin, Lin Liang, Yuzhuo Qi, Yali Zhu, and et al. 2021. "Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens" Animals 11, no. 6: 1523. https://doi.org/10.3390/ani11061523
APA StyleZhang, G., Wang, H., Zhang, J., Tang, X., Raheem, A., Wang, M., Lin, W., Liang, L., Qi, Y., Zhu, Y., Jia, Y., Cui, S., & Qin, T. (2021). Modulatory Effects of Bacillus subtilis on the Performance, Morphology, Cecal Microbiota and Gut Barrier Function of Laying Hens. Animals, 11(6), 1523. https://doi.org/10.3390/ani11061523







