Pre- and Post-Slaughter Methodologies to Estimate Body Fat Reserves in Lactating Saanen Goats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Pre-Slaughter Measurements
2.2.1. Somatic Measurements and Body Condition Score
2.2.2. Measurement of Fat Thickness Using Ultrasound
2.3. Post-Slaughter Measurements
2.3.1. Slaughter Procedures, Hot Carcass, and Empty Body Weight
2.3.2. Carcass Measurements
2.3.3. Fat Content on Carcass and Non-Carcass Components
2.4. Statistical Analysis
3. Results
3.1. Pre-Slaughter Measurements
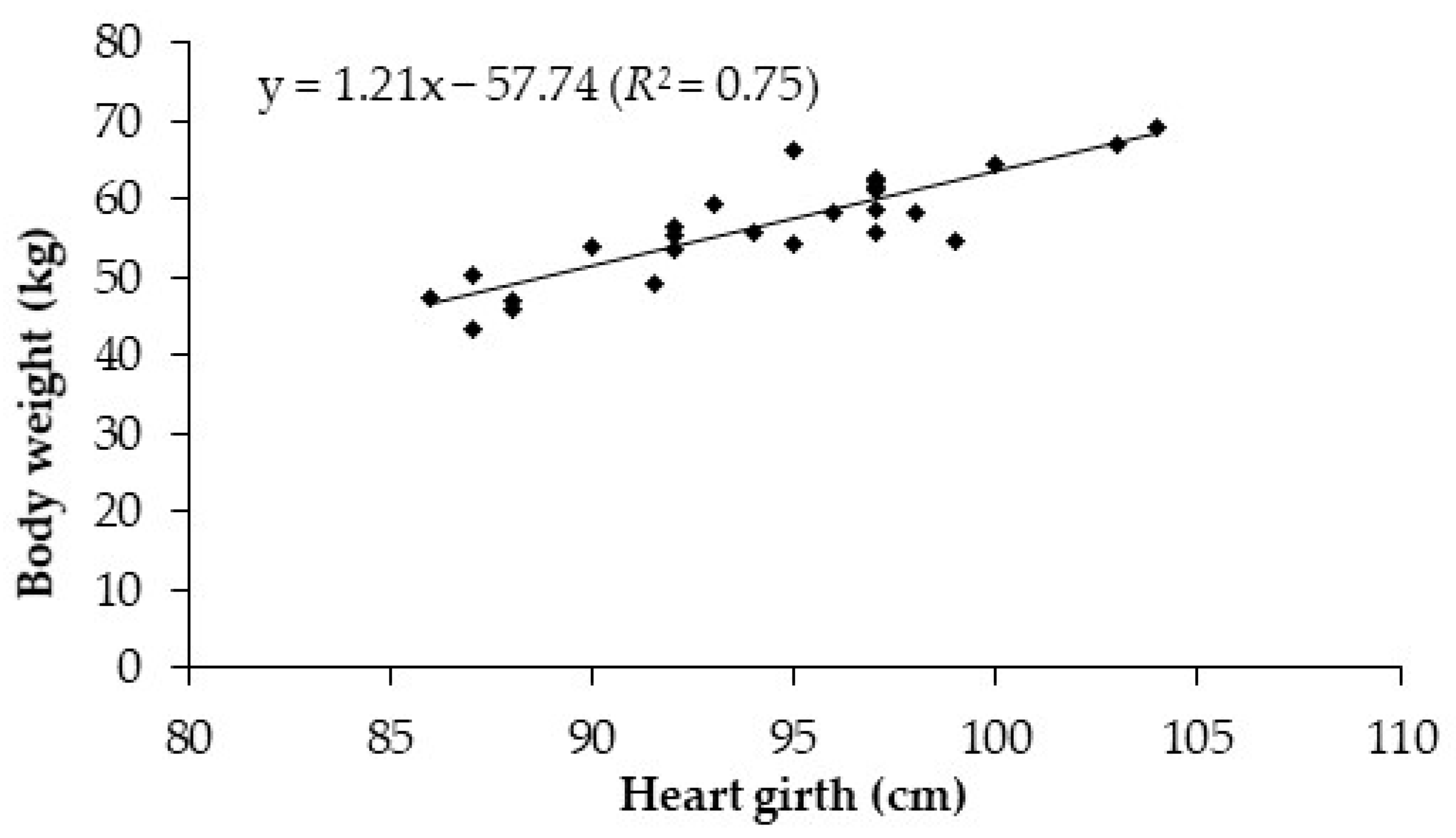
3.1.1. Somatic Measurements
3.1.2. Body Weight
3.1.3. Body Condition Score
3.1.4. Ultrasound Measurements
3.1.5. Multiple Regressions
3.2. Post-Slaughter Measurements
3.2.1. Hot Carcass Weight
3.2.2. Empty Body Weight
3.2.3. Digital Caliper Measurements
3.2.4. Multiple Regressions
4. Discussion
- (1)
- OM: 4.79 + 0.13 × BW + 1.57 × BCSl − 0.16 × WH − 0.15 × RL (R2 = 0.55);
- (2)
- MES: 1.57 + 0.05 × BW − 0.04 × WH (R2 = 0.56);
- (3)
- PR: 2.79 + 0.08 × BW − 0.07 × WH − 0.09 × PW (R2 = 0.48);
- (4)
- ORG: 1.21 + 0.02 × BW − 0.06 × CD (R2 = 0.60);
- (5)
- CARC: −3.31 + 0.12 × BW + 3.19 × BCSl − 0.07 × RH − 0.12 × SW (R2 = 0.87);
- (6)
- NC: 1.65 + 0.25 × BW + 2.61 × BCSl − 0.16 × WH − 0.31 × PW (R2 = 0.69);
- (7)
- TOT: −4.21 + 0.36 × BW + 6.12 × BCSl − 0.21 × WH − 0.38 × SW (R2 = 0.79).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eknæs, M.; Kolstad, K.; Volden, H.; Hove, K. Changes in body reserves and milk quality throughout lactation in dairy goats. Small Rumin. Res. 2006, 63, 1–11. [Google Scholar] [CrossRef]
- Ngwa, A.T.; Dawson, L.J.; Puchala, R.; Detweiler, G.; Merkel, R.C.; Wang, Z.; Tesfai, K.; Sahlu, T.; Ferrell, C.L.; Goetsch, A.L. Effects of stage of lactation and dietary forage level on body composition of Alpine dairy goats. J. Dairy Sci. 2009, 92, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Cannas, A.; Tedeschi, L.O.; Fox, D.G.; Pell, A.N.; Van Soest, P.J. A mechanistic model for predicting the nutrient requirements and feed biological values for sheep. J. Anim. Sci. 2004, 82, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.; Chizzotti, M.L.; Busato, K.C.; Rodrigues, R.T.S.; da Silva, I.F.; Queiroz, M.A.Á. Indirect methods for predicting body composition of Boer crossbreds and indigenous goats from the Brazilian semiarid. Trop. Anim. Health Prod. 2015, 47, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Akdag, F.; Teke, B.; Meral, Y.; Arslan, S.; Ugurlu, M. Prediction of carcass composition by ultrasonic measurement and the effect of region and age on ultrasonic measurements. Small Rumin. Res. 2015, 133, 82–87. [Google Scholar] [CrossRef]
- Scholz, A.M.; Bünger, L.; Kongsro, J.; Baulain, U.; Mitchell, A.D. Non-invasive methods for the determination of body and carcass composition in livestock: Dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: Invited review. Animal 2015, 9, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Díaz, E.; Salazar-Cuytun, R.; Chay-Canul, A.J.; Garcia Herrera, R.A.; Piñeiro-Vázquez, Á.T.; Magaña Monforte, J.G.; Tedeschi, L.O.; Cruz-Hernández, A.; Gómez-Vázquez, A. Determination of carcass traits in Pelibuey ewes using biometric measurements. Small Rumin. Res. 2017, 147, 115–119. [Google Scholar] [CrossRef]
- Cam, M.A.; Olfaz, M.; Soydan, E. Possibilities of using morphometrics characteristics as a tool for body weight prediction in Turkish hair goats (Kilkeci). Asian J. Anim. Vet. Adv. 2010, 5, 52–59. [Google Scholar] [CrossRef][Green Version]
- Hervieu, J.; Morand-Fehr, P.; Schmidely, P.; Fedele, V.; Delfa, R. Mesures anatomiques permettant d’expliquer les variations des notes sternales, lombaires et caudales utilisées pour estimer l’état corporel des chèvres laitières. In Etat Corporel des Brebis et Chèvres, Proceedings of CIHEAM, Zaragoza; Purroy, A., Ed.; 1991; pp. 43–56. Available online: https://om.ciheam.org/article.php?IDPDF=92605094 (accessed on 18 May 2021).
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. (Camb.) 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Ngwa, A.T.; Dawson, L.J.; Puchala, R.; Detweiler, G.; Merkel, R.C.; Tovar-Luna, I.; Sahlu, T.; Ferrell, C.L.; Goetsch, A.L. Urea space and body condition score to predict body composition of meat goats. Small Rumin. Res. 2007, 73, 27–36. [Google Scholar] [CrossRef]
- Mendizabal, J.A.; Delfa, R.; Arana, A.; Purroy, A. A comparison of different pre- and post-slaughter measurements for estimating fat reserves in Spanish Blanca Celtibérica goats. Can. J. Anim. Sci. 2010, 90, 437–444. [Google Scholar] [CrossRef]
- Teixeira, A.; Joy, M.; Delfa, R. In vivo estimation of goat carcass composition and body fat partition by real-time ultrasonography. J. Anim. Sci. 2008, 86, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Rocher, F.; Kirton, A.H.; Mercer, G.J.K.; Duganzich, D.M. Carcass composition of New Zealand Saanen goats slaughtered at different weights. Small Rumin. Res. 1992, 7, 161–173. [Google Scholar] [CrossRef]
- Härter, C.J.; Silva, H.G.O.; Lima, L.D.; Castagnino, D.S.; Rivera, A.R.; Neto, O.B.; Gomes, R.A.; Canola, J.C.; Resende, K.T.; Teixeira, I.A.M.A. Ultrasonographic measurements of kidney fat thickness and Longissimus muscle area in predicting body composition of pregnant goats. Anim. Prod. Sci. 2014, 54, 1481–1485. [Google Scholar] [CrossRef]
- McGregor, B.A. Relationships between live weight, body condition, dimensional and ultrasound scanning measurements and carcass attributes in adult Angora goats. Small Rumin. Res. 2017, 147, 8–17. [Google Scholar] [CrossRef]
- Slippers, S.C.; Letty, B.A.; de Villiers, J.F. Prediction of the body weight of Nguni goats. S. Afr. J. Anim. Sci. 2000, 30 (Suppl. 1), 127–128. [Google Scholar] [CrossRef]
- Teixeira, A.; Delfa, R.; Colomer-Rocher, F. Relationships between fat depots and body condition score or tail fatness in the Rasa Aragonesa breed. Anim. Sci. 1989, 49, 275–280. [Google Scholar] [CrossRef]
- Colomer-Rocher, F.; Morand-Fehr, P.; Kirton, A.H. Standard methods and procedures for goat carcass evaluation, jointing and tissue separation. Livest. Prod. Sci. 1987, 17, 149–159. [Google Scholar] [CrossRef]
- Delfa, R.; González, C.; Teixeira, A.; Gosalvez, L.F.; Tor, M. Relationships between body fat depots, carcass composition, live weight and body condition scores in Blanca Celtibérica goats. In Body Condition of Sheep and Goats: Methodological Aspects and Applications, Proceeding of CIHEAM, Zaragoza; Purroy, A., Ed.; 1995; pp. 109–119. Available online: https://om.ciheam.org/article.php?IDPDF=96605600 (accessed on 18 May 2021).

| Item | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Somatic measurements (cm) | ||||
| Wither height | 70.9 | 65.0 | 77.0 | 3.4 |
| Rump height | 73.6 | 69.0 | 82.0 | 3.3 |
| Rump length | 21.6 | 17.5 | 28.0 | 3.0 |
| Pelvis width | 18.7 | 16.0 | 25.0 | 1.9 |
| Chest depth | 35.1 | 29.0 | 39.0 | 2.1 |
| Shoulder width | 18.1 | 13.5 | 24.0 | 2.5 |
| Heart girth | 94.2 | 86.0 | 104.0 | 4.8 |
| Body length | 74.7 | 68.0 | 90.0 | 4.7 |
| Sternum height | 35.3 | 28.0 | 44.0 | 3.9 |
| BW (kg) | 56.4 | 43.6 | 69.4 | 6.8 |
| BCSl (scale 0–5) | 2.64 | 1.84 | 2.96 | 0.3 |
| BCSs (scale 0–5) | 2.64 | 1.75 | 3.00 | 0.3 |
| HCW (kg) | 24.3 | 18.1 | 30.1 | 3.4 |
| EBW (kg) | 47.6 | 36.1 | 59.9 | 6.8 |
| FTUSl (mm) | 2.27 | 1.08 | 3.50 | 0.6 |
| FTDCl (mm) | 2.13 | 1.47 | 3.60 | 0.5 |
| FTUSs (mm) | 22.68 | 9.70 | 27.90 | 4.5 |
| FTDCs (mm) | 22.10 | 9.79 | 29.82 | 4.7 |
| FTUSpr (cm) | 1.21 | 0.44 | 2.34 | 0.1 |
| Fat depot weight (kg) | ||||
| Omental | 1.83 | 0.30 | 4.38 | 1.3 |
| Mesenteric | 0.94 | 0.46 | 1.54 | 0.3 |
| Perirenal | 0.72 | 0.07 | 2.02 | 0.6 |
| Organ fat (heart, liver, and lungs) | 0.22 | 0.08 | 1.06 | 0.2 |
| Total fat (kg) | ||||
| Carcass | 4.61 | 1.33 | 6.58 | 1.3 |
| Non-carcass | 5.56 | 2.16 | 9.38 | 2.1 |
| Carcass and non-carcass | 10.17 | 3.49 | 15.93 | 3.2 |
| Item 1 | Intercept ± Standard Error | b ± Standard Error | R2 | RSD | p-Value |
|---|---|---|---|---|---|
| Heart girth | |||||
| OM | −9.26 ± 4.58 | 0.12 ± 0.05 | 0.20 | 1.17 | 0.023 |
| MES | −2.96 ± 1.06 | 0.04 ± 0.01 | 0.36 | 0.27 | 0.001 |
| PR | −3.83 ± 2.17 | 0.05 ± 0.02 | 0.16 | 0.56 | 0.046 |
| ORG | −1.47 ± 0.67 | 0.02 ± 0.01 | 0.21 | 0.17 | 0.019 |
| CARC | −12.49 ± 3.82 | 0.18 ± 0.04 | 0.46 | 0.98 | 0.001 |
| NC | −19.27 ± 6.58 | 0.26 ± 0.07 | 0.37 | 1.68 | 0.001 |
| TOT | −31.76 ± 9.95 | 0.44 ± 0.10 | 0.43 | 2.55 | 0.001 |
| BW at slaughter | |||||
| OM | −3.82 ± 1.87 | 0.10 ± 0.03 | 0.28 | 1.11 | 0.005 |
| MES | −0.86 ± 0.43 | 0.03 ± 0.01 | 0.43 | 0.26 | 0.001 |
| PR | −1.89 ± 0.86 | 0.05 ± 0.01 | 0.28 | 0.51 | 0.005 |
| ORG | −0.49 ± 0.29 | 0.01 ± 0.005 | 0.21 | 0.17 | 0.020 |
| CARC | −3.65 ± 1.45 | 0.15 ± 0.02 | 0.58 | 0.86 | <0.001 |
| NC | −6.55 ± 2.57 | 0.21 ± 0.04 | 0.49 | 1.52 | <0.001 |
| TOT | −10.20 ± 3.80 | 0.36 ± 0.07 | 0.55 | 2.25 | <0.001 |
| BCS lumbar (scale 0–5) | |||||
| OM | −3.95 ± 2.38 | 2.21 ± 0.89 | 0.20 | 1.17 | 0.022 |
| MES | −0.42 ± 0.63 | 0.52 ± 0.24 | 0.17 | 0.31 | 0.038 |
| PR | −1.73 ± 1.12 | 0.93 ± 0.42 | 0.17 | 0.55 | 0.037 |
| ORG | −0.38 ± 0.37 | 0.23 ± 0.14 | 0.10 | 0.18 | 0.116 |
| CARC | −5.79 ± 1.64 | 3.94 ± 0.62 | 0.63 | 0.81 | <0.001 |
| NC | −6.30 ± 3.57 | 4.50 ± 1.34 | 0.32 | 1.76 | 0.003 |
| TOT | −12.08 ± 5.07 | 8.44 ± 1.91 | 0.45 | 2.50 | 0.001 |
| BCS sternal (scale 0–5) | |||||
| OM | −3.08 ± 2.37 | 1.87 ± 0.89 | 0.16 | 1.20 | 0.047 |
| MES | −0.49 ± 0.60 | 0.55 ± 0.22 | 0.20 | 0.30 | 0.023 |
| PR | −1.45 ± 1.10 | 0.83 ± 0.42 | 0.14 | 0.56 | 0.057 |
| ORG | −0.28 ± 0.37 | 0.19 ± 0.14 | 0.07 | 0.19 | 0.183 |
| CARC | −4.76 ± 1.76 | 3.55 ± 0.66 | 0.54 | 0.90 | <0.001 |
| NC | −4.95 ± 3.59 | 3.99 ± 1.35 | 0.27 | 1.82 | 0.007 |
| TOT | −9.71 ± 5.21 | 7.54 ± 1.96 | 0.38 | 2.64 | 0.001 |
| Item 1 | Intercept ± Standard Error | b ± Standard Error | R2 | RSD | p-Value |
|---|---|---|---|---|---|
| Lumbar fat thickness (FTUSl) | |||||
| OM | −0.46 ± 0.92 | 1.04 ± 0.39 | 0.23 | 1.16 | 0.012 |
| MES | 0.37 ± 0.24 | 0.27 ± 0.10 | 0.23 | 0.30 | 0.012 |
| PR | −0.38 ± 0.42 | 0.50 ± 0.18 | 0.25 | 0.53 | 0.008 |
| ORG | −0.003 ± 0.14 | 0.10 ± 0.06 | 0.10 | 0.18 | 0.120 |
| CARC | 2.07 ± 0.92 | 1.13 ± 0.39 | 0.27 | 1.16 | 0.007 |
| NC | 1.10 ± 1.40 | 2.01 ± 0.59 | 0.33 | 1.76 | 0.002 |
| TOT | 3.16 ± 2.23 | 3.14 ± 0.95 | 0.32 | 2.81 | 0.002 |
| Sternal fat thickness (FTUSs) | |||||
| OM | −1.81 ± 1.05 | 0.16 ± 0.05 | 0.35 | 1.06 | 0.001 |
| MES | 0.17 ± 0.29 | 0.03 ± 0.01 | 0.24 | 0.29 | 0.011 |
| PR | −1.01 ± 0.48 | 0.08 ± 0.02 | 0.37 | 0.48 | 0.001 |
| ORG | 0.02 ± 0.19 | 0.01 ± 0.01 | 0.05 | 0.19 | 0.278 |
| CARC | −0.04 ± 0.88 | 0.21 ± 0.04 | 0.55 | 0.89 | <0.001 |
| NC | −1.11 ± 1.57 | 0.30 ± 0.07 | 0.44 | 1.59 | 0.001 |
| TOT | −1.15 ± 2.33 | 0.51 ± 0.10 | 0.51 | 2.36 | <0.001 |
| Perirenal fat thickness (FTUSpr) | |||||
| OM | −0.53 ± 0.26 | 2.23 ± 0.22 | 0.81 | 0.57 | <0.001 |
| MES | 0.48 ± 0.11 | 0.45 ± 0.09 | 0.49 | 0.24 | <0.001 |
| PR | −0.40 ± 0.10 | 1.06 ± 0.09 | 0.86 | 0.22 | <0.001 |
| ORG | 0.14 ± 0.09 | 0.08 ± 0.07 | 0.05 | 0.19 | 0.283 |
| CARC | 2.88 ± 0.46 | 1.64 ± 0.49 | 0.42 | 1.01 | 0.001 |
| NC | 1.94 ± 0.51 | 3.44 ± 0.43 | 0.73 | 1.11 | <0.001 |
| TOT | 4.82 ± 0.93 | 5.07 ± 0.78 | 0.63 | 2.03 | <0.001 |
| Item 1 | Intercept ± Standard Error | b ± Standard Error | R2 | RSD | p-Value |
|---|---|---|---|---|---|
| Hot carcass weight (HCW) | |||||
| OM | −4.00 ± 1.39 | 0.24 ± 0.06 | 0.43 | 0.99 | 0.001 |
| MES | −0.81 ± 0.31 | 0.07 ± 0.01 | 0.58 | 0.22 | <0.001 |
| PR | −1.95 ± 0.65 | 0.11 ± 0.03 | 0.42 | 0.46 | 0.001 |
| ORG | −0.32 ± 0.25 | 0.02 ± 0.01 | 0.17 | 0.18 | 0.037 |
| CARC | −3.55 ± 0.80 | 0.34 ± 0.03 | 0.82 | 0.57 | <0.001 |
| NC | −5.89 ± 1.83 | 0.47 ± 0.07 | 0.63 | 1.30 | <0.001 |
| TOT | −9.44 ± 2.43 | 0.81 ± 0.10 | 0.74 | 1.73 | <0.001 |
| Empty body weight (EBW) | |||||
| OM | −4.30 ± 1.34 | 0.13 ± 0.03 | 0.47 | 0.95 | 0.001 |
| MES | −0.86 ± 0.30 | 0.04 ± 0.01 | 0.61 | 0.21 | <0.001 |
| PR | −2.08 ± 0.63 | 0.06 ± 0.01 | 0.46 | 0.44 | 0.001 |
| ORG | −0.42 ± 0.24 | 0.01 ± 0.01 | 0.23 | 0.17 | 0.012 |
| CARC | −3.20 ± 0.95 | 0.16 ± 0.02 | 0.74 | 0.67 | <0.001 |
| NC | −6.44 ± 1.69 | 0.25 ± 0.03 | 0.68 | 1.20 | <0.001 |
| TOT | −9.64 ± 2.40 | 0.42 ± 0.05 | 0.74 | 1.70 | <0.001 |
| Lumbar fat thickness (FTDCl) | |||||
| OM | 1.52 ± 1.12 | 0.16 ± 0.51 | 0.01 | 1.31 | 0.760 |
| MES | 0.75 ± 0.29 | 0.10 ± 0.13 | 0.02 | 0.33 | 0.473 |
| PR | 0.72 ± 0.52 | 0.01 ± 0.24 | 0.01 | 0.61 | 0.976 |
| ORG | −0.10 ± 0.15 | 0.15 ± 0.07 | 0.17 | 0.18 | 0.036 |
| CARC | 3.42 ± 1.11 | 0.56 ± 0.51 | 0.05 | 1.29 | 0.279 |
| NC | 4.65 ± 1.82 | 0.45 ± 0.83 | 0.01 | 2.12 | 0.591 |
| TOT | 8.07 ± 2.86 | 1.02 ± 1.31 | 0.02 | 3.32 | 0.444 |
| Sternal fat thickness (FTDCs) | |||||
| OM | −1.97 ± 1.03 | 0.17 ± 0.04 | 0.37 | 1.03 | 0.001 |
| MES | 0.09 ± 0.29 | 0.04 ± 0.01 | 0.28 | 0.29 | 0.005 |
| PR | −1.09 ± 0.47 | 0.08 ± 0.02 | 0.40 | 0.47 | 0.001 |
| ORG | −0.08 ± 0.18 | 0.01 ± 0.01 | 0.11 | 0.18 | 0.099 |
| CARC | −0.34 ± 0.82 | 0.22 ± 0.04 | 0.61 | 0.82 | <0.001 |
| NC | −1.55 ± 1.50 | 0.32 ± 0.07 | 0.50 | 1.51 | <0.001 |
| TOT | −1.89 ± 2.20 | 0.54 ± 0.10 | 0.57 | 2.21 | <0.001 |
| Step | Dependent Variable (y) 1 | Independent Variables (x) | Intercept ± Standard Error 2 | b ± Standard Error | R2 | RSD |
|---|---|---|---|---|---|---|
| 1 | OM | FTUSpr | −7.77 ± 2.85 | 2.10 ± 0.27 | 0.79 | 0.65 |
| 2 | HG | 0.08 ± 0.03 | 0.85 | 0.57 | ||
| 1 | MES | FTUSpr | −2.43 ± 1.13 | 0.35 ± 0.11 | 0.46 | 0.26 |
| 2 | HG | 0.03 ± 0.01 | 0.62 | 0.23 | ||
| 1 | PR | FTUSpr | −1.66 ± 0.50 | 0.97 ± 0.11 | 0.84 | 0.26 |
| 2 | BW | 0.02 ± 0.01 | 0.88 | 0.23 | ||
| 1 | ORG | FTUSl | −0.21 ± 0.15 | 0.07 ± 0.03 | 0.09 | 0.19 |
| 2 | BW | 0.004 ± 0.003 | 0.40 | 0.07 | ||
| 1 | CARC | FTUSpr | −7.63 ± 1.14 | 0.80 ± 0.23 | 0.47 | 1.09 |
| 2 | BW | 0.09 ± 0.02 | 0.79 | 0.70 | ||
| 3 | BCSl | 2.25 ± 0.47 | 0.92 | 0.46 | ||
| 1 | NC | FTUSpr | −18.21 ± 3.57 | 2.59 ± 0.36 | 0.70 | 1.25 |
| 2 | HG | 0.19 ± 0.04 | 0.89 | 0.76 | ||
| 3 | BCSl | 1.32 ± 0.75 | 0.91 | 0.71 | ||
| 1 | TOT | FTUSpr | −15.80 ± 2.85 | 3.22 ± 0.58 | 0.62 | 2.28 |
| 2 | BW | 0.22 ± 0.05 | 0.86 | 1.44 | ||
| 3 | BCSl | 3.79 ± 1.18 | 0.92 | 1.14 |
| Step | Dependent Variable (y) 1 | Independent Variable(s) (x) | Intercept ± Standard Error 2 | b ± Standard Error | R2 | RSD |
|---|---|---|---|---|---|---|
| 1 | OM | EBW | −4.46 ± 1.31 | 0.09 ± 0.03 | 0.47 | 0.95 |
| 2 | FTDCs | 0.08 ± 0.05 | 0.52 | 0.92 | ||
| 1 | MES | EBW | −0.86 ± 0.30 | 0.04 ± 0.01 | 0.61 | 0.21 |
| 1 | PR | EBW | −2.16 ± 0.60 | 0.04 ± 0.02 | 0.46 | 0.44 |
| 2 | FTDCs | 0.04 ± 0.02 | 0.53 | 0.43 | ||
| 1 | ORG | EBW | −0.54 ± 0.24 | 0.01 ± 0.005 | 0.23 | 0.17 |
| 2 | FTDCl | 0.11 ± 0.07 | 0.32 | 0.16 | ||
| 1 | CARC | HCW | −3.83 ± 0.55 | 0.36 ± 0.07 | 0.82 | 0.57 |
| 2 | FTDCs | 0.12 ± 0.02 | 0.91 | 0.40 | ||
| 3 | EBW | -0.06 ± 0.04 | 0.92 | 0.38 | ||
| 1 | NC | EBW | −6.70 ± 1.58 | 0.19 ± 0.04 | 0.68 | 1.20 |
| 2 | FTDCs | 0.14 ± 0.06 | 0.74 | 1.12 | ||
| 1 | TOT | HCW | −10.44 ± 2.00 | 0.60 ± 0.10 | 0.74 | 1.73 |
| 2 | FTDCs | 0.27 ± 0.08 | 0.83 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knupp, L.S.; Lunesu, M.F.; Costa, R.G.; Ledda, M.; Knupp, S.N.R.; Acciaro, M.; Decandia, M.; Molle, G.; Francesconi, A.H.D.; Cannas, A. Pre- and Post-Slaughter Methodologies to Estimate Body Fat Reserves in Lactating Saanen Goats. Animals 2021, 11, 1440. https://doi.org/10.3390/ani11051440
Knupp LS, Lunesu MF, Costa RG, Ledda M, Knupp SNR, Acciaro M, Decandia M, Molle G, Francesconi AHD, Cannas A. Pre- and Post-Slaughter Methodologies to Estimate Body Fat Reserves in Lactating Saanen Goats. Animals. 2021; 11(5):1440. https://doi.org/10.3390/ani11051440
Chicago/Turabian StyleKnupp, Leonardo Sidney, Mondina Francesca Lunesu, Roberto Germano Costa, Mauro Ledda, Sheila Nogueira Ribeiro Knupp, Marco Acciaro, Mauro Decandia, Giovanni Molle, Ana Helena Dias Francesconi, and Antonello Cannas. 2021. "Pre- and Post-Slaughter Methodologies to Estimate Body Fat Reserves in Lactating Saanen Goats" Animals 11, no. 5: 1440. https://doi.org/10.3390/ani11051440
APA StyleKnupp, L. S., Lunesu, M. F., Costa, R. G., Ledda, M., Knupp, S. N. R., Acciaro, M., Decandia, M., Molle, G., Francesconi, A. H. D., & Cannas, A. (2021). Pre- and Post-Slaughter Methodologies to Estimate Body Fat Reserves in Lactating Saanen Goats. Animals, 11(5), 1440. https://doi.org/10.3390/ani11051440







