Behavioral Implications of the Complete Absence of Guests on a Zoo-Housed Gorilla Troop
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Subjects
2.3. Procedures
2.4. Data Analysis
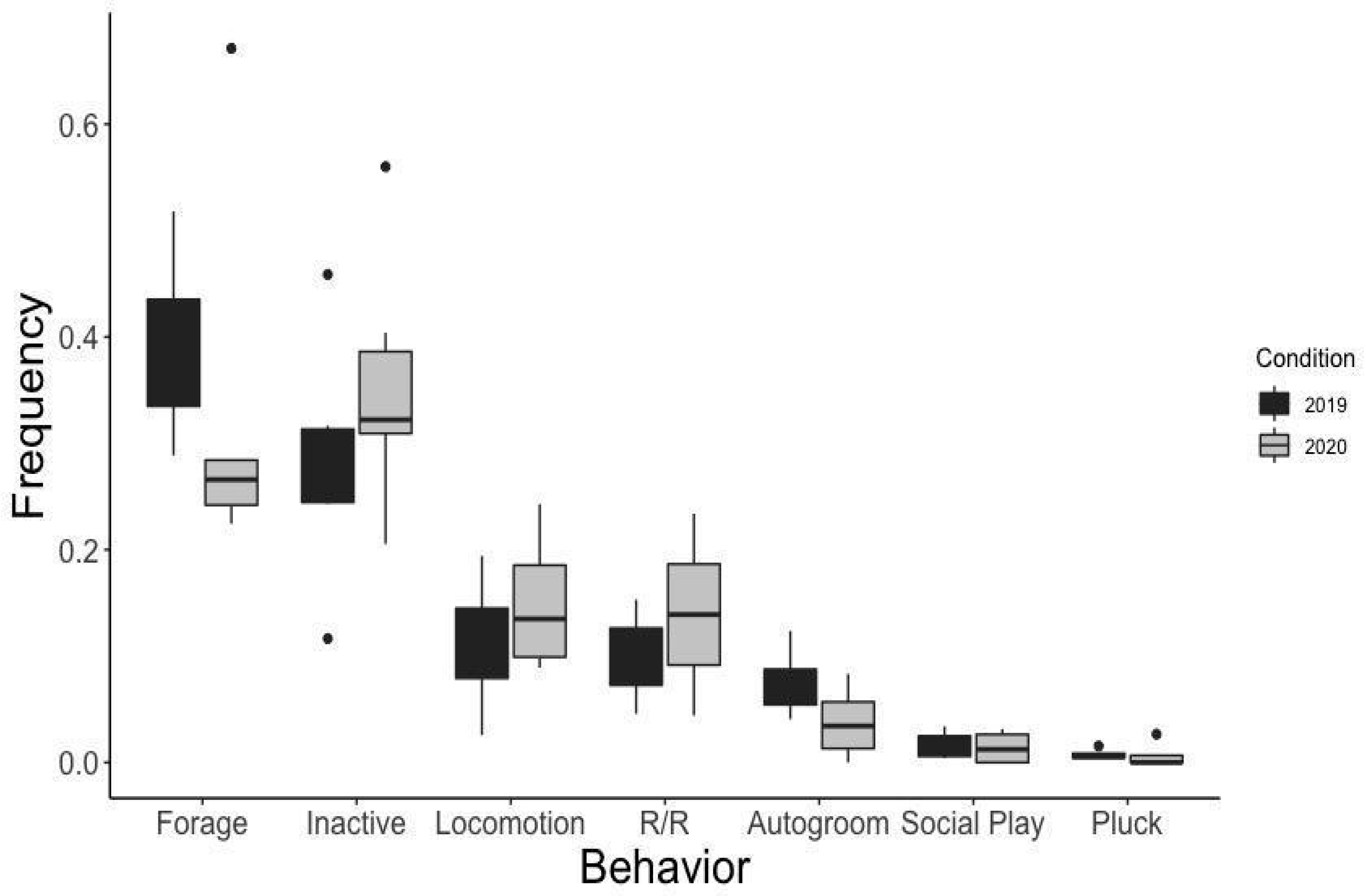
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maple, T.; Perdue, B.M. Zoo Animal Welfare; Springer: Berlin, Geramny, 2013. [Google Scholar]
- Ward, S.J.; Sherwin, S.; Clark, F.E. Advances in applied zoo animal welfare science. J. Appl. Anim. Welf. Sci. 2018, 21, 23–33. [Google Scholar] [CrossRef]
- Hill, S.P.; Broom, D.M. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Hosey, G.R. Zoo animals and their human audiences: What is the visitor effect? Anim. Welf. 2000, 9, 343–357. [Google Scholar]
- Davey, G. Visitors’ effects on the welfare of animals in the zoo: A review. J. Appl. Anim. Welf. Sci. 2007, 10, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.M.G.; Thyssen, A.; Laevens, H.; Vervaecke, H. The influence of zoo visitor numbers on the behaviour of harbour seals (Phoca vitulina). J. Zoo Aquar. Res. 2013, 1, 31–34. [Google Scholar]
- Larsen, M.J.; Sherwin, S.L.; Rault, J. Number of nearby visitors and noise level affect vigilance in captive koalas. Appl. Anim. Behav. Sci. 2014, 154, 76–82. [Google Scholar] [CrossRef]
- Thicks, S. Is there a visitor effect on Abyssinian ground hornbills (Bucorvus abyssinicus), Papuan wreathed hornbills (Aceros plicatus), wrinkled hornbills (Aceros corrugates) and toco tucans (Ramphastos toco) in a captive zoo environment? Plymouth Stud. Sci. 2008, 1, 30–55. [Google Scholar]
- Sherwin, S.L.; Harvey, T.J.; Magrath, M.J.L.; Butler, K.L.; Fanson, K.V.; Hemsworth, P.H. Effects of visual contact with zoo visitors on black-capped capuchin welfare. Appl. Anim. Behav. Sci. 2015, 167, 65–73. [Google Scholar] [CrossRef]
- López-Álvarez, J.; Sanjorge, Y.; Soloaga, S.; Crailsheim, D.; Llorente, M. Looking for visitor’s effect in sanctuaries: Implications of guided visitor groups on the behavior of the chimpanzees at Fundació Mona. Animals 2019, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.K.; Hopper, L.M.; Fultz, A.L.; Ross, S.R. Understanding the behavior of sanctuary-housed chimpanzees during public programs. Anthrozoös 2020, 33, 481–495. [Google Scholar] [CrossRef]
- Choo, Y.; Todd, P.A.; Li, D. Visitor effects on zoo orangutans in two novel, naturalistic enclosures. Appl. Anim. Behav. Sci. 2011, 133, 78–86. [Google Scholar] [CrossRef]
- Cooke, C.M.; Schillace, M.A. Behavioral responses to the zoo environment by white handed gibbons. Appl. Anim. Behav. Sci. 2007, 106, 125–133. [Google Scholar] [CrossRef]
- Stoinski, T.S.; Jaicks, H.F.; Drayton, L.A. Visitor effects on the behavior of captive western lowland gorillas: The importance of individual differences in examining welfare. Zoo Biol. 2012, 31, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Blaney, E.C.; Wells, D.L. The influence of a camouflage net barrier on the behaviour, welfare and public perceptions of zoo-housed gorillas. Anim. Welf. 2004, 13, 111–118. [Google Scholar]
- Lenczewski, M.; Halfdanardottir, M.R.; Margulis, S. Now you see me (Now you don’t): A visual barrier study on a zoo-housed group of western lowland gorillas. Artic. Bones Discourse 2017, 7, 77–91. [Google Scholar]
- Clark, F.E.; Fitzpatrick, M.; Hartley, A.; King, A.J.; Lee, T.; Routh, A.; Walker, S.L.; George, K. Relationship between behavior, adrenal activity, and environment in zoo-housed Western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2012, 31, 306–321. [Google Scholar] [CrossRef]
- Wood, W. Interactions among environmental enrichment, viewing crowds, and zoo chimpanzees (Pan troglodytes). Zoo Biol. 1998, 17, 211–230. [Google Scholar] [CrossRef]
- Wells, D.L. A note on the influence of visitors on the behaviour and welfare of zoo-housed gorillas. Appl. Anim. Behav. Sci. 2005, 93, 13–17. [Google Scholar] [CrossRef]
- Chamove, A.S.; Hosey, G.R.; Schaetzel, P. Visitors excite primates in zoos. Zoo Biol. 1988, 7, 359–369. [Google Scholar] [CrossRef]
- Lewis, R.N.; Chang, Y.; Ferguson, A.; Lee, T.; Clifforde, L.; Abeyesinghe, S.M. The effect of visitors on the behavior of zoo-housed western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2020, 39, 283–296. [Google Scholar] [CrossRef]
- Carder, G.; Semple, S. Visitor effects on anxiety in two captive groups of western lowland gorillas. Appl. Anim. Behav. Sci. 2008, 115, 211–220. [Google Scholar] [CrossRef]
- Davis, N.; Schaffner, C.M.; Smith, T.E. Evidence that zoo visitors influence HPA activity in spider monkeys (Ateles geoffroyii rufiventris). Appl. Anim. Behav. Sci. 2005, 90, 131–141. [Google Scholar] [CrossRef]
- Kuhar, C.W. Group differences in captive gorillas’ reaction to large crowds. Appl. Anim. Behav. Sci. 2008, 110, 377–385. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 15 March 2021).
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Woolway, E.E.; Goodenough, A.E. Effects of visitor numbers on captive European red squirrels (Sciurus vulgaris) and impacts on visitor experience. Zoo Biol. 2017, 36, 112–119. [Google Scholar] [CrossRef] [PubMed]

| Individual | DOB | Sex | Observations Used/Total 2019 | Observations Used/Total 2020 |
|---|---|---|---|---|
| Koga (AM) | 14 Aug 1987 | M | 17/21 | 20/27 |
| Sidney (AF1) | 6 Apr 1997 | F | 18/20 | 20/25 |
| Lily (AF2) | 19 Dec 2000 | F | 19/20 | 21/22 |
| Amari (SAF) | 8 Oct 2010 | F | 17/21 | 19/24 |
| Nyah (JF) | 4 Sept 2013 | F | 17/25 | 20/25 |
| Kayin (JM) | 10 Jan 2016 | M | 16/18 | 20/25 |
| State Behavior Analyzed for This Study | |
|---|---|
| Inactive | Sitting, standing, or laying while not engaged in physical activity |
| Locomotion | Movement on the ground terrestrially (T) or arboreally (A) utilizing Sclimbing structures in the exhibit |
| Auto-groom | Scratching, itching, rubbing, or self-grooming |
| R/R | Regurgitation and reingestion seen when a gorilla regurgitates food and consumes it again |
| Pluck | Pulling hair out |
| Social play | Any number of chasing, pulling, or biting contributing to ruff and tumble play |
| Forage | Searching or eating substrate |
| Out of view | The subject gorilla is beyond the observer’s field of view |
| Individual | Behavior | ||||||
|---|---|---|---|---|---|---|---|
| Forage | Inactive | Locomote | R/R | Auto-Groom | Social Play | Pluck | |
| Koga (AM) | 0.0585 | 0.02348 | 0.0319 | n/a | 0.0095 * | n/a | 0.1863 |
| Sidney (AF1) | 0.2259 | 0.7636 | 0.247 | 0.6546 | 0.7982 | 0.3531 | 0.3531 |
| Lily (AF2) | 0.1011 | 0.3125 | 0.9373 | 0.8598 | 0.0653 | 0.3028 | 0.3028 |
| Amari (SAF) | 0.0292 | 0.01003 * | 0.4245 | n/a | 0.9375 | n/a | 0.0795 |
| Nyah (JF) | 0.0849 | 0.3286 | 0.4321 | n/a | 0.5664 | 0.3222 | n/a |
| Kayin (JM) | 0.2691 | 0.08796 | 0.2262 | n/a | 0.5036 | 0.59 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, M.E.; Robinson, C.M.; Margulis, S.W. Behavioral Implications of the Complete Absence of Guests on a Zoo-Housed Gorilla Troop. Animals 2021, 11, 1346. https://doi.org/10.3390/ani11051346
Miller ME, Robinson CM, Margulis SW. Behavioral Implications of the Complete Absence of Guests on a Zoo-Housed Gorilla Troop. Animals. 2021; 11(5):1346. https://doi.org/10.3390/ani11051346
Chicago/Turabian StyleMiller, Megan E., Caeley M. Robinson, and Susan W. Margulis. 2021. "Behavioral Implications of the Complete Absence of Guests on a Zoo-Housed Gorilla Troop" Animals 11, no. 5: 1346. https://doi.org/10.3390/ani11051346
APA StyleMiller, M. E., Robinson, C. M., & Margulis, S. W. (2021). Behavioral Implications of the Complete Absence of Guests on a Zoo-Housed Gorilla Troop. Animals, 11(5), 1346. https://doi.org/10.3390/ani11051346






