The Genetic Diversity and Structure of the European Turtle Dove Streptopelia turtur
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Isolation, Amplification and Sequencing
2.3. Data Analysis
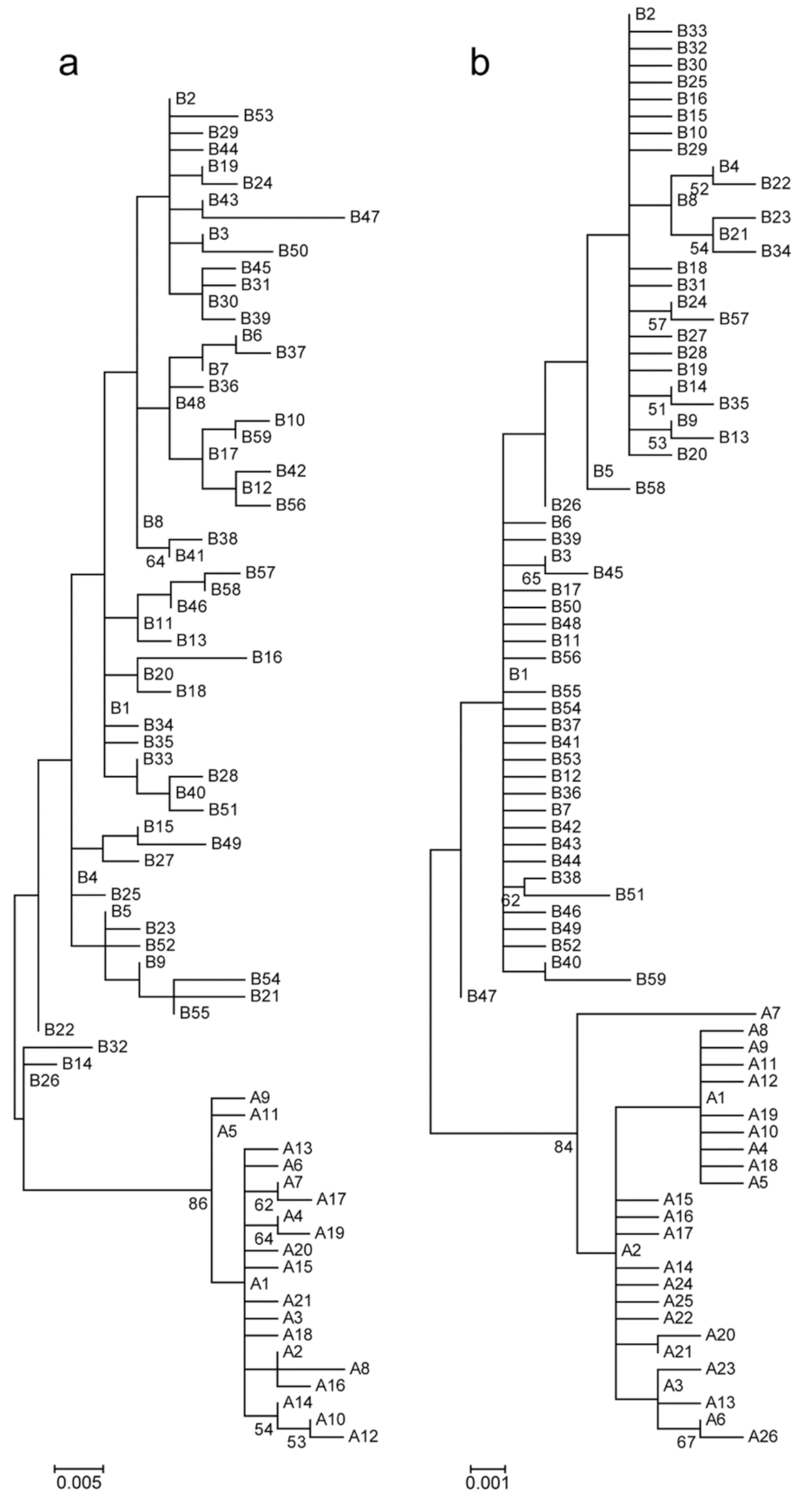
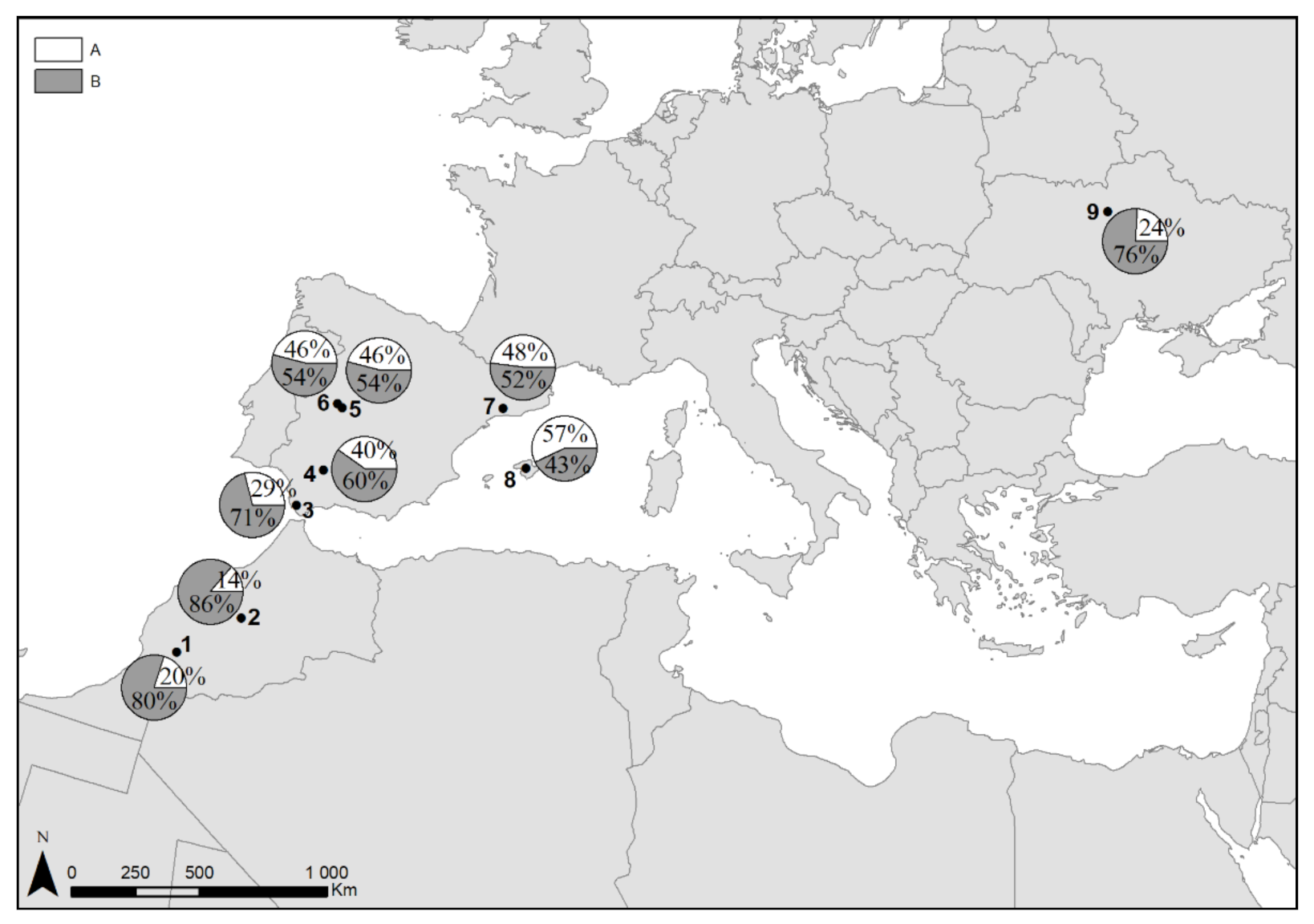
3. Results
3.1. Genetic Variation
3.2. Population Genetic Structure
4. Discussion
4.1. Population Genetic Studies in Columbidae
4.2. Genetic Diversity in the Turtle Dove
4.3. Evolutionary Lineages of Turtle Doves
4.4. Species Management and Conservation Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cramp, S. Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford, UK, 1985; Volume 4, pp. 353–363. [Google Scholar]
- Winkler, D.; Billerman, S.; Lovette, I. Pigeons and Doves (Columbidae), Version 1.0. In Birds of the World; Billerman, S., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Fisher, I.; Ashpole, J.; Scallan, D.; Proud, T.; Carboneras, C. International Single Species Action Plan for the Conservation of the European Turtle Dove Streptopelia turtur (2018 to 2028); European Commission: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Marx, M.; Korner-Nievelgert, F.; Quillfeldt, P. Analysis of Ring Recoveries of European Turtle Doves Streptopelia turtur—Flyways, Migration Timing and Origin of Hunted Birds. Acta Ornithol. 2016, 51, 55–70. [Google Scholar] [CrossRef]
- Poluda, A. (Ed.) Encyclopedia of Migratory Wild Animals of Ukraine; Academy of Sciences of Ukraine: Kyiv, Ukraine, 2018; pp. 412–414. (In Ukrainian) [Google Scholar]
- BirdLife International. Streptopelia turtur; Amended Version of 2017 Assessment; The IUCN Red List of Threatened Species; BirdLife International: Cambridge, UK, 2017; Available online: http://www.birdlife.org (accessed on 17 December 2020).
- Calderón, L.; Campagna, L.; Wilke, T.; Lormee, H.; Eraud, C.; Dunn, J.C.; Rocha, G.; Zehtindjiev, P.; Bakaloudis, D.E.; Metzger, B.; et al. Genomic Evidence of Demographic Fluctuations and Lack of Genetic Structure Across Flyways in a Long Distance Migrant, the European Turtle Dove. BMC Evol. Biol. 2016, 16, 237. [Google Scholar] [CrossRef]
- Cibois, A.; Thibault, J.-C.; Bonillo, C.; Filardi, C.E.; Watling, D.; Pasquet, E. Phylogeny and Biogeography of the Fruit Doves (Aves: Columbidae). Mol. Phylogenet. Evol. 2014, 70, 442–453. [Google Scholar] [CrossRef]
- Cibois, A.; Thibault, J.-C.; Bonillo, C.; Filardi, C.E.; Pasquet, E. Phylogeny and Biogeography of the Imperial Pigeons (Aves: Columbidae) in the Pacific Ocean. Mol. Phylogenet. Evol. 2017, 110, 19–26. [Google Scholar] [CrossRef]
- Bruxaux, J.; Gabrielli, M.; Ashari, H.; Prŷs-Jones, R.; Joseph, L.; Milá, B.; Besnard, G.; Thébaud, C. Recovering the Evolutionary History of Crowned Pigeons (Columbidae: Goura): Implications for the Biogeography and Conservation of New Guinean Lowland Birds. Mol. Phylogenet. Evol. 2018, 120, 248–258. [Google Scholar] [CrossRef]
- Bigi, D.; Mucci, N.; Mengoni, C.; Baldaccini, E.N.; Randi, E. Genetic Investigation of Italian Domestic Pigeons Increases Knowledge about the Long-bred History of Columba livia (Aves: Columbidae). Ital. J. Zool. 2016, 83, 173–182. [Google Scholar] [CrossRef]
- Domyan, E.T.; Shapiro, M.D. Pigeonetics Takes Flight: Evolution, Development, and Genetics of Intraspecific Variation. Dev. Biol. 2017, 427, 241–250. [Google Scholar] [CrossRef]
- Ramadan, S.; Dawod, A.; El-Garhy, O.; Nowier, A.M.; Eltanany, M.; Inoue-Murayama, M. Genetic Characterisation of 11 Microsatellite loci in Egyptian Pigeons (Columba livia domestica) and their Cross-species Amplification in other Columbidae Populations. Vet. World 2018, 11, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Carlen, E.; Munshi-South, J. Widespread Genetic Connectivity of Feral Pigeons across the Northeastern Megacity. Evol. Appl. 2020, 14, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Bagi, Z.; Dimopoulos, E.A.; Loukovitis, D.; Eraud, C.; Kusza, S. MtDNA Genetic Diversity and Structure of Eurasian Collared Dove (Streptopelia decaocto). PLoS ONE 2018, 13, e0193935. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Luikart, G.; Aitken, S. Conservation and the Genetics of Populations, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ando, H.; Ogawa, H.; Kaneko, S.; Takano, H.; Seki, S.-I.; Suzuki, H.; Horikoshi, K.; Isagi, Y. Genetic Structure of the Critically Endangered Red-Headed Wood Pigeon Columba janthina nitens and Its Implications for the Management of Threatened Island Populations. IBIS 2014, 156, 153–164. [Google Scholar] [CrossRef]
- Clements, J.F.; Schulenberg, T.S.; Iliff, M.J.; Roberson, D.; Fredericks, T.A.; Sullivan, B.L.; Wood, C.L. The eBird/Clements Checklist of Birds of the World; Cornell University Press: New York, NY, USA, 2015; Available online: http://www.birds.cornell.edu/clementschecklist/ (accessed on 17 December 2020).
- Telleria, J.L.; Carbonell, R.; Fandos, G.; Tena, E.; Onrubia, A.; Qninba, J.I.; Aguirre, J.I.; Hernández-Téllez, I.; Martín, C.A.; Ramírez, Á. Distribution of the European Turtle Dove (Streptopelia turtur) at the Edge of the South-Western Palearctic: Transboundary Differences and Conservation Prospects. Eur. J. Wildl. Res. 2020, 66, 74. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and Rapid Salt-extraction of High Quality Genomic DNA for PCR-based Techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- Butkauskas, D.; Švažas, S.; Sruoga, A.; Bea, A.; Grishanov, G. Variability of Haplotypes among Different Populations of Woodpigeon (Columba palumbus) in Southern Europe and in the Eastern Baltic Region. Acta Zool. Litu. 2008, 18, 77–82. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Villesen, P. FaBox: An Online Toolbox for Fasta Sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (Version 3.0): An Integrated Software Package for Population Genetics Data Analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Dupanloup, I.; Schneider, S.; Excoffier, L. A Simulated Annealing Approach to Define the Genetic Structure of Populations. Mol. Ecol. 2002, 11, 2571–2581. [Google Scholar] [CrossRef]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; Tanksley, S.M.; Parker, P.G. Morphological Variation and Genetic Structure of Galapagos Dove (Zenaida galapagoensis) Populations: Issues in Conservation for the Galapagos bird fauna. Wilson J. Ornithol. 2006, 118, 194–207. [Google Scholar] [CrossRef]
- Monceau, K.; Cézilly, F.; Moreau, J.; Motreuil, S.; Wattier, R. Colonisation and Diversification of the Zenaida Dove (Zenaida aurita) in the Antilles: Phylogeography, Contemporary Gene Flow and Morphological Divergence. PLoS ONE 2013, 8, e82189. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.-I.; Takano, H.; Kawakami, K.; Kotaka, N.; Endo, A.; Takehara, K. Distribution and Genetic Structure of the Japanese Wood Pigeon (Columba janthina) Endemic to the Islands of East Asia. Conserv. Genet. 2007, 8, 1109–1121. [Google Scholar] [CrossRef]
- Goldberg, J.; Trewick, S.A.; Powlesland, R.G. Population Structure and Biogeography of Hemiphaga Pigeons (Aves: Columbidae) on Islands in the New Zealand Region. J. Biogeogr. 2011, 38, 285–298. [Google Scholar] [CrossRef]
- Butkauskas, D.; Švažas, S.; Bea, A.; Prakas, P.; Olano, I.; Grishanov, G.; Mischenko, A.; Kozulin, A.; Stanevicius, S.; Baldi, A.; et al. Designation of Flyways and Genetic Structure of Woodpigeon Columba palumbus in Europe and Morocco. Eur. J. Wildl. Res. 2019, 65, 91. [Google Scholar] [CrossRef]
- Baptista, L.; Trail, P.; Horblit, H. Family Columbidae (Pigeons). In Handbook of the Birds of the World; del Hoyo, T., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1997; Volume 4, pp. 60–243. [Google Scholar]
- Wenink, P.W.; Baker, A.J.; Rosner, H.-U.; Tilanus, M.G.J. Global Mitochondrial DNA Phylogeography of Holarctic Breeding Dunlins (Calidris alpina). Evolution 1996, 50, 318–330. [Google Scholar] [CrossRef]
- Shephard, J.M.; Ogden, R.; Tryjanowski, P.; Olsson, O.; Galbusera, P. Is Population Structure in the European White Stork Determined by Flyway Permeability Rather than Translocation History? Ecol. Evol. 2013, 3, 4881–4895. [Google Scholar] [CrossRef]
- Wilson, R.E.; Ely, C.R.; Talbot, S.L. Flyway Structure in the Circumpolar Greater White-Fronted Goose. Ecol. Evol. 2018, 8, 8490–8507. [Google Scholar] [CrossRef]

| Sample | n | S | h | Hd ± SD | π ± SD |
|---|---|---|---|---|---|
| D-loop | |||||
| Taroudant (Southwestern Morocco) | 25 | 20 | 17 | 0.950 ± 0.029 | 0.01443 ± 0.00201 |
| Beni Mellal (Central Morocco) | 29 | 20 | 21 | 0.978 ± 0.014 | 0.01234 ± 0.00184 |
| Vejer de Frontera (Strait of Gibraltar coast) | 31 | 15 | 17 | 0.931 ± 0.029 | 0.01331 ± 0.00110 |
| Palma del Rio (Southern Spain) | 20 | 14 | 9 | 0.832 ± 0.063 | 0.01461 ± 0.00094 |
| Almaraz (Central Spain 1) | 35 | 22 | 15 | 0.909 ± 0.027 | 0.01615 ± 0.00085 |
| Pereleda de Roman (Central Spain 2) | 28 | 20 | 17 | 0.950 ± 0.024 | 0.01612 ± 0.00081 |
| Catalonia (Eastern Spain) | 25 | 23 | 18 | 0.927 ± 0.045 | 0.01655 ± 0.00101 |
| Balearic Islands (Spain) | 23 | 13 | 13 | 0.877 ± 0.061 | 0.01387 ± 0.00119 |
| Central Ukraine | 42 | 18 | 23 | 0.950 ± 0.018 | 0.01364 ± 0.00138 |
| A haplogroup | 90 | 20 | 21 | 0.659 ± 0.057 | 0.00286 ± 0.00039 |
| B haplogroup | 168 | 59 | 32 | 0.948 ± 0.008 | 0.00722 ± 0.00032 |
| Overall | 258 | 44 | 80 | 0.937 ± 0.009 | 0.01502 ± 0.00034 |
| cytb | |||||
| Taroudant (Southwestern Morocco) | 25 | 22 | 12 | 0.797 ± 0.077 | 0.00486 ± 0.00094 |
| Beni Mellal (Central Morocco) | 29 | 24 | 19 | 0.958 ± 0.021 | 0.00520 ± 0.00078 |
| Vejer de Frontera (Strait of Gibraltar coast) | 31 | 17 | 10 | 0.854 ± 0.035 | 0.00583 ± 0.00056 |
| Palma del Rio (Southern Spain) | 20 | 20 | 12 | 0.921 ± 0.042 | 0.00696 ± 0.00068 |
| Almaraz (Central Spain 1) | 35 | 21 | 14 | 0.859 ± 0.038 | 0.00623 ± 0.00037 |
| Pereleda de Roman (Central Spain 2) | 28 | 21 | 12 | 0.905 ± 0.030 | 0.00639 ± 0.00043 |
| Catalonia (Eastern Spain) | 25 | 18 | 11 | 0.883 ± 0.042 | 0.00656 ± 0.00040 |
| Balearic Islands (Spain) | 23 | 20 | 11 | 0.877 ± 0.049 | 0.00650 ± 0.00065 |
| Central Ukraine | 42 | 23 | 17 | 0.900 ± 0.028 | 0.00521 ± 0.00053 |
| Comino * (Malta) | 8 | 15 | 8 | 1.000 ± 0.063 | 0.00637 ± 0.00128 |
| Monfrague * (Central Spain) | 10 | 16 | 10 | 1.000 ± 0.045 | 0.00717 ± 0.00093 |
| Dobrich * (Bulgaria) | 14 | 14 | 7 | 0.879 ± 0.058 | 0.00623 ± 0.00077 |
| Essex, Norfolk * (UK) | 14 | 15 | 7 | 0.857 ± 0.065 | 0.00591 ± 0.00067 |
| Pitou-Charente, Auvergne, Marne * (France) | 15 | 21 | 13 | 0.981 ± 0.031 | 0.00728 ± 0.00101 |
| Evros * (Greece) | 16 | 19 | 11 | 0.950 ± 0.036 | 0.00656 ± 0.00073 |
| Isla Ventotene * (Italy) | 17 | 17 | 9 | 0.875 ± 0.058 | 0.00719 ± 0.00051 |
| A haplogroup | 128 | 30 | 30 | 0.745 ± 0.036 | 0.00215 ± 0.00017 |
| B haplogroup | 224 | 51 | 63 | 0.848 ± 0.018 | 0.00277 ± 0.00010 |
| Overall | 352 | 76 | 93 | 0.905 ± 0.009 | 0.00628 ± 0.00014 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 Southwestern Morocco | 0.013 | 0.037 | 0.036 | 0.029 | 0.072 | 0.106 | 0.152 | 0.007 | |
| 2 Central Morocco | −0.008 | 0.095 | 0.113 | 0.105 | 0.158 | 0.179 | 0.234 | −0.002 | |
| 3 Strait of Gibraltar coast | 0.012 | 0.027 | −0.018 | −0.014 | 0.016 | −0.001 | 0.023 | 0.062 | |
| 4 Southern Spain | 0.033 | 0.085 | −0.012 | −0.034 | −0.015 | −0.018 | 0.001 | 0.074 | |
| 5 Central Spain 1 | 0.060 | 0.107 | 0.004 | −0.026 | -0.016 | −0.011 | 0.009 | 0.059 | |
| 6 Central Spain 2 | 0.071 | 0.120 | 0.011 | −0.020 | −0.021 | −0.017 | −0.008 | 0.102 | |
| 7 Eastern Spain | 0.074 | 0.122 | 0.011 | −0.030 | −0.026 | −0.029 | −0.035 | 0.127 | |
| 8 Balearic Islands | 0.146 | 0.207 | 0.060 | 0.008 | −0.005 | −0.016 | −0.019 | 0.177 | |
| 9 Central Ukraine | −0.017 | 0.005 | 0.005 | 0.027 | 0.050 | 0.062 | 0.063 | 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakas, P.; Butkauskas, D.; Švažas, S.; Bea, A.; Yanenko, V.; Ragauskas, A.; Vaitkuvienė, D. The Genetic Diversity and Structure of the European Turtle Dove Streptopelia turtur. Animals 2021, 11, 1283. https://doi.org/10.3390/ani11051283
Prakas P, Butkauskas D, Švažas S, Bea A, Yanenko V, Ragauskas A, Vaitkuvienė D. The Genetic Diversity and Structure of the European Turtle Dove Streptopelia turtur. Animals. 2021; 11(5):1283. https://doi.org/10.3390/ani11051283
Chicago/Turabian StylePrakas, Petras, Dalius Butkauskas, Saulius Švažas, Antonio Bea, Vadym Yanenko, Adomas Ragauskas, and Daiva Vaitkuvienė. 2021. "The Genetic Diversity and Structure of the European Turtle Dove Streptopelia turtur" Animals 11, no. 5: 1283. https://doi.org/10.3390/ani11051283
APA StylePrakas, P., Butkauskas, D., Švažas, S., Bea, A., Yanenko, V., Ragauskas, A., & Vaitkuvienė, D. (2021). The Genetic Diversity and Structure of the European Turtle Dove Streptopelia turtur. Animals, 11(5), 1283. https://doi.org/10.3390/ani11051283








