Ultrasound-Guided Funicular Block: Ropivacaine Injection into the Tissue around the Spermatic Cord to Improve Analgesia during Orchiectomy in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics
2.3. Pre-Surgery Procedure
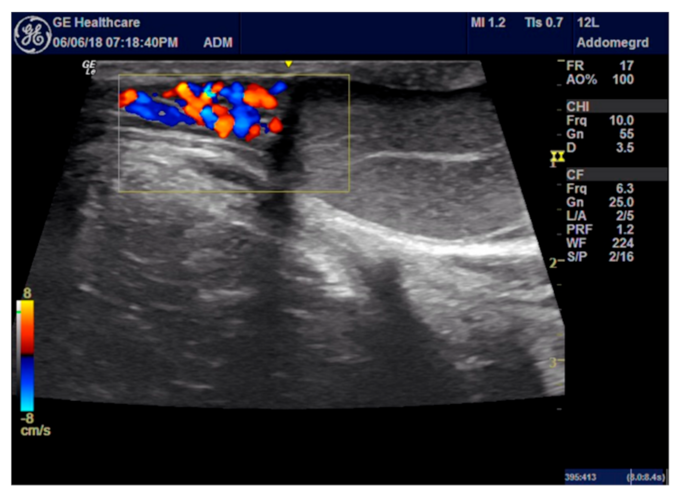
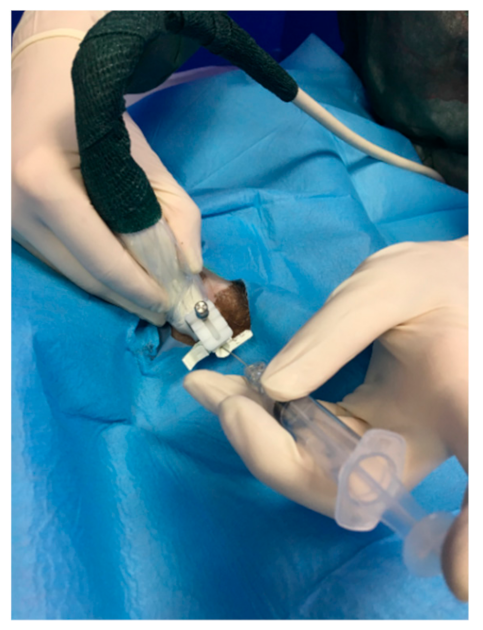
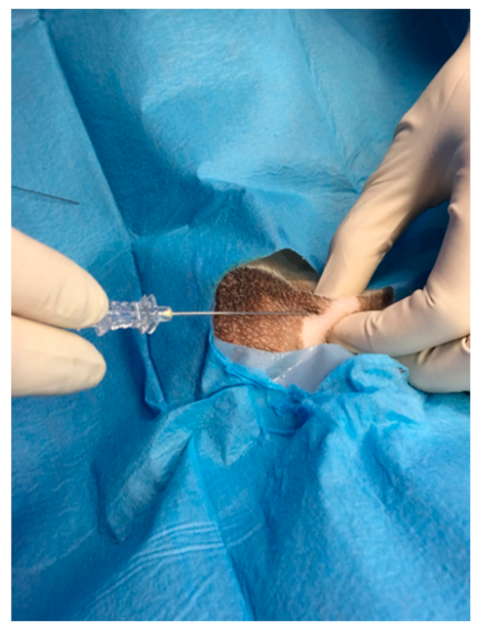
2.4. ROP Group Protocol
2.5. Control Group Protocol
2.6. Surgery Procedure
2.7. Postoperative Pain Evaluation
2.8. Data Analysis
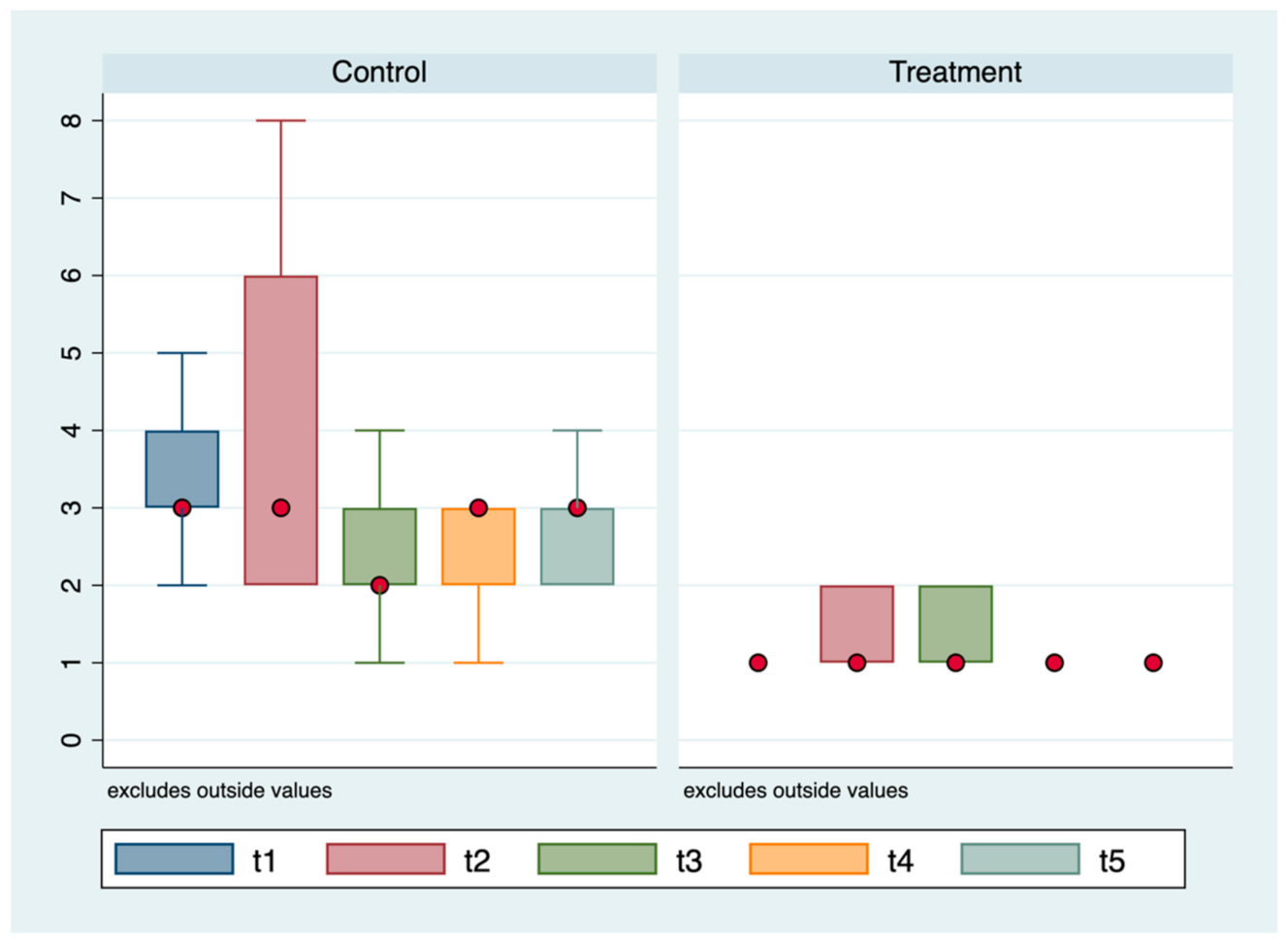
3. Results
3.1. ROP Group
3.2. Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, B.D. Analgesia and sedation in the critically ill. J. Vet. Emerg. Crit. Care 2005, 15, 285–294. [Google Scholar] [CrossRef]
- Gwendolyn, L.C.; Carrol, M.S. How to manage perioperative pain. Vet. Med. 1996, 51, 353–357. [Google Scholar]
- Cicirelli, V.; Debidda, P.; Maggio, N.; Caira, M.; Mrenoshki, D.; Aiudi, G.; Lacalandra, G. Use of Spinal Anaesthesia with Anaesthetic Block of Intercostal Nerves Compared to a Continuous Infusion of Sufentanyl to Improve Analgesia in Cats Undergoing Unilateral Mastectomy. Animals 2021, 11, 887. [Google Scholar] [CrossRef]
- Adin, C.A. Complications of ovariohysterectomy and orchiectomy in companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Austin, B.R.; Ko, J.C.; Payton, M.E.; Weil, A.B.; Inoue, T. Evaluation of dexmedetomidine and ketamine in combination with opioids as injectable anesthesia for castration in dogs. J. Am. Vet. Med. Assoc. 2011, 238, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Wenger, S.; Moens, Y.; Jäggin, N.; Schatzmann, U. Evaluation of the analgesic effect of lidocaine and bupivacaine used to provide a brachial plexus block for forelimb surgery in 10 dogs. Vet. Rec. 2005, 156, 639–642. [Google Scholar] [CrossRef]
- Grubb, T.; Lobprise, H. Local and regional anaesthesia in dogs and cats: Descriptions of specific local and regional techniques. Vet. Med. Sci. 2020, 6, 218–234. [Google Scholar] [CrossRef]
- Ingvast-Larsson, C.; Holgersson, A.; Bondesson, U.; Lagerstedt, A.-S.; Olsson, K. Clinical pharmacology of methadone in dogs. Vet. Anaesth. Analg. 2010, 37, 48–56. [Google Scholar] [CrossRef]
- Cardoso, C.G.; Marques, D.R.; da Silva, T.H.; de Mattos-Junior, E. Cardiorespiratory, sedative and antinociceptive effects of dexmedetomidine alone or in combination with methadone, morphine or tramadol in dogs. Vet. Anaesth. Analg. 2014, 41, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.L.; Bohling, M.W.; Bushby, P.A.; Howe, L.M.; Griffin, B.; Levy, J.K.; Eddlestone, S.M.; Weedon, J.R.; Appel, L.D.; Rigdon-Brestle, Y.K.; et al. The Association of Shelter Veterinarians veterinary medical care guidelines for spay-neuter programs. J. Am. Vet. Med. Assoc. 2008, 233, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Zawistowski, S.; Miller, L. Shelter Medicine for Veterinarians and Staff; Iowa State University Press: Ames, IA, USA, 2004. [Google Scholar]
- Clarke, K.W.; Hall, L.W. A survey of anaesthesia in small animal practice: AVA/BSAVA report. J. Assoc. Vet. Anaesth. Great Br. Irel. 1990, 17, 4–10. [Google Scholar] [CrossRef]
- Archibald, J. Male genital system. In Canine Surgery, 2nd ed.; American Veterinary Publications: Santa Barbara, CA, USA, 1974; pp. 727–729. [Google Scholar]
- Fossum, T.W. Surgery of the reproductive and genital systems. In Small Animal Surgery, 2nd ed.; Elsevier: Philadelphia, PA, USA, 2002; pp. 619–620. [Google Scholar]
- Howe, L.M. Surgical methods of contraception and sterilization. Theriogenology 2006, 66, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Egger, C.M.; Glerum, L.E.; Allen, S.W.; Haag, M. Plasma fentanyl concentrations in awake cats and cats undergoing anesthesia and ovariohysterectomy using transdermal administration. Vet. Anaesth. Analg. 2003, 30, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rømsing, J.; Mysager, S.; Vilmann, P.; Sonne, J.; Larsen, N.E.; Østergaard, D. Postoperative analgesia is not different after local vs systemic administration of meloxicam in patients undergoing inguinal hernia repair. Can. J. Anesth. 2001, 48, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Bekker, A.; Kloepping, C.; Collingwood, S. Meloxicam in the management of post-operative pain: Narrative review. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, W.E.; Mogoa, E.M.; Mwangi, J.N.; Mbuthia, P.G.; Mbugua, S.W. A systematic review of analgesia practices in dogs undergoing ovariohysterectomy. Vet. World 2018, 11, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.M.; Escobar, A.; Maguilnik, S. Comparison of analgesia provided by lidocaine, lidocaine-morphine or lidocaine-tramadol delivered epidurally in dogs following orchiectomy. Vet. Anaesth. Analg. 2010, 37, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, C.; Kluge, K.; Lanza-Perea, M.; Bruhl-Day, R.; Guerrero, K.S.K. Comparison of intraperitoneal ropivacaine and bupivacaine for postoperative analgesia in dogs undergoing ovariohysterectomy. Vet. Anaesth. Analg. 2018, 45, 865–870. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control Group (n = 25) | ROP group (n = 25) | Total (n = 50) | p-Value |
|---|---|---|---|---|
| Propofol administration; n (%) | 25 (100.0) | 22 (88.0) | 47 (94.0) | 0.235 |
| N. doses; n (%) | ||||
| 9/25 (36.0) | 4/22 (18.2) | 13 (27.7) | 0.173 |
| Dosage dose 1 (mg/kg); n (%) | ||||
| 1/25 (4.0) 22/25 (88.0) 2/25 (8.0) | 6/22 (27.3) 16/22 (72.7) 0/22 (0.0) | 7/47 (14.9) 38/47 (80.9) 2/47 (4.2) | 0.025 |
| Dosage dose 2 (mg/kg); n (%) | ||||
| 7/9 (77.8) 2/9 (22.2) | 2/4 (50.0) 2/4 (50.0) | 9/13 (69.2) 4/13 (30.8) | 0.317 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicirelli, V.; Debidda, P.; Maggio, N.; Caira, M.; Lacalandra, G.M.; Aiudi, G.G. Ultrasound-Guided Funicular Block: Ropivacaine Injection into the Tissue around the Spermatic Cord to Improve Analgesia during Orchiectomy in Dogs. Animals 2021, 11, 1275. https://doi.org/10.3390/ani11051275
Cicirelli V, Debidda P, Maggio N, Caira M, Lacalandra GM, Aiudi GG. Ultrasound-Guided Funicular Block: Ropivacaine Injection into the Tissue around the Spermatic Cord to Improve Analgesia during Orchiectomy in Dogs. Animals. 2021; 11(5):1275. https://doi.org/10.3390/ani11051275
Chicago/Turabian StyleCicirelli, Vincenzo, Pasquale Debidda, Nicola Maggio, Michele Caira, Giovanni M. Lacalandra, and Giulio G. Aiudi. 2021. "Ultrasound-Guided Funicular Block: Ropivacaine Injection into the Tissue around the Spermatic Cord to Improve Analgesia during Orchiectomy in Dogs" Animals 11, no. 5: 1275. https://doi.org/10.3390/ani11051275
APA StyleCicirelli, V., Debidda, P., Maggio, N., Caira, M., Lacalandra, G. M., & Aiudi, G. G. (2021). Ultrasound-Guided Funicular Block: Ropivacaine Injection into the Tissue around the Spermatic Cord to Improve Analgesia during Orchiectomy in Dogs. Animals, 11(5), 1275. https://doi.org/10.3390/ani11051275







