Long Intergenic Non-Coding RNAs in the Mammary Parenchyma and Fat Pad of Pre-Weaning Heifer Calves: Identification and Functional Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Library Construction
2.2. Databases
2.3. Alignment and Assembly of RNA-Seq Data
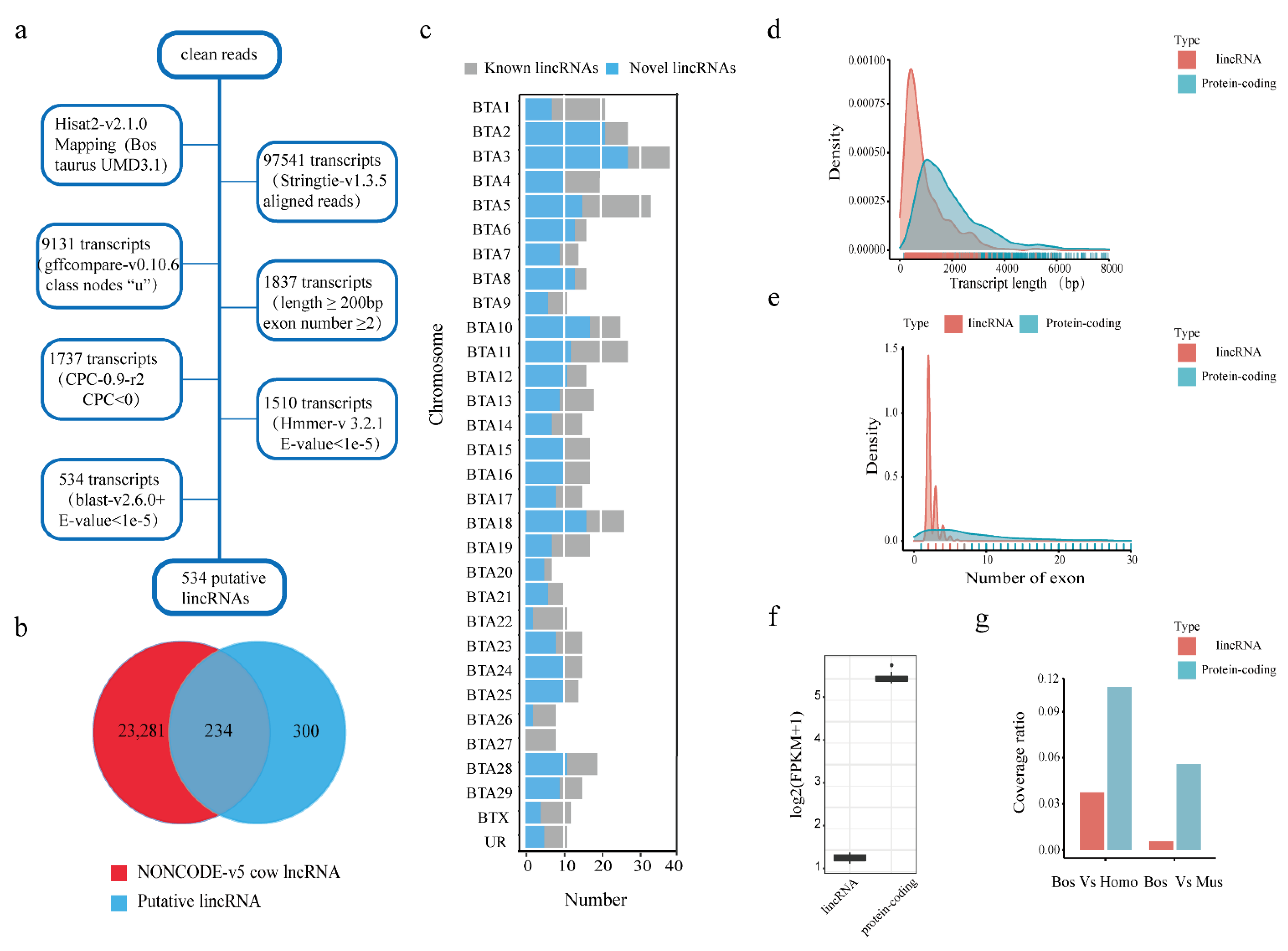
2.4. Identification and Characterization of Putative LincRNAs
2.5. Express Analysis mRNA and Putative LincRNAs in MFP and PAR
2.6. Neighboring Gene Identification and Correlation Analysis of DELs of MFP and PAR
2.7. GO Ontology and Pathway Analysis
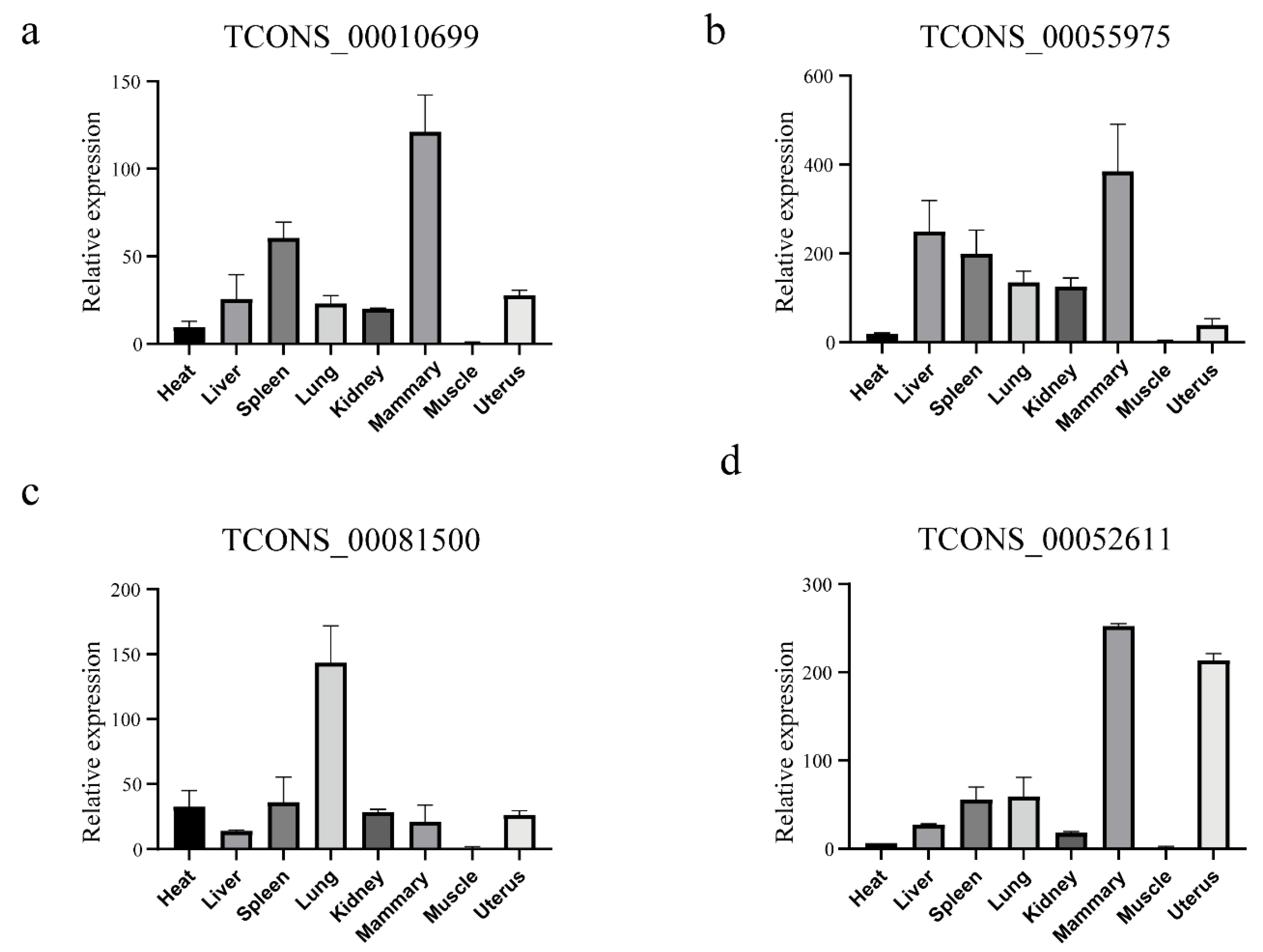
2.8. Quantification of LincRNAs through QRT-PCR
3. Results
3.1. Identification and Characterization of the Putative LincRNAs
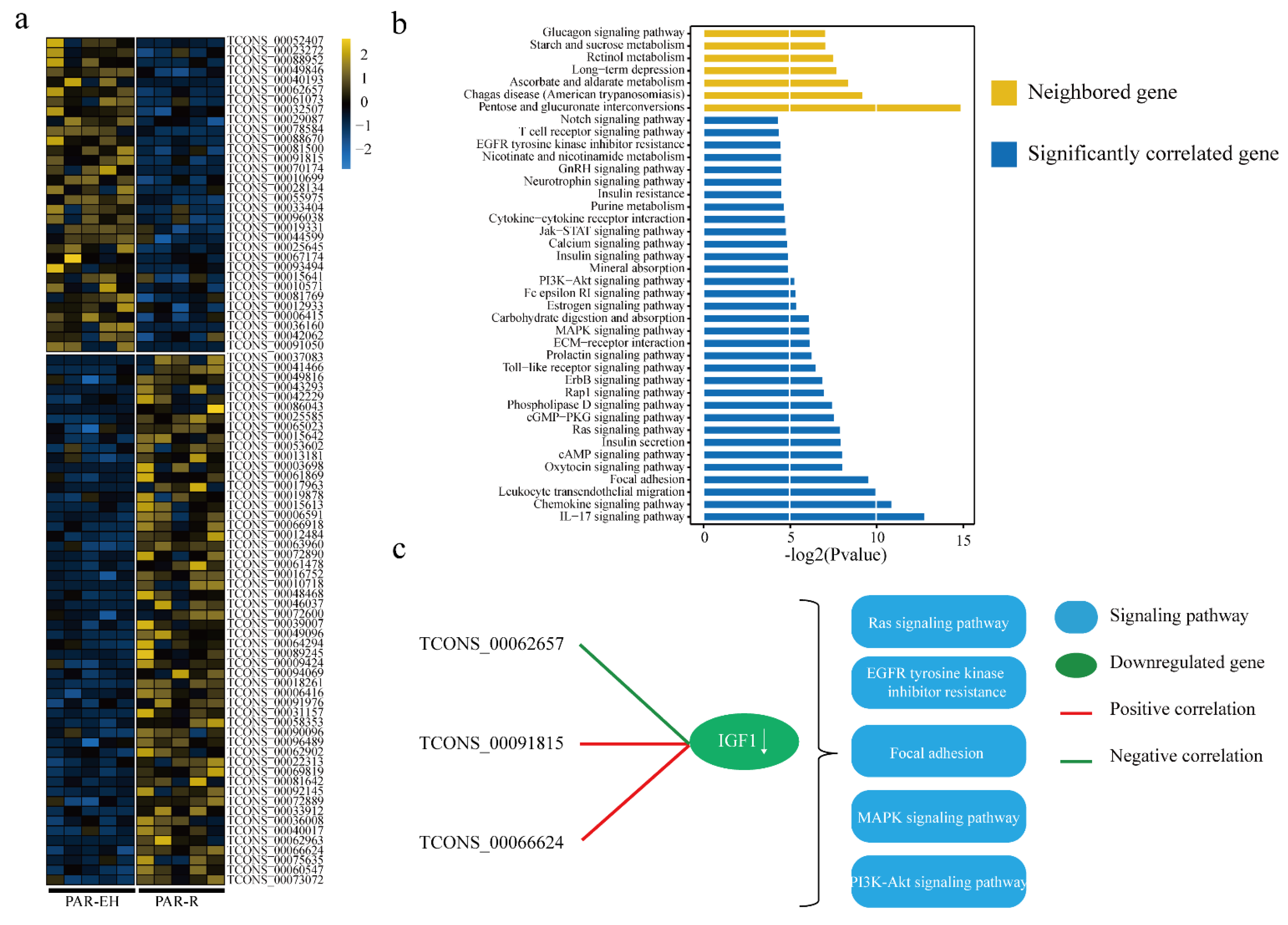
3.2. Expression Analysis and Functional Prediction of DELs in MFP
3.3. Expression Analysis and Functional Prediction of DELs in PAR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Hovey, R.C.; Aimo, L. Diverse and active roles for adipocytes during mammary gland growth and function. J. Mammary Gland. Biol. Neoplasia 2010, 15, 279–290. [Google Scholar] [CrossRef]
- Lohakare, J.D.; Sudekum, K.H.; Pattanaik, A.K. Nutrition-induced changes of growth from birth to first calving and its impact on mammary development and first-lactation Milk yield in dairy heifers: A review. Asian Australas. J. Anim. Sci. 2012, 25, 1338–1350. [Google Scholar] [CrossRef]
- Piantoni, P.; Bionaz, M.; Graugnard, D.E.; Daniels, K.M.; Everts, R.E.; Rodriguez-Zas, S.L.; Lewin, H.A.; Hurley, H.L.; Akers, M.; Loor, J.J. Functional and gene network analyses of transcriptional signatures characterizing pre-weaned bovine mammary parenchyma or fat pad uncovered novel inter-tissue signaling networks during development. BMC Genom. 2010, 11, 331. [Google Scholar] [CrossRef]
- Watson, C.J.; Khaled, W.T. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 2008, 135, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Butner, J.D.; Chuang, Y.L.; Simbawa, E.; Al-Fhaid, A.S.; Mahmoud, S.R.; Cristini, V.; Wang, Z. A hybrid agent-based model of the developing mammary terminal end bud. J. Theor. Biol. 2016, 407, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Kertz, A.F.; Hill, T.M.; Quigley, J.D.; Heinrichs, A.J., 3rd; Linn, J.G.; Drackley, J.K. A 100-Year Review: Calf nutrition and management. J. Dairy Sci. 2017, 100, 10151–10172. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Beermann, J.; Kirste, D.; Iwanov, K.; Lu, D.; Kleemiß, F.; Kumarswamy, R.; Schimmel, K.; Bär, C.; Thum, T. A large shRNA library approach identifies lncRNA Ntep as an essential regulator of cell proliferation. Cell Death Differ. 2017, 25, 307–318. [Google Scholar] [CrossRef]
- Guttman, M.; Garber, M.; Levin, J.Z.; Donaghey, J.; Robinson, J.; Adiconis, X.; Fan, L.; Koziol, M.J.; Gnirke, A.; Nusbaum, C.; et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 2010, 28, 503–510. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Chen, D.; Zhang, B.; Tian, R.R.; Wu, J.; Zhang, Y.; Xu, K.; Yang, L.-M.; Cheng, C.; et al. Annotation and cluster analysis of spatiotemporal- and sex-related lncRNA expression in rhesus macaque brain. Genome Res. 2017, 27, 1608–1620. [Google Scholar] [CrossRef]
- Standaert, L.; Adriaens, C.; Radaelli, E.; Van Keymeulen, A.; Blanpain, C.; Hirose, T.; Nakagawa, S.; Marina, J.-C. The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA 2014, 20, 1844–1849. [Google Scholar] [CrossRef]
- Hansji, H.; Leung, E.Y.; Baguley, B.C.; Finlay, G.J.; Cameron-Smith, D.; Figueiredo, V.C.; Askarian-Amiri, M.E. ZFAS1: A long noncoding RNA associated with ribosomes in breast cancer cells. Biol. Direct. 2016, 11, 62. [Google Scholar] [CrossRef]
- Shore, A.N.; Kabotyanski, E.B.; Roarty, K.; Smith, M.A.; Zhang, Y.; Creighton, C.J.; Dinger, M.E.; Rosen, J.M. Pregnancy-induced noncoding RNA (PINC) associates with polycomb repressive complex 2 and regulates mammary epithelial differentiation. PLoS Genet. 2012, 8, e1002840. [Google Scholar] [CrossRef]
- Tong, C.; Chen, Q.; Zhao, L.; Ma, J.; Ibeagha-Awemu, E.M.; Zhao, X. Identification and characterization of long intergenic noncoding RNAs in bovine mammary glands. BMC Genom. 2017, 18, 468. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Li, R.; Dudemaine, P.L.; Do, D.N.; Bissonnette, N. Transcriptome Analysis of Long Non-Coding RNA in the Bovine Mammary Gland Following Dietary Supplementation with Linseed Oil and Safflower Oil. Int. J. Mol. Sci. 2018, 19, 3610. [Google Scholar] [CrossRef]
- Yang, B.; Jiao, B.; Ge, W.; Zhang, X.; Wang, S.; Zhao, H.; Wang, X. Transcriptome sequencing to detect the potential role of long non-coding RNAs in bovine mammary gland during the dry and lactation period. BMC Genom. 2018, 19, 605. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Bucktrout, R.E.; Zhan, S.; Geiger, A.; McCann, J.C.; Akers, R.M.; Loor, J.J. Higher plane of nutrition pre-weaning enhances Holstein calf mammary gland development through alterations in the parenchyma and fat pad transcriptome. BMC Genom. 2018, 19, 900. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, L.; Guo, J.; Niu, Y.; Wu, Y.; Li, H.; Zhao, L.; Li, X.; Teng, X.; Sun, X.; et al. NONCODEV5: A comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018, 46, D308–D314. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Zhao, W.; Mu, Y.; Ma, L.; Wang, C.; Tang, Z.; Yang, S.; Zhou, R.; Hu, X.; Li, M.; Li, K. Systematic identification and characterization of long intergenic non-coding RNAs in fetal porcine skeletal muscle development. Sci. Rep. 2015, 5, 8957. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Smith, A.A.T.; Ferreira da Silva, M.; Matthey-Doret, C.; Rueedi, R.; Sonmez, R.; Ding, D.; Kutalik, Z.; Bergmann, S.; Marques, A.C. cis-Acting Complex-Trait-Associated lincRNA Expression Correlates with Modulation of Chromosomal Architecture. Cell Rep. 2017, 18, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Fu, Y.; Liu, J. Chemical reprogramming and transdifferentiation. Curr. Opin. Genet. Dev. 2017, 46, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Casero, D.; Sandoval, S.; Seet, C.S.; Scholes, J.; Zhu, Y.; Ha, V.L.; Luong, A.; Parekh, C.; Crooks, G.M. Long non-coding RNA profiling of human lymphoid progenitor cells reveals transcriptional divergence of B cell and T cell lineages. Nat. Immunol. 2015, 16, 1282–1291. [Google Scholar] [CrossRef]
- Sejrsen, K.; Huber, J.T.; Tucker, H.A.; Akers, R.M. Influence of nutrition of mammary development in pre- and postpubertal heifers. J. Dairy Sci. 1982, 65, 793–800. [Google Scholar] [CrossRef]
- Anderson, K.L.; Nagaraja, T.G.; Morrill, J.L. Ruminal metabolic development in calves weaned conventionally or early. J. Dairy Sci. 1987, 70, 1000–1005. [Google Scholar] [CrossRef]
- Van Amburgh, M.E.; Galton, D.M.; Bauman, D.E.; Everett, R.W.; Fox, D.G.; Chase, L.E.; Erb, H.N. Effects of three prepubertal body growth rates on performance of Holstein heifers during first lactation. J. Dairy Sci. 1998, 81, 527–538. [Google Scholar] [CrossRef]
- Moallem, U.; Werner, D.; Lehrer, H.; Zachut, M.; Livshitz, L.; Yakoby, S.; Shamay, A. Long-term effects of ad libitum whole milk prior to weaning and prepubertal protein supplementation on skeletal growth rate and first-lactation milk production. J. Dairy Sci. 2010, 93, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Soberon, F.; Raffrenato, E.; Everett, R.W.; Van Amburgh, M.E. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Hare, K.S.; Leal, L.N.; Romao, J.M.; Hooiveld, G.J.; Soberon, F.; Berends, H.; Van Amburgh, M.E.; Martin-Tereso, J.; Steele, M.A. Preweaning nutrient supply alters mammary gland transcriptome expression relating to morphology, lipid accumulation, DNA synthesis, and RNA expression in Holstein heifer calves. J. Dairy Sci. 2019, 102, 2618–2630. [Google Scholar] [CrossRef] [PubMed]
- Holliday, H.; Baker, L.A.; Junankar, S.R.; Clark, S.J.; Swarbrick, A. Epigenomics of mammary gland development. Breast Cancer Res. 2018, 20, 100. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, Z.Z.; Lu, Z.J.; Zhao, S.; Mathews, D.H. Discovery of Novel ncRNA Sequences in Multiple Genome Alignments on the Basis of Conserved and Stable Secondary Structures. PLoS ONE 2015, 10, e0130200. [Google Scholar] [CrossRef]
- Kim, H.K.; Youn, B.S.; Shin, M.S.; Namkoong, C.; Park, K.H.; Baik, J.H.; Kim, J.B.; Park, J.-Y.; Lee, K.U.; Kim, Y.-B.; et al. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes 2010, 59, 2772–2780. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Nicholls, S.J.; Langsted, A.; Ray, K.K.; Tybjærg-Hansen, A. Advances in lipid-lowering therapy through gene-silencing technologies. Nat. Rev. Cardiol. 2018, 15, 261–272. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, M.; Mattapally, S.; Chen, S.; Zhang, J. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circ. Res. 2018, 122, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, G.E.; Trott, J.F.; Hovey, R.C. Mammary gland development--It’s not just about estrogen. J. Dairy Sci. 2016, 99, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef] [PubMed]

| DELs ID | Neighboring Gene Name | Change (EH vs R) | Tissue |
|---|---|---|---|
| TCONS_00049867 | ACVR2B | up | MFP |
| TCONS_00055163 | LAMA1 | down | MFP |
| TCONS_00027412 | LOC100847119 | up | MFP |
| TCONS_00063960 | PYGM | up | PAR |
| TCONS_00033404 | HRC | up | PAR |
| TCONS_00043293 | THEMIS2 | down | PAR |
| TCONS_00040017 | LIMS2 | up | PAR |
| TCONS_00049846 | STAC | up | PAR |
| TCONS_00091815 | PDE10A | down | PAR |
| TCONS_00016752 | HCK | down | PAR |
| TCONS_00090096 | NTRK2 | down | PAR |
| TCONS_00039007 | MRC2 | down | PAR |
| TCONS_00033404 | KCNA7 | down | PAR |
| TCONS_00015641 | PTGIS | up | PAR |
| TCONS_00015642 | PTGIS | up | PAR |
| TCONS_00042229 | SCN7A | up | PAR |
| TCONS_00036160 | ACE | up | PAR |
| TCONS_00033404 | LHB | down | PAR |
| TCONS_00040017 | MYO7B | up | PAR |
| TCONS_00072600 | GIMAP1 | down | PAR |
| TCONS_00036008 | PYY | up | PAR |
| TCONS_00033404 | NTF4 | down | PAR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Ahmad, S.; Zhang, Y.; Hua, G.; Yi, J. Long Intergenic Non-Coding RNAs in the Mammary Parenchyma and Fat Pad of Pre-Weaning Heifer Calves: Identification and Functional Analysis. Animals 2021, 11, 1268. https://doi.org/10.3390/ani11051268
Zhang S, Ahmad S, Zhang Y, Hua G, Yi J. Long Intergenic Non-Coding RNAs in the Mammary Parenchyma and Fat Pad of Pre-Weaning Heifer Calves: Identification and Functional Analysis. Animals. 2021; 11(5):1268. https://doi.org/10.3390/ani11051268
Chicago/Turabian StyleZhang, Shengchao, Sibtain Ahmad, Yuxia Zhang, Guohua Hua, and Jianming Yi. 2021. "Long Intergenic Non-Coding RNAs in the Mammary Parenchyma and Fat Pad of Pre-Weaning Heifer Calves: Identification and Functional Analysis" Animals 11, no. 5: 1268. https://doi.org/10.3390/ani11051268
APA StyleZhang, S., Ahmad, S., Zhang, Y., Hua, G., & Yi, J. (2021). Long Intergenic Non-Coding RNAs in the Mammary Parenchyma and Fat Pad of Pre-Weaning Heifer Calves: Identification and Functional Analysis. Animals, 11(5), 1268. https://doi.org/10.3390/ani11051268






