Systematic Review and Quality Evaluation Using ARRIVE 2.0 Guidelines on Animal Models Used for Periosteal Distraction Osteogenesis
Abstract
Simple Summary
Abstract
1. Introduction
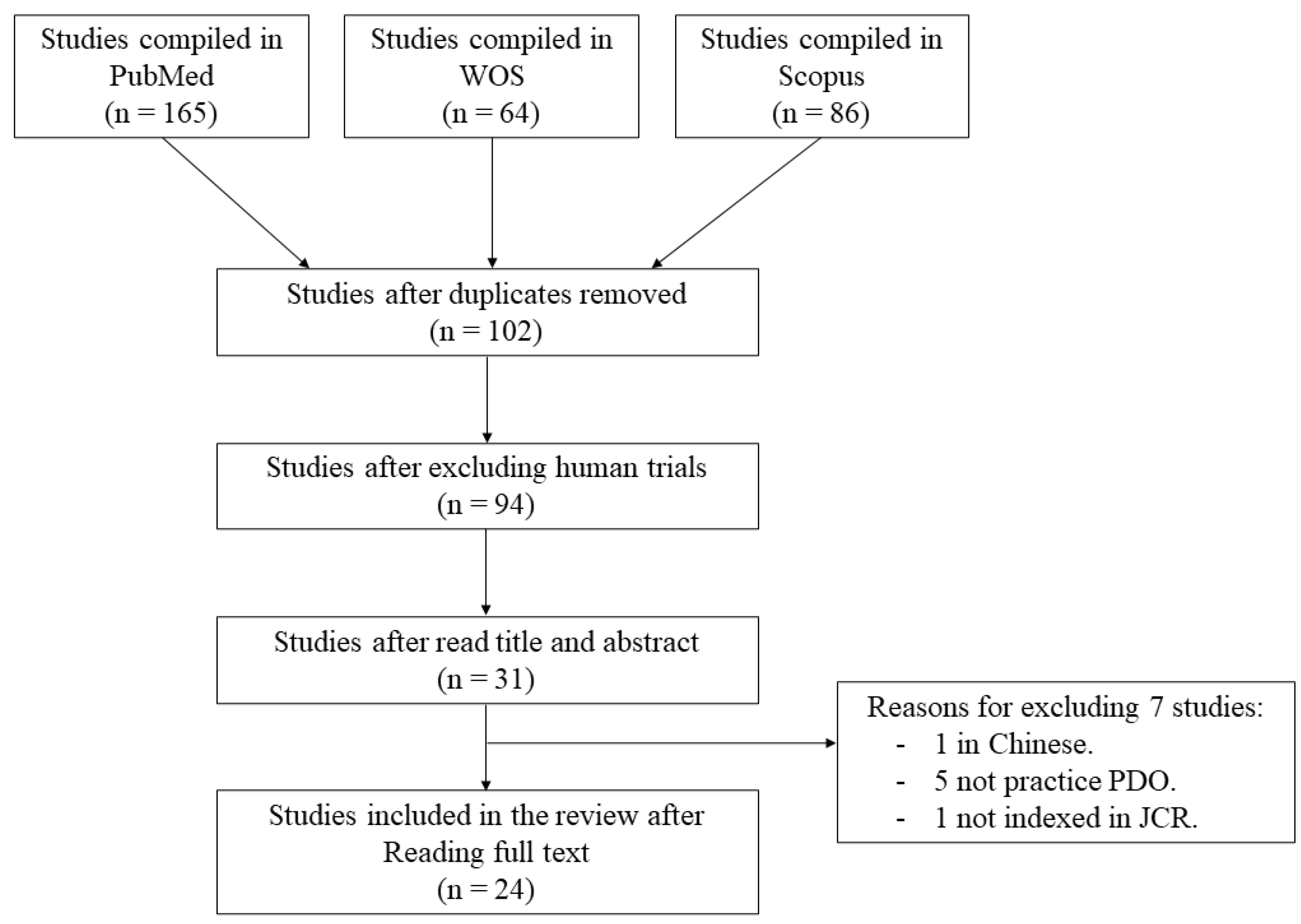
2. Materials and Methods
2.1. Search Strategy
- -
- Animal model AND preclinical studies AND (periosteal distraction osteogenesis OR osteogenesis distraction OR periosteum).
- -
- Periosteal distraction osteogenesis AND (bone augmentation OR bone regeneration).
- -
- Animal AND periosteal distraction.
2.2. PICO Methodology
2.3. Inclusion Criteria.
- Experimental studies of PDO aimed at bone regeneration with animals used as biological models.
- Studies indexed in JCR (Journal Citation Reports).
- Articles in English.
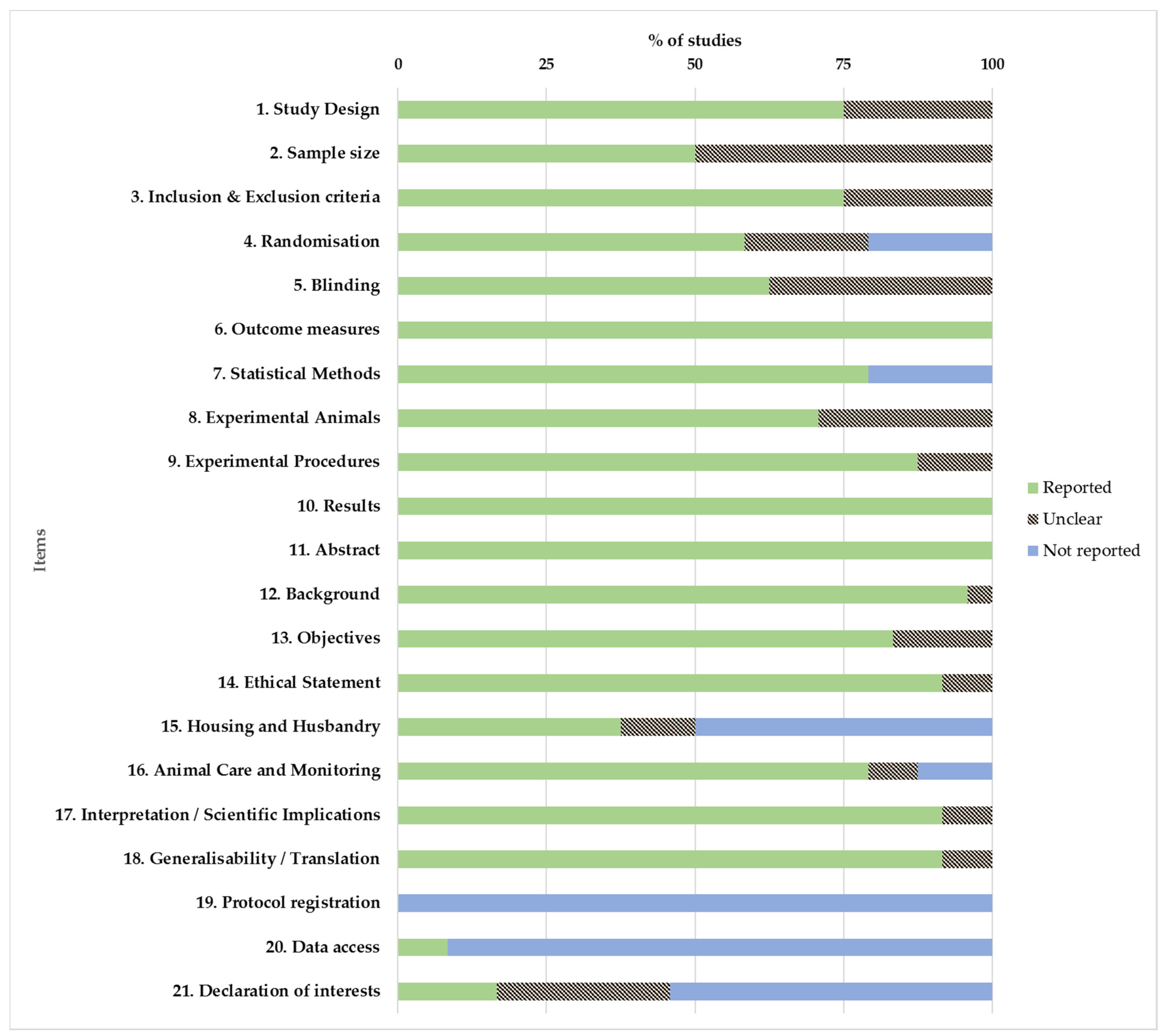
2.4. Quality Assessment and Risk of Bias
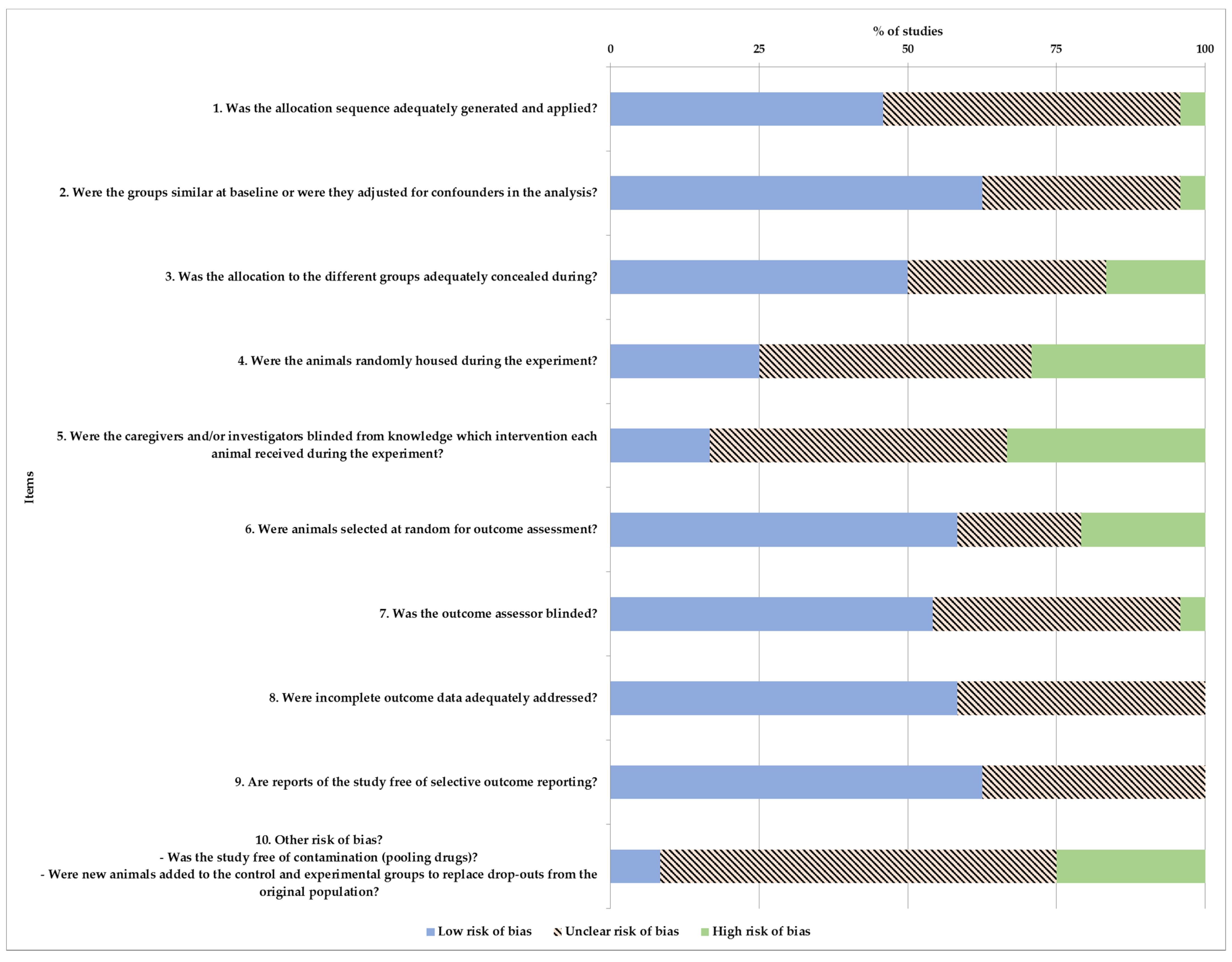
2.5. Risk of Bias
2.6. Analysis and Extraction of Parameters of Interest
2.7. Statistical Analysis
3. Results
3.1. Indication and Location for Distraction
3.2. Device Details
3.3. PDO Protocol
3.4. Evaluation Methods and Results
3.5. Complications and Treatment
3.6. Quality Assessment of Selected Studies
3.7. Risk of Bias in Studies
4. Discussion
4.1. Animal Models and Complications
4.2. Protocol
4.3. Strengths and Limitations of the Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, D.; Wang, Y.; Han, D. Periosteal Distraction Osteogenesis: An Effective Method for Bone Regeneration. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Tudor, C.; Bumiller, L.; Birkholz, T.; Stockmann, P.; Wiltfang, J.; Kessler, P. Static and dynamic periosteal elevation: A pilot study in a pig model. Int. J. Oral Maxillofac. Surg. 2010, 39, 897–903. [Google Scholar] [CrossRef] [PubMed]
- García, M.P.S.; Martín, J.M.M.; Torreira, M.G.; Martínez, M.D.M.M.; García-García, A. Periosteal distraction as bone regenerative alternative. Biomed. Res. 2018, 29, 2766–2772. [Google Scholar] [CrossRef]
- Kessler, P.; Bumiller, L.; Schlegel, A.; Birkholz, T.; Neukam, F.W.; Wiltfang, J. Dynamic periosteal elevation. Br. J. Oral Maxillofac. Surg. 2007, 45, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, W.; Wang, Y.; Wang, C.; Zhang, X.; Li, Q.; Han, D. Three-Dimensional-Printed Poly-L-Lactic Acid Scaffolds with Different Pore Sizes Influence Periosteal Distraction Osteogenesis of a Rabbit Skull. Biomed. Res. Int. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Kung, L.; Jones, C.; Casap, N. Induced osteogenesis by periosteal distraction. J. Oral Maxillofac. Surg. 2002, 60, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.; Emerson, M.; Altman, D. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. Animals 2014, 4, 35–44. [Google Scholar] [CrossRef]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- de Vries, R.B.M.; Hooijmans, C.R.; Langendam, M.W.; van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid.-Based Preclin. Med. 2015, 2, e00007. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Critical size defects for bone regeneration experiments in rabbit calvariae: Systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral Implants Res. 2015, 26, 915–930. [Google Scholar] [CrossRef]
- Schwarz, F.; Iglhaut, G.; Becker, J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J. Clin. Periodontol. 2012, 39, 63–72. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Enislidis, G.; Fock, N.; Millesi-Schobel, G.; Klug, C.; Wittwer, G.; Yerit, K.; Ewers, R. Analysis of complications following alveolar distraction osteogenesis and implant placement in the partially edentulous mandible. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 100, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, O.E.; Erdogan, O.; Namli, H.; Sencar, L. Effects of local simvastatin on periosteal distraction osteogenesis in rabbits. Br. J. Oral Maxillofac. Surg. 2015, 53, e18–e22. [Google Scholar] [CrossRef] [PubMed]
- Pripatnanont, P.; Balabid, F.; Pongpanich, S.; Vongvatcharanon, S. Effect of osteogenic periosteal distraction by a modified Hyrax device with and without platelet-rich fibrin on bone formation in a rabbit model: A pilot study. Int. J. Oral Maxillofac. Surg. 2015, 44, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Lethaus, B.; Tudor, C.; Bumiller, L.; Birkholz, T.; Wiltfang, J.; Kessler, P. Guided bone regeneration: Dynamic procedures versus static shielding in an animal model. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2010, 95, 126–130. [Google Scholar] [CrossRef]
- Sato, K.; Haruyama, N.; Shimizu, Y.; Hara, J.; Kawamura, H. Osteogenesis by gradually expanding the interface between bone surface and periosteum enhanced by bone marrow stem cell administration in rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, O.; Kon, K.; Kasugai, S. Evaluation of a biodegradable novel periosteal distractor. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2012, 100, 882–889. [Google Scholar] [CrossRef]
- Zakaria, O.; Madi, M.; Kasugai, S. Induced osteogenesis using a new periosteal distractor. J. Oral Maxillofac. Surg. 2012, 70, e225–e234. [Google Scholar] [CrossRef]
- Saulacic, N.; Schaller, B.; Iizuka, T.; Buser, D.; Hug, C.; Bosshardt, D.D. Analysis of new bone formation induced by periosteal distraction in a rat calvarium model. Clin. Implant. Dent. Relat. Res. 2013, 15, 283–291. [Google Scholar] [CrossRef]
- Saulacic, N.; Hug, C.; Bosshardt, D.D.; Schaller, B.; Buser, D.; Haeniwa, H.; Iizuka, T. Relative Contributions of Osteogenic Tissues to New Bone Formation in Periosteal Distraction Osteogenesis: Histological and Histomorphometrical Evaluation in a Rat Calvaria. Clin. Implant. Dent. Relat. Res. 2013, 15, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Saulacic, N.; Nakahara, K.; Iizuka, T.; Haga-Tsujimura, M.; Hofstetter, W.; Scolozzi, P. Comparison of two protocols of periosteal distraction osteogenesis in a rabbit calvaria model. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2016, 104, 1121–1131. [Google Scholar] [CrossRef]
- Nakahara, K.; Haga-Tsujimura, M.; Iizuka, T.; Saulacic, N. Periosteum-Induced Bone Formation by Distraction Osteogenesis: Histologic and Microcomputed Tomography Analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Haga-Tsujimura, M.; Sawada, K.; Mottini, M.; Schaller, B.; Saulacic, N. Periosteal distraction osteogenesis versus immediate periosteal elevation in a rat model: Histological and micro-CT analysis. J. Cranio-Maxillofac. Surg. 2017, 45, 620–627. [Google Scholar] [CrossRef]
- Sencimen, M.; Aydintug, Y.S.; Ortakoglu, K.; Karslioglu, Y.; Gunhan, O.; Gunaydin, Y. Histomorphometrical analysis of new bone obtained by distraction osteogenesis and osteogenesis by periosteal distraction in rabbits. Int. J. Oral Maxillofac. Surg. 2007, 36, 235–242. [Google Scholar] [CrossRef]
- Estrada, J.I.C.; Saulacic, N.; Vazquez, L.; Lombardi, T.; Ramirez, J.U.C.; Bernard, J.P. Periosteal distraction osteogenesis: Preliminary experimental evaluation in rabbits and dogs. Br. J. Oral Maxillofac. Surg. 2007, 45, 402–405. [Google Scholar] [CrossRef]
- Casap, N.; Venezia, N.B.; Wilensky, A.; Samuni, Y. VEGF facilitates periosteal distraction-induced osteogenesis in rabbits: A micro-computerized tomography study. Tissue Eng.-Part A 2008, 14, 247–253. [Google Scholar] [CrossRef]
- Altuǧ, H.A.; Aydintuǧ, Y.S.; Şençimen, M.; Günhan, Ö.; Ortakoǧlu, K.; Bayar, G.R.; Doǧan, N. Histomorphometric analysis of different latency periods effect on new bone obtained by periosteal distraction: An experimental study in the rabbit model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kuroiwa, Y.; Naitoh, M.; Ariji, E.; Sugita, Y.; Maeda, H.; Kurita, K. Image analysis of lateral alveolar ridge augmentation using periosteal distraction osteogenesis. J. Hard Tissue Biol. 2014, 23, 125–130. [Google Scholar] [CrossRef][Green Version]
- Suer, B.T.; Ortakoglu, K.; Gunaydin, Y.; Sencimen, M.; Mutlu, I.; Dogan, N.; Kaya, A. Effects of the hyperbaric oxygen on de novo bone formation during periosteal distraction. J. Craniofac. Surg. 2014, 25, 1740–1745. [Google Scholar] [CrossRef]
- Oda, T.; Kinoshita, K.; Ueda, M. Effects of Cortical Bone Perforation on Periosteal Distraction: An Experimental Study in the Rabbit Mandible. J. Oral Maxillofac. Surg. 2009, 67, 1478–1485. [Google Scholar] [CrossRef]
- Bayar, G.R.; Gunaydin, Y.; Ortakoglu, K.; Gunhan, O.; Aydintug, Y.S.; Sencimen, M. Histomorphometric analysis of new bone obtained by osteogenic periosteal distraction in ovariectomized rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 472–479. [Google Scholar] [CrossRef]
- García-González, M.; Muñoz, F.; González-Cantalapiedra, A.; López-Peña, M.; Saulacic, N. Does the Animal Model Influence in Vertical Alveolar Distraction? A Systematic Review of the Literature. Animals 2020, 10, 2347. [Google Scholar] [CrossRef]
- Saulacic, N.; Somosa Martín, M.; de los Angeles Leon Camacho, M.; García García, A. Complications in Alveolar Distraction Osteogenesis: A Clinical Investigation. J. Oral Maxillofac. Surg. 2007, 65, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.; Sun, Z. Mandibular distraction osteogenesis assisted by cell-based tissue engineering: A systematic review. Orthod. Craniofacial Res. 2015, 18, 39–49. [Google Scholar] [CrossRef]
- Martínez-González, J.M.; Cano-Sánchez, J.; Campo-Trapero, J.; Gonzalo-Lafuente, J.C.; Díaz-Regañón, J.; Vázquez-Piñeiro, M.T. Evaluation of minipigs as an animal model for alveolar distraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.; Gao, G.G.; Shen, X.C.; McLaren, S.G.; Skinner, R.A.; Badger, T.M.; Lumpkin, C.K. The effect of aging on distraction osteogenesis in the rat. J. Orthop. Res. 2001, 19, 421–427. [Google Scholar] [CrossRef]
- Mofid, M.M.; Manson, P.N.; Robertson, B.C.; Tufaro, A.P.; Elias, J.J.; Vander Kolk, C.A. Craniofacial distraction osteogenesis: A review of 3278 cases. Plast. Reconstr. Surg. 2001, 108, 1103–1114. [Google Scholar] [CrossRef]
- Jensen, O.T.; Cockrell, R.; Kuhike, L.; Reed, C. Anterior maxillary alveolar distraction osteogenesis: A prospective 5-year clinical study. Int. J. Oral Maxillofac. Implants 2002, 17, 52–68. [Google Scholar] [PubMed]
- Pérez-Sayáns, M.; Martínez-Martín, J.M.; Chamorro-Petronacci, C.; Gallas-Torreira, M.; Marichalar-Mendía, X.; García-García, A. 20 years of alveolar distraction: A systematic review of the literature. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e742–e751. [Google Scholar] [CrossRef]
- Kilkenny, C.; Parsons, N.; Kadyszewski, E.; Festing, M.F.W.; Cuthill, I.C.; Fry, D.; Hutton, J.; Altman, D.G. Survey of the Quality of Experimental Design, Statistical Analysis and Reporting of Research Using Animals. PLoS ONE 2009, 4, e7824. [Google Scholar] [CrossRef] [PubMed]
- Ulm, C.; Kneissel, M.; Schedle, A.; Solar, P.; Matejka, M.; Schneider, B.; Donath, K. Characteristic features of trabecular bone in edentulous maxillae. Clin. Oral Implants Res. 1999, 10, 459–467. [Google Scholar] [CrossRef]
| Author | Year | Animal | Breed | Age | Sex | Weight (kg) | N° Patients | Evaluation Method |
|---|---|---|---|---|---|---|---|---|
| Schmidt et al. [6] | 2002 | Rabbit | New Zealand | Adult | Male | 3.9 ± 0.39 | 10 | Histologic and histomorphometric |
| Sencimen et al. [27] | 2007 | Rabbit | New Zealand | Adult | Male | 3.55 ± 0.65 | 36 | Histologic and histomorphometric |
| Estrada et al. [28] | 2007 | Dog | - | 20 months-old | - | 16 | 4 | Radiographic and histologic |
| Casap et al. [29] | 2008 | Rabbit | New Zealand | 10 months-old | Male | 2.9 | 10 | Micro-CT and histomorphometric |
| Oda et al. [33] | 2009 | Rabbit | Japanese | Adult | Male | 3.2–3.7 | 25 | Radiographic and histologic |
| Altuğ et al. [30] | 2011 | Rabbit | New Zealand | Adult | - | 4.15 ± 0.55 | 36 | Histologic and histomorphometric |
| Bayar et al. [34] | 2012 | Rabbit | New Zealand | 6 months-old | Female | 3.6 | 36 | Histologic and histomorphometric |
| Inoue et al. [31] | 2014 | Dog | - | 1–2 years-old | Female | 10 to 15 | 4 | Micro-CT |
| Suer et al. [32] | 2014 | Rabbit | New Zealand | Adult | Male | 3.7 ± 0.55 | 24 | Radiologic, photodensitometric and histologic |
| Kahraman et al. [16] | 2015 | Rabbit | New Zealand | Adult | - | 3.05 ± 0.15 | 20 | Radiographic, micro-CT, histologic and histomorphometric |
| Pripatnanont et al. [17] | 2015 | Rabbit | New Zealand | Adult | Male | 3.5 | 12 | Micro-CT, Histologic and histomorphometric |
| Kessler et al. [4] | 2006 | Minipig | Göttingen | 2–3 months-old | Female | 20–25 | 6 | Micro-CT and histologic |
| Estrada et al. [28] | 2007 | Rabbit | New Zealand | 4 months-old | - | 3.5 | 12 | Radiographic and histologic |
| Lethaus et al. [18] | 2010 | Minipig | - | Adult | Female | 34 ± 4.8 | 9 | Histologic and micro-CT |
| Sato et al. [19] | 2010 | Rabbit | New Zealand | 3–4 months-old | Male | 3 | 8 | Micro-CT, histologic and inmunohistochemistry |
| Tudor et al. [2] | 2010 | Minipig | Gottingen | 23 months-old | Female | 24 ± 4.8 | 9 | Histologic and micro-CT |
| Zakaria et al. [20] | 2012 | Rabbit | Japanese | Adult | Male | 2.5–3 | 8 | Histologic and micro-CT |
| Zakaria et al. [21] | 2012 | Rabbit | Japanese | 1.5 months-old | Male | 2.5–3 | 12 | Histologic and micro-CT |
| Saulacic et al. [22] | 2013 | Rat | - | - | - | - | 16 | Histologic and histomorphometric |
| Saulacic et al. [23] | 2013 | Rat | Wistar | Adult | Male | 0.4 | 48 | Histologic and histomorphometric |
| Saulacic et al. [24] | 2016 | Rabbit | New Zealand | Adult | Female | 3 | 60 | Histologic and micro-CT |
| Nakahara et al. [25] | 2016 | Rat | Wistar | Adult | Male | 0.3 | 28 | Histologic and micro-CT |
| Nakahara et al. [26] | 2017 | Rat | Wistar | Adult | Male | 0.3 | 30 | Histologic and micro-CT |
| Zhao et al. [5] | 2020 | Rabbit | New Zealand | 1.5–2 months-old | Male | 2.5–3 | 18 | Histologic and micro-CT |
| Author | Year | Animal | Breed | Distractor | N° Devices | Anatomical Region | Latency Period (Days) | Distraction Period (Days) | Frecuency/Rate | Consolidation Period (Days or Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| Schmidt et al. [6] | 2002 | Rabbit | New Zealand | U-shaped body, Synthes Maxillofacial, Paoli, Pa | 10 | Lateral surface of the mandible (E) | 7 | 15 | 7 mm over 15 days | 4, 5, 6, 8 w |
| Sencimen et al. [27] | 2007 | Rabbit | New Zealand | U-shaped device | 18 | Lateral surface of the mandible (E) | 7 | 10 | 0.25 mm/12 h | 15, 30, 60 d |
| Estrada et al. [28] | 2007 | Dog | - | Titanium plate, Tracper TM Serf. Décines, France | 12 | Intraoral in the four quadrants (I) | 10 | 22 | 0.22 mm/d | 90 d |
| Casap et al. [29] | 2008 | Rabbit | New Zealand | U-shaped device | 10 | Mandible (E) | 14 | 7 | 1 mm/d | 60 d |
| Oda et al. [33] | 2009 | Rabbit | Japanese | Titanium mesh, M-TAM, Stryker Leibinger, Kalamazoo, MI | 25 | Mandible (E) | 7 | 8 | 0.5 mm/d | 4 and 8 w |
| Altuğ et al. [30] | 2011 | Rabbit | New Zealand | U-Shaped device | 36 | Mandible (E) | 1 or 7 | 10 | 0.25 mm/12 h | 15, 30, 60 d |
| Bayar et al. [34] | 2012 | Rabbit | New Zealand | U-shaped device | 36 | Mandibular corpus (E) | 7 | 10 | 0.25 mm/12 h | 15, 30, 60 d |
| Inoue et al. [31] | 2014 | Dog | - | Titanium plate | 6 | Mandible PM1-M1 (I) | 24 | 6 | 0.5 mm/d | 8 w |
| Suer et al. [32] | 2014 | Rabbit | New Zealand | U-shaped device | 24 | Lateral surface of the mandible (E) | 7 | 6 | 0.25 mm/12 h | 4 and 8 w |
| Kahraman et al. [16] | 2015 | Rabbit | New Zealand | Titanium mesh | 20 | Lower border of the mandible (E) | 7 | 10 | 0.35 mm/d | 45 d |
| Pripatnanont et al. [17] | 2015 | Rabbit | New Zealand | Modified Hyrax device, Leone S.p.A., Firenze, Italy | 12 | Ramus and body of Mandible (E) | 3 | 7 | 0.5 mm/12 h | 4 and 8 w |
| Kessler et al. [4] | 2006 | Minipig | Goettingen | Titanium mesh | 6 | Forehead region | 5 | 10 | 0.5 mm/d | 7, 17, 45 d |
| Estrada et al. [28] | 2007 | Rabbit | New Zealand | Titanium plate | 12 | Forehead region | 10 | 22 | 0.25 mm/d, 0.5 mm/d | 10, 20, 30, 40, 50, 60 d |
| Lethaus et al. [18] | 2010 | Minipig | - | Laser-perforated titanium mesh | 18 | Forehead region | 3 | 5, 10, 15 | 0.5 mm/12 h | 2, 4, 6 w |
| Sato et al. [19] | 2010 | Rabbit | New Zealand | Titanium plate | 8 | Calvaria | 7 | 20 | 0.5 mm/d | 3 w |
| Tudor et al. [2] | 2010 | Minipig | Gottingen | Laser-perforated titanium mesh, KLS Martin, Tuttligen, Germany | 9 | Forhead region | 3 | 5, 10, 15 | 0.5 mm/12 h | 2, 4, 6 w |
| Zakaria et al. [20] | 2012 | Rabbit | Japanese | Biodegradable PLLA mesh | 8 | Calvaria | 7 | 5 | 0.5 mm/12 h | 4, 6 w |
| Zakaria et al. [21] | 2012 | Rabbit | Japanese | Titanium mesh | 12 | Calvaria | 7 | 5 | 0.5 mm/12 h | 4, 6 w |
| Saulacic et al. [22] | 2013 | Rat | - | Hemispherical disc | 16 | Calvaria | 7 | 10 | 0.4 mm/d | 10, 20 d |
| Saulacic et al. [23] | 2013 | Rat | Wistar | Titanium plate | 48 | Calvaria | 7 | 10 | 0.2 mm/d | 7 |
| Saulacic et al. [24] | 2016 | Rabbit | New Zealand | U-shaped device | 60 | Calvaria | 7 | 10 | 0.25 mm/d 0.5 mm/d | 10, 17, 24, 31, 77 d |
| Nakahara et al. [25] | 2016 | Rat | Wistar | Titanium plate | 28 | Calvaria | 7 | 10 | 0.1 mm/d | 10 d |
| Nakahara et al. [26] | 2017 | Rat | Wistar | Titanium plate | 30 | Calvaria | 7 | 10 | 0.1 mm/d | 17, 31, 45 d |
| Zhao et al. [5] | 2020 | Rabbit | New Zealand | Biodegradable PLLA mesh | 18 | Calvaria | 7 | 5 | 0.1 mm/d | 8 w |
| Author | Year | Animal Model | Patient Number | Distractor | Complications | No Animals Affected | Period | Major or Minor | Treatment/Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Schmidt et al. [6] | 2002 | Rabbit | 10 | U-shaped body, Synthes Maxillofacial, Paoli, Pa | Lost device | 1 | Latency | Major | Animal excluded |
| Sencimen et al. [27] | 2007 | Rabbit | 36 | U-shaped device | N | N | N | N | N |
| Estrada et al. [28] | 2007 | Dog | 4 | Titanium plate, Tracper TM Serf. Décines, France | Dehiscence | 4 | Distraction | Major | Device remove |
| Casap et al. [29] | 2008 | Rabbit | 10 | U-shaped device | Severe infection/Body weight loss > 15% | 1 and 1 | Latency | Major/Major | Animal excluded |
| Oda et al. [33] | 2009 | Rabbit | 25 | Titanium mesh, M-TAM, Stryker Leibinger, Kalamazoo, MI | Screw loss | 1 | Consolidation | Major | Animal excluded |
| Altuğ et al. [30] | 2011 | Rabbit | 36 | U-Shaped device | N | N | N | N | N |
| Bayar et al. [34] | 2012 | Rabbit | 36 | U-shaped device | ? | ? | ? | ? | ? |
| Inoue et al. [31] | 2014 | Dog | 4 | Titanium plate | N | N | N | N | N |
| Suer et al. [32] | 2014 | Rabbit | 24 | U-shaped device | N | N | N | N | N |
| Kahraman et al. [16] | 2015 | Rabbit | 20 | Titanium mesh | N | N | N | N | N |
| Pripatnanont et al. [17] | 2015 | Rabbit | 12 | Modified Hyrax device, Leone S.p.A., Firenze, Italy | Slight Device displacement | 2 | Consolidation | Minor | Neck collar and conservative |
| Kessler et al. [4] | 2006 | Minipig | 6 | Titanium mesh | N | N | N | N | N |
| Estrada et al. [28] | 2007 | Rabbit | 12 | Titanium plate | Severe infection | 12. | Consolidation | Major/Major | Animal excluded |
| Lethaus et al. [18] | 2010 | Minipig | 9 | Laser-perforated titanium mesh | Severe Device displacement | 1 | Consolidation | Major | Animal excluded |
| Sato et al. [19] | 2010 | Rabbit | 8 | Titanium plate | ? | ? | ? | ? | ? |
| Tudor et al. [2] | 2010 | Minipig | 9 | Laser-perforated titanium mesh, KLS Martin, Tuttligen, Germany | Slight Device displacement | 3 | Consolidation | Minor | Conservative |
| Zakaria et al. [20] | 2012 | Rabbit | 8 | Biodegradable PLLA mesh | Mild Infection | 2 | Latency | Minor | Conservative |
| Zakaria et al. [21] | 2012 | Rabbit | 12 | Titanium mesh | N | N | N | N | N |
| Saulacic et al. [22] | 2013 | Rat | 16 | Hemispherical disc | Post-operative death/Lost device | 1 and 1 | Surgery/Distraction | Major/Major | Animal excluded |
| Saulacic et al. [23] | 2013 | Rat | 48 | Titanium plate | Severe infection/Lost device | 1 and 2 | Consolidation | Major/Major | Animal excluded |
| Saulacic et al. [24] | 2016 | Rabbit | 60 | U-shaped device | N | N | N | N | N |
| Nakahara et al. [25] | 2016 | Rat | 28 | Titanium plate | Post-operative death/Lost device | 1 and 1 | Surgery/Latency | Major/Major | Animal excluded |
| Nakahara et al. [26] | 2017 | Rat | 30 | Titanium plate | N | N | N | N | N |
| Zhao et al. [5] | 2020 | Rabbit | 18 | Biodegradable PLLA mesh | ? | ? | ? | ? | ? |
| Author | Year | Animal Model | Coefficient | Quality |
|---|---|---|---|---|
| Schmidt et al. [6] | 2002 | Rabbit | 0.72 | Average |
| Sencimen et al. [27] | 2007 | Rabbit | 0.69 | Average |
| Estrada et al. [28] | 2007 | Dog | 0.66 | Average |
| Casap et al. [29] | 2008 | Rabbit | 0.77 | Average |
| Oda et al. [33] | 2009 | Rabbit | 0.79 | Average |
| Altuğ et al. [30] | 2011 | Rabbit | 0.62 | Average |
| Bayar et al. [34] | 2012 | Rabbit | 0.67 | Average |
| Inoue et al. [31] | 2014 | Dog | 0.67 | Average |
| Suer et al. [32] | 2014 | Rabbit | 0.89 | Excellent |
| Kahraman et al. [16] | 2015 | Rabbit | 0.84 | Excellent |
| Pripatnanont et al. [17] | 2015 | Rabbit | 0.96 | Excellent |
| Kessler et al. [4] | 2006 | Minipig | 0.65 | Average |
| Estrada et al. [28] | 2007 | Rabbit | 0.67 | Average |
| Lethaus et al. [18] | 2010 | Minipig | 0,72 | Average |
| Sato et al. [19] | 2010 | Rabbit | 0.62 | Average |
| Tudor et al. [2] | 2010 | Minipig | 0.79 | Average |
| Zakaria et al. [20] | 2012 | Rabbit | 0.67 | Average |
| Zakaria et al. [21] | 2012 | Rabbit | 0.67 | Average |
| Saulacic et al. [22] | 2013 | Rat | 0.97 | Excellent |
| Saulacic et al. [23] | 2013 | Rat | 0.84 | Excellent |
| Saulacic et al. [24] | 2016 | Rabbit | 0.86 | Excellent |
| Nakahara et al. [25] | 2016 | Rat | 0.89 | Excellent |
| Nakahara et al. [26] | 2017 | Rat | 0.91 | Excellent |
| Zhao et al. [5] | 2020 | Rabbit | 0.86 | Excellent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-González, M.; Muñoz, F.; González-Cantalapiedra, A.; López-Peña, M.; Saulacic, N. Systematic Review and Quality Evaluation Using ARRIVE 2.0 Guidelines on Animal Models Used for Periosteal Distraction Osteogenesis. Animals 2021, 11, 1233. https://doi.org/10.3390/ani11051233
García-González M, Muñoz F, González-Cantalapiedra A, López-Peña M, Saulacic N. Systematic Review and Quality Evaluation Using ARRIVE 2.0 Guidelines on Animal Models Used for Periosteal Distraction Osteogenesis. Animals. 2021; 11(5):1233. https://doi.org/10.3390/ani11051233
Chicago/Turabian StyleGarcía-González, Mario, Fernando Muñoz, Antonio González-Cantalapiedra, Mónica López-Peña, and Nikola Saulacic. 2021. "Systematic Review and Quality Evaluation Using ARRIVE 2.0 Guidelines on Animal Models Used for Periosteal Distraction Osteogenesis" Animals 11, no. 5: 1233. https://doi.org/10.3390/ani11051233
APA StyleGarcía-González, M., Muñoz, F., González-Cantalapiedra, A., López-Peña, M., & Saulacic, N. (2021). Systematic Review and Quality Evaluation Using ARRIVE 2.0 Guidelines on Animal Models Used for Periosteal Distraction Osteogenesis. Animals, 11(5), 1233. https://doi.org/10.3390/ani11051233







