Plasmids Expressing shRNAs Specific to the Nucleocapsid Gene Inhibit the Replication of Porcine Deltacoronavirus In Vivo
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Virus and Cells
2.3. shRNA Design and Selection
2.4. Construction of shRNA Plasmids
2.5. Generation of LLC-PK1 Cells Stably Expressing shRNA and Virus Infection
2.6. Cell Viability Determination (MTS Assay)
2.7. Virus Titration (TCID50 Determination)
2.8. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.9. Western Blotting
2.10. Animal Challenge Experiments
2.11. Statistical Analysis
3. Results
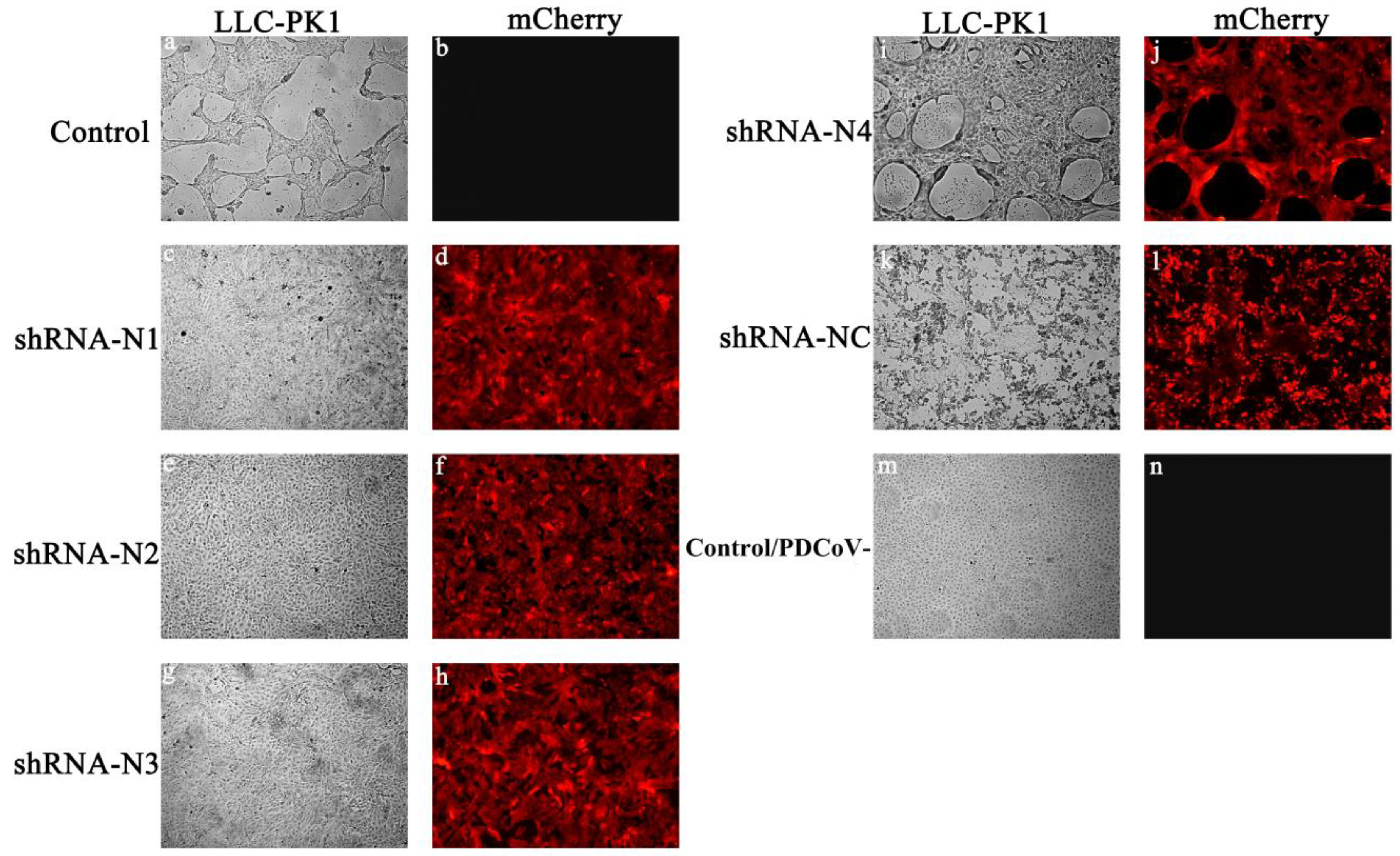
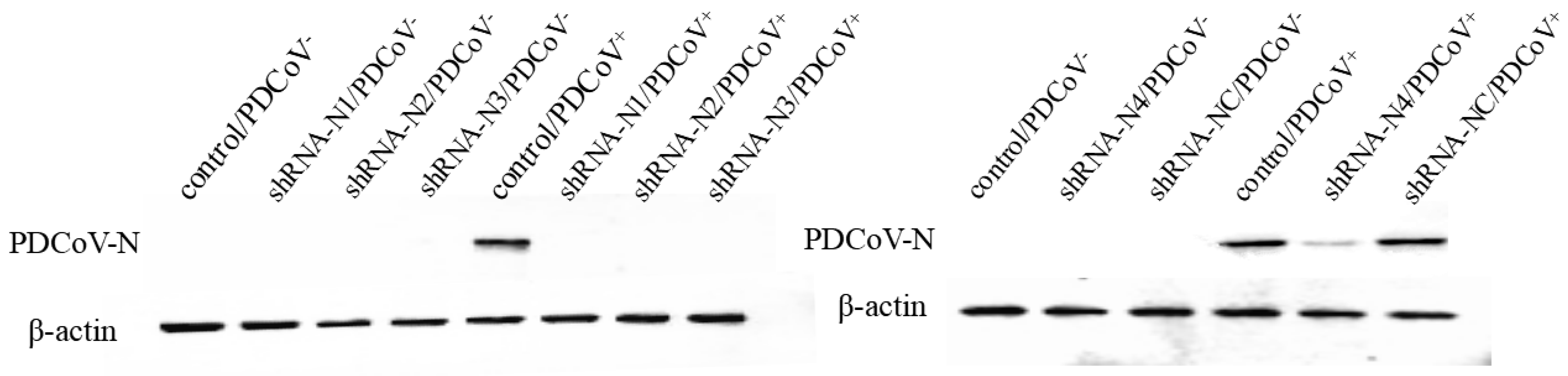
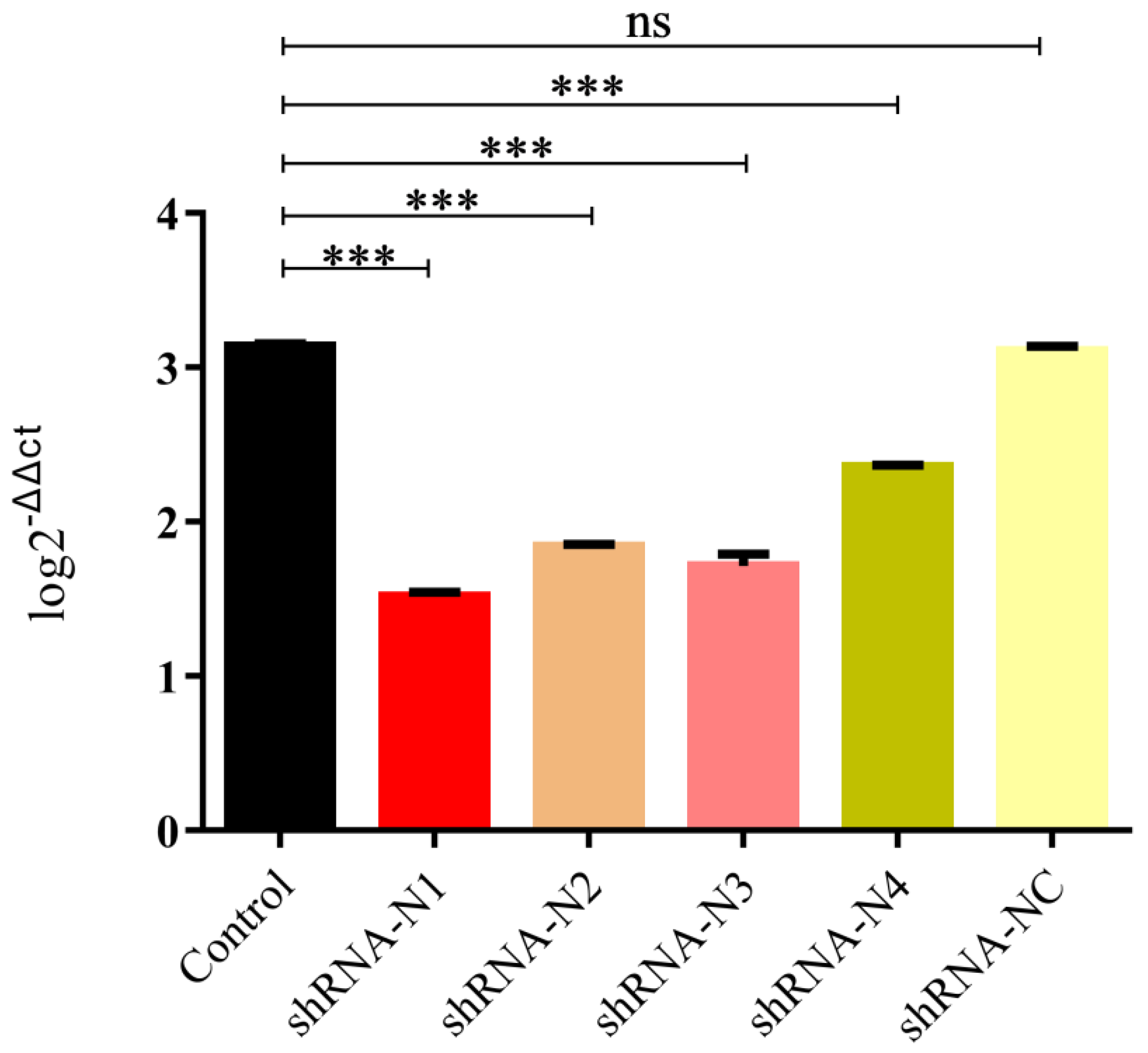
3.1. Generation of LLC-PK1 Cell Lines Stably Expressing shRNA and Exploration of the Antiviral Properties of shRNA
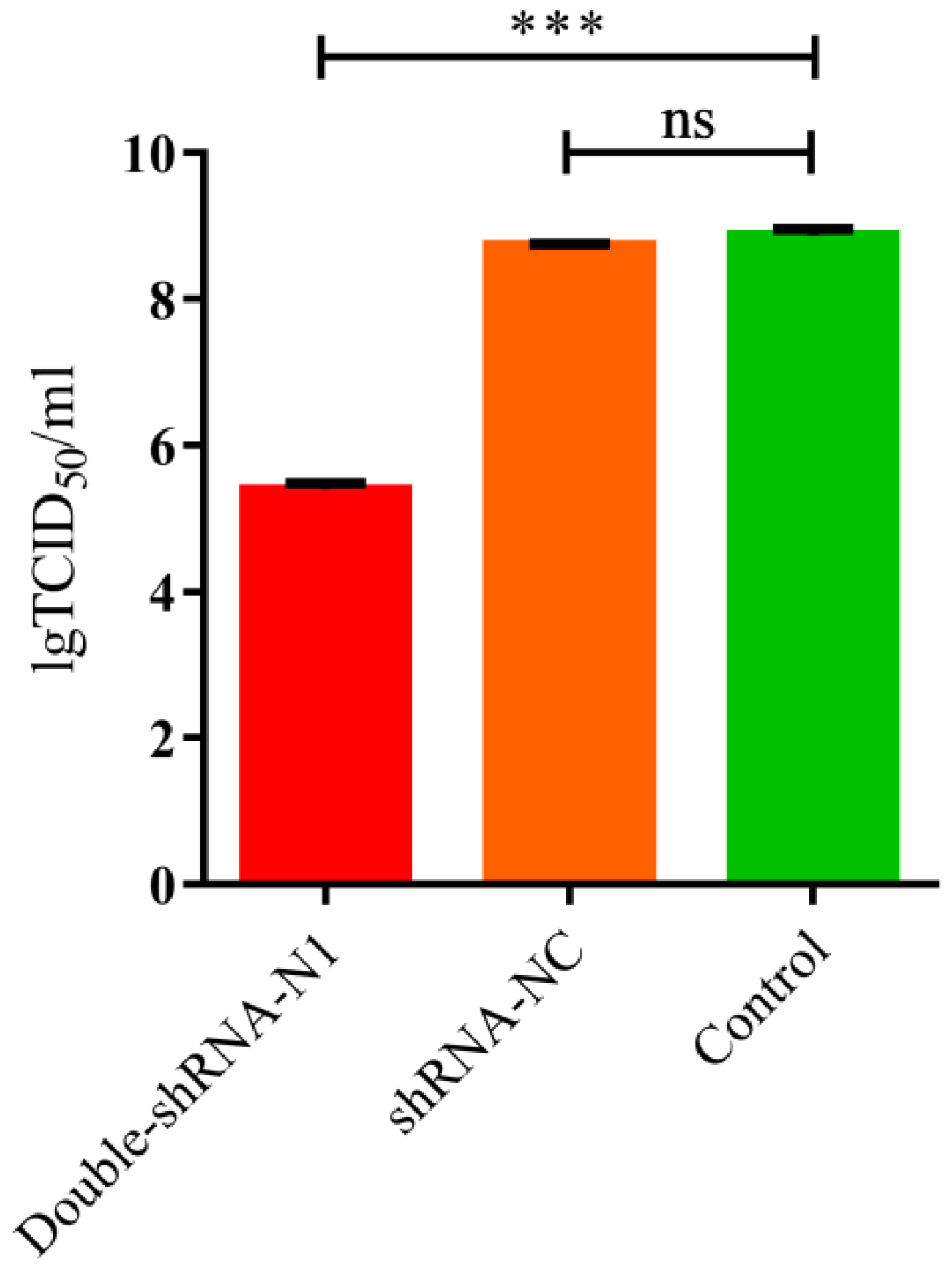
3.2. Anti-PDCoV Activity of pSil-Double-shRNA-N1-Mcherry in Infected LLC-PK1 Cells
3.3. Inhibition Effects of pSil-Double-shRNA-N1-Mcherry Plasmids on PDCoV Replication in Neonatal Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Chan, J.F.; To, K.K.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Dong, B.Q.; Liu, W.; Fan, X.H.; Vijaykrishna, D.; Tang, X.C.; Gao, F.; Li, L.F.; Li, G.J.; Zhang, J.X.; Yang, L.Q.; et al. Detection of a novel and highly divergent coronavirus from asian leopard cats and Chinese ferret badgers in Southern China. J. Virol. 2007, 81, 6920–6926. [Google Scholar] [CrossRef]
- Marthaler, D.; Jiang, Y.; Collins, J.; Rossow, K. Complete Genome Sequence of Strain SDCV/USA/Illinois121/2014, a Porcine Deltacoronavirus from the United States. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Marthaler, D.; Raymond, L.; Jiang, Y.; Collins, J.; Rossow, K.; Rovira, A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg. Infect. Dis. 2014, 20, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Byrum, B.; Zhang, Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014, 20, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Byrum, B.; Zhang, Y. Porcine coronavirus HKU15 detected in 9 US states, 2014. Emerg. Infect. Dis. 2014, 20, 1594–1595. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Fang, L.; Zeng, S.; Sun, Q.; Chen, H.; Xiao, S. Porcine Deltacoronavirus in Mainland China. Emerg. Infect. Dis. 2015, 21, 2254–2255. [Google Scholar] [CrossRef] [PubMed]
- Janetanakit, T.; Lumyai, M.; Bunpapong, N.; Boonyapisitsopa, S.; Chaiyawong, S.; Nonthabenjawan, N.; Kesdaengsakonwut, S.; Amonsin, A. Porcine Deltacoronavirus, Thailand, 2015. Emerg. Infect. Dis. 2016, 22, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, C. Complete Genome Characterization of Korean Porcine Deltacoronavirus Strain KOR/KNU14-04/2014. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Lorsirigool, A.; Saeng-Chuto, K.; Temeeyasen, G.; Madapong, A.; Tripipat, T.; Wegner, M.; Tuntituvanont, A.; Intrakamhaeng, M.; Nilubol, D. The first detection and full-length genome sequence of porcine deltacoronavirus isolated in Lao PDR. Arch. Virol. 2016, 161, 2909–2911. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhou, X.; Peng, Q.; Chen, Y.; Zhang, F.; Huang, T.; Zhang, T.; Li, A.; Huang, D.; Wu, Q.; et al. Newly Emerged Porcine Deltacoronavirus Associated with Diarrhoea in Swine in China: Identification, Prevalence and Full-Length Genome Sequence Analysis. Transbound. Emerg. Dis. 2015, 62, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016, 226, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hulswit, R.J.G.; Kenney, S.P.; Widjaja, I.; Jung, K.; Alhamo, M.A.; van Dieren, B.; van Kuppeveld, F.J.M.; Saif, L.J.; Bosch, B.J. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc. Natl. Acad. Sci. USA 2018, 115, e5135–e5143. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Hu, H.; Eyerly, B.; Lu, Z.; Chepngeno, J.; Saif, L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015, 21, 650–654. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liang, X.; Lou, F.; Oglesbee, M.; Krakowka, S.; Li, J. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio 2015, 6, e00064. [Google Scholar] [CrossRef]
- McCaffrey, A.P.; Nakai, H.; Pandey, K.; Huang, Z.; Salazar, F.H.; Xu, H.; Wieland, S.F.; Marion, P.L.; Kay, M.A. Inhibition of hepatitis B virus in mice by RNA interference. Nat. Biotechnol. 2003, 21, 639–644. [Google Scholar] [CrossRef]
- Hiscox, J.A.; Wurm, T.; Wilson, L.; Britton, P.; Cavanagh, D.; Brooks, G. The coronavirus infectious bronchitis virus nucleoprotein localizes to the nucleolus. J. Virol. 2001, 75, 506–512. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C. Functional characterization and proteomic analysis of the nucleocapsid protein of porcine deltacoronavirus. Virus Res. 2015, 208, 136–145. [Google Scholar] [CrossRef]
- McBride, R.; van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Vaucheret, H.; Beclin, C.; Fagard, M. Post-transcriptional gene silencing in plants. J. Cell Sci. 2001, 114, 3083–3091. [Google Scholar] [PubMed]
- Lau, T.S.; Li, Y.; Kameoka, M.; Ng, T.B.; Wan, D.C. Suppression of HIV replication using RNA interference against HIV-1 integrase. FEBS Lett. 2007, 581, 3253–3259. [Google Scholar] [CrossRef]
- Li, B.J.; Tang, Q.; Cheng, D.; Qin, C.; Xie, F.Y.; Wei, Q.; Xu, J.; Liu, Y.; Zheng, B.J.; Woodle, M.C.; et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005, 11, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.Y.; Zhao, G.Y.; Huang, J.D.; Jin, D.Y.; Yuen, K.Y.; Zheng, B.J. Small interfering RNA targeting m2 gene induces effective and long term inhibition of influenza A virus replication. PLoS ONE 2009, 4, e5671. [Google Scholar] [CrossRef]
- Li, J.; Dai, Y.; Liu, S.; Guo, H.; Wang, T.; Ouyang, H.; Tu, C. In vitro inhibition of CSFV replication by multiple siRNA expression. Antiviral. Res. 2011, 91, 209–216. [Google Scholar] [CrossRef]
- Oh, J.N.; Choi, K.H.; Lee, C.K. Multi-resistance strategy for viral diseases and in vitro short hairpin RNA verification method in pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, F.; Hua, X.; Cui, L.; Zhang, W.; Shen, Y.; Yan, Y.; Chen, P.; Ding, D.; Mou, J.; et al. Inhibition of porcine transmissible gastroenteritis virus (TGEV) replication in mini-pigs by shRNA. Virus Res. 2010, 149, 51–55. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, C.; Guo, P.; Liu, Z.; Zhang, J. Effective inhibition of porcine epidemic diarrhea virus by RNA interference in vitro. Virus Genes 2015, 51, 252–259. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Bi, Z.; Song, D.; Zhang, F.; Lei, D.; Luo, S.; Li, Z.; Gong, W.; Huang, D.; et al. Significant inhibition of re-emerged and emerging swine enteric coronavirus in vitro using the multiple shRNA expression vector. Antiviral. Res. 2019, 166, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Dong, H.; Zhu, Y.; Shi, H.; Chen, J.; Shi, D.; Feng, L. Characterization of Two Monoclonal Antibodies That Recognize Linker Region and Carboxyl Terminal Domain of Coronavirus Nucleocapsid Protein. PLoS ONE 2016, 11, e0163920. [Google Scholar] [CrossRef] [PubMed]
- Caplen, N.J.; Taylor, J.P.; Statham, V.S.; Tanaka, F.; Fire, A.; Morgan, R.A. Rescue of polyglutamine-mediated cytotoxicity by double-stranded RNA-mediated RNA interference. Hum. Mol. Genet. 2002, 11, 175–184. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Xiangji, L.; Feng, X.; Qingbao, C.; Weifeng, T.; Xiaoqing, J.; Baihe, Z.; Feng, S.; Hongyang, W.; Mengchao, W. Knockdown of HBV surface antigen gene expression by a lentiviral microRNA-based system inhibits HBV replication and HCC growth. J. Viral. Hepat. 2011, 18, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors Applied for RNAi-Based Antiviral Therapy. Viruses 2020, 12, 924. [Google Scholar] [CrossRef]
- Brummelkamp, T.R.; Bernards, R.; Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002, 296, 550–553. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Y.M.; Dong, L.J.; Cheng, M.; Wang, J.; Tang, Q.H.; Wang, G. In vitro inhibition of transmissible gastroenteritis coronavirus replication in swine testicular cells by short hairpin RNAs targeting the ORF 7 gene. Virol. J. 2012, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhou, J.; Yang, Z.; Cui, L.; Zhang, W.; Yuan, C.; Yang, S.; Zhu, J.; Hua, X. RNA interference inhibits hepatitis E virus mRNA accumulation and protein synthesis in vitro. Vet. Microbiol. 2010, 142, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; York, J.P.; Zhang, P. A transgenic approach for RNA interference-based genetic screening in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 2252–2256. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Cao, L.; Wang, P.; Qing, J.; Zheng, Q.; Shang, L.; Yin, Z.; Sun, Y. A Conserved Inhibitory Mechanism of a Lycorine Derivative against Enterovirus and Hepatitis C Virus. Antimicrob. Agents Chemother. 2016, 60, 913–924. [Google Scholar] [CrossRef]
- Lambeth, L.S.; Van Hateren, N.J.; Wilson, S.A.; Nair, V. A direct comparison of strategies for combinatorial RNA interference. BMC Mol. Biol. 2010, 11, 77. [Google Scholar] [CrossRef][Green Version]
- Khan, A.A.; Betel, D.; Miller, M.L.; Sander, C.; Leslie, C.S.; Marks, D.S. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat. Biotechnol. 2009, 27, 549–555. [Google Scholar] [CrossRef] [PubMed]






| Name | Sequence (5′ to 3′) | Position |
|---|---|---|
| N1 | 5′-TTTAATAGAAGTGTCAGCC-3′ | 24305-24321 |
| N2 | 5′-TAAATACCTGAGAAATGGC-3′ | 24594-24610 |
| N3 | 5′-TGTTAACAGATTGAGATCC-3′ | 24468-24484 |
| N4 | 5′-TATACTTAAGATTTCCTCC-3′ | 24225-24241 |
| NC | 5′-TAACATAGGGCAATTTAGC-3′ |
| Name | Sequence |
|---|---|
| N a | F: 5′-ATCGACCACATGGCTCCAA-3′ |
| R: 5′-CAGCTCTTGCCCATGTAGCTT-3′ | |
| β-actin | F: 5′-CTCTTCCAGCCCTCCTTCC-3′ |
| R: 5′-GGTCCTTGCGGATGTCG-3′ |
| Group | ||||||
|---|---|---|---|---|---|---|
| P + V | P | N + V | N | C + V | C | |
| Plasmid | pSil-double-shRNA-N1-mcherry | pSil-double-shRNA-N1-mcherry | pSil-shRNA-NC-mcherry | pSil-shRNA-NC-mcherry | PBS | PBS |
| Vector dosage | 3 mg/1 mL/piglet | 3 mg/1 mL/piglet | 3 mg/1 mL/piglet | 3 mg/1 mL/piglet | 1 mL/piglet | 1 mL/piglet |
| PDCoV | PDCoV | PBS | PDCoV | PBS | PDCoV | PBS |
| Viral dosage | 105 TCID50/10 mL/piglet | 10 mL/piglet | 105 TCID50/10 mL/piglet | 10 mL/piglet | 105 TCID50/10 mL/piglet | 10 mL/piglet |
| Group | V/C Ratio | |
|---|---|---|
| Duodenum | P + V | 6.56 |
| P | 15.45 | |
| N + V | 2.76 | |
| N | 5.52 | |
| C + V | 1.12 | |
| C | 16.54 | |
| Jejunum | P + V | 8.45 |
| P | 11.38 | |
| N + V | 2.46 | |
| N | 11.79 | |
| C + V | 0.94 | |
| C | 13.97 | |
| Ileum | P + V | 9.68 |
| P | 11.46 | |
| N + V | 2.23 | |
| N | 12.86 | |
| C + V | 1.29 | |
| C | 13.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Li, H.; Bi, Z.; Li, K.; Li, Z.; Song, D.; Ding, Z.; He, H.; Wu, Q.; Huang, D.; et al. Plasmids Expressing shRNAs Specific to the Nucleocapsid Gene Inhibit the Replication of Porcine Deltacoronavirus In Vivo. Animals 2021, 11, 1216. https://doi.org/10.3390/ani11051216
Gu J, Li H, Bi Z, Li K, Li Z, Song D, Ding Z, He H, Wu Q, Huang D, et al. Plasmids Expressing shRNAs Specific to the Nucleocapsid Gene Inhibit the Replication of Porcine Deltacoronavirus In Vivo. Animals. 2021; 11(5):1216. https://doi.org/10.3390/ani11051216
Chicago/Turabian StyleGu, Jun, Hao Li, Zhen Bi, Kai Li, Zhiquan Li, Deping Song, Zhen Ding, Houjun He, Qiong Wu, Dongyan Huang, and et al. 2021. "Plasmids Expressing shRNAs Specific to the Nucleocapsid Gene Inhibit the Replication of Porcine Deltacoronavirus In Vivo" Animals 11, no. 5: 1216. https://doi.org/10.3390/ani11051216
APA StyleGu, J., Li, H., Bi, Z., Li, K., Li, Z., Song, D., Ding, Z., He, H., Wu, Q., Huang, D., Gan, P., Ye, Y., & Tang, Y. (2021). Plasmids Expressing shRNAs Specific to the Nucleocapsid Gene Inhibit the Replication of Porcine Deltacoronavirus In Vivo. Animals, 11(5), 1216. https://doi.org/10.3390/ani11051216






