Complete Blood Count Analysis on Beef Cattle Exposed to Fescue Toxicity and Rumen-Protected Niacin Supplementation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
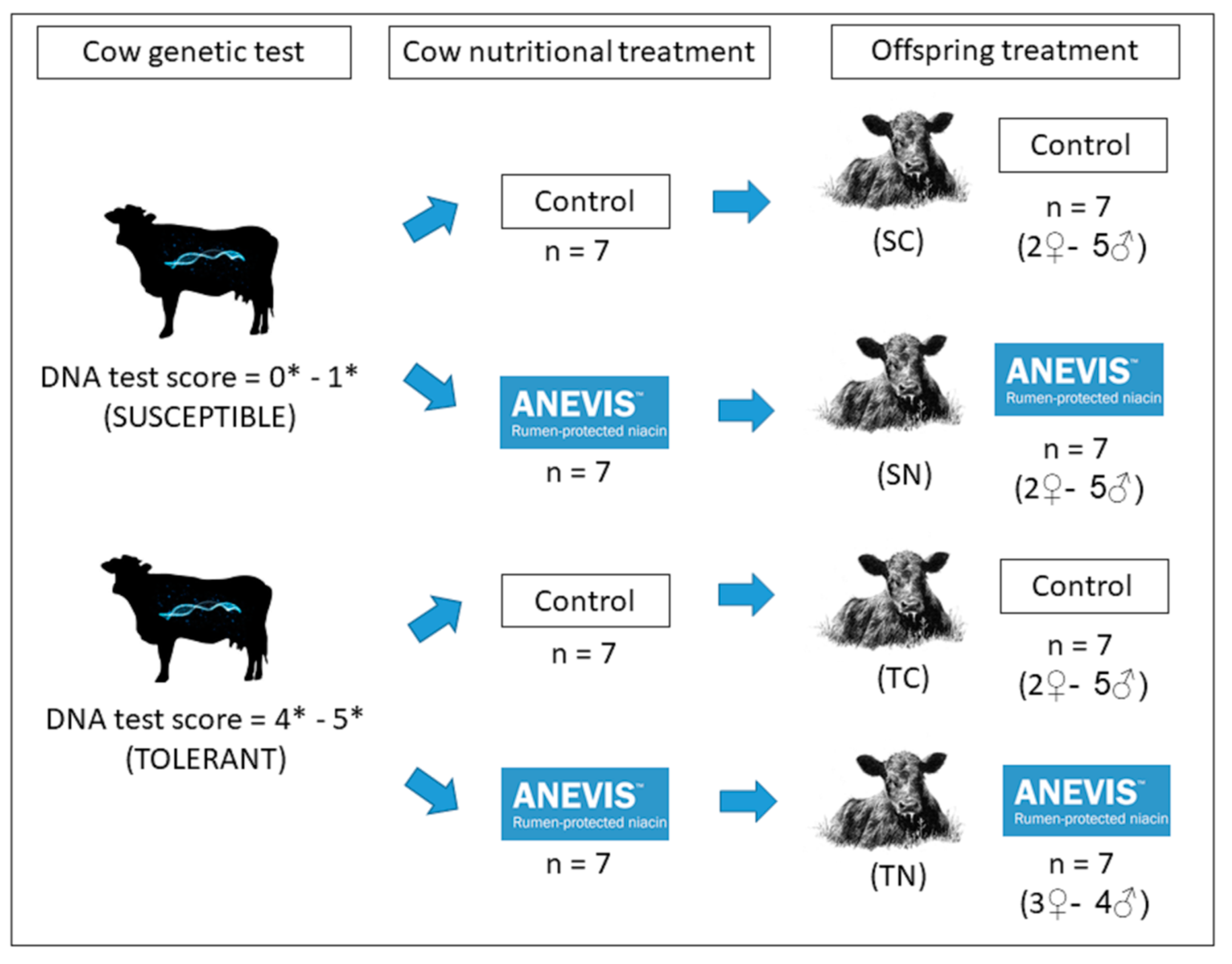
2.1. Animals and Experimental Design
2.2. Animal Performance
2.3. Complete Blood Counting Analysis
2.4. Statistical Analyses
3. Results
3.1. Complete Blood Count
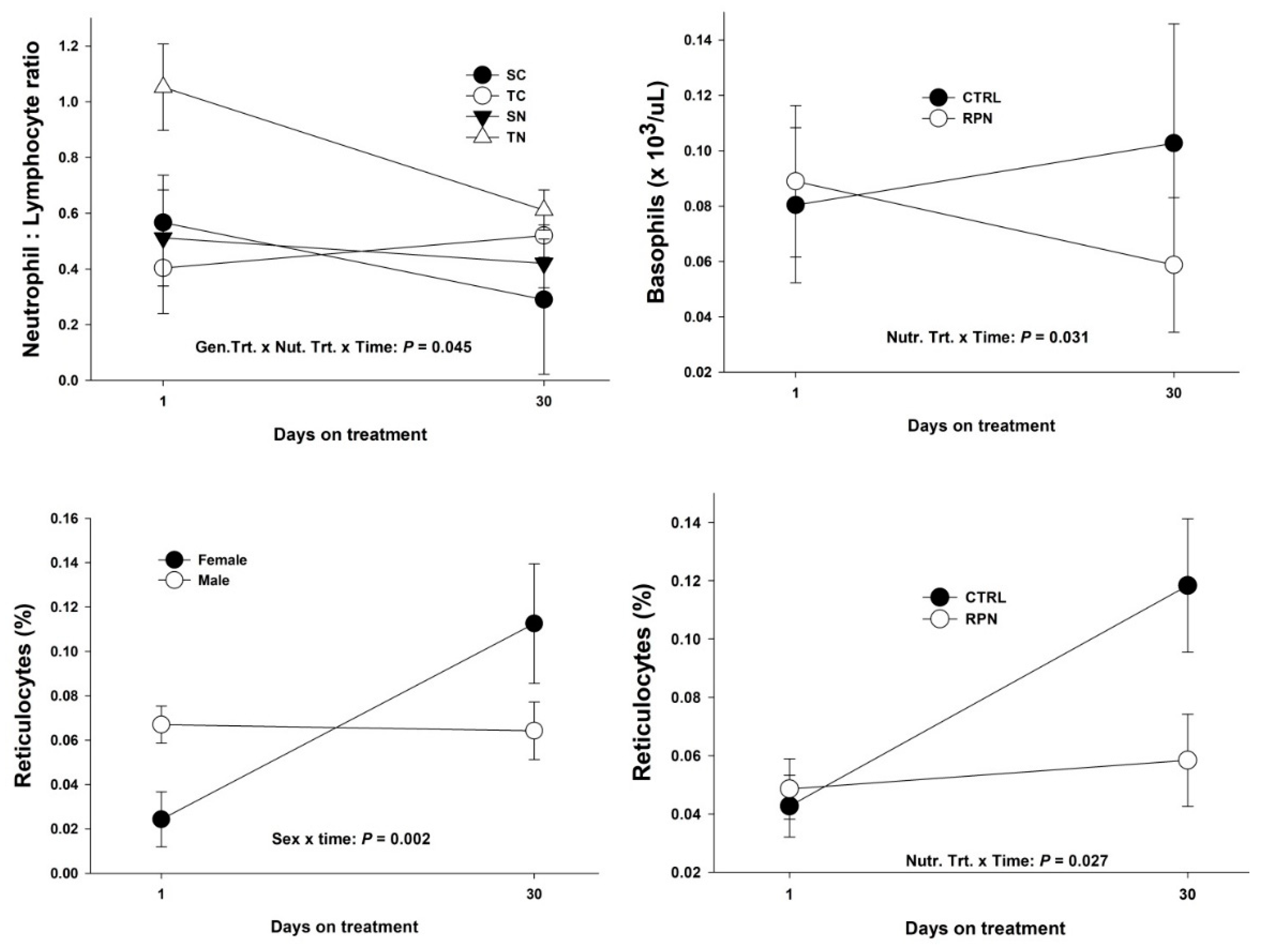
3.1.1. Neutrophil to Lymphocyte Ratio, Reticulocytes and Basophils
3.1.2. Hematocrit, Hemoglobin, Red Blood Cell Distribution Width, and White Blood Cells
3.1.3. Mean Corpuscular Hemoglobin, Mean Corpuscular Volume, Neutrophils and White Blood Cells
3.1.4. Endophyte-Infected Tall Fescue Seeds Effect on Hematocrit, Hemoglobin, Red Blood Cells, and Reticulocytes
3.1.5. Sex Effects on Red Blood Cell Distribution Width and Rumen-Protected Niacin Effect on Mean Corpuscular Hemoglobin and Mean Corpuscular Volume
3.2. Animal Performance
Body Weight, Average Daily Gain, Rectal Temperature (RT), Respiration Rate (RR), and Hair Shedding Score (HS)
4. Discussion
4.1. Complete Blood Count
4.1.1. Red Blood Cells Indices and Niacin
4.1.2. White Blood Cells (WBC), Neutrophil:Lymphocyte Ratio, Neutrophils and Basophils
4.1.3. Reticulocytes and Red Blood Cells Distribution Width
4.2. Animal Performance
4.2.1. Body Weight and Average Daily Gain
4.2.2. Rectal Temperature and Respiration Rate
4.2.3. Hair Shedding Score
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kallenbach, R.L.; Bishop Hurley, G.J.; Massie, M.D.; Rottinghaus, G.E.; West, C.P. Herbage mass, nutritive value, and ergovaline concentration of stockpiled tall fescue. Crop Sci. 2003, 43, 1001–1005. [Google Scholar] [CrossRef]
- Stuedemann, J.A.; Hoveland, C.S. Fescue endophyte: History and impact on animal agriculture. J. Prod. Agric. 1988, 1, 39–44. [Google Scholar] [CrossRef]
- Bacon, C.W.; Siegel, M.R. Endophyte Parasitism of Tall Fescue. J. Prod. Agric. 1988, 1, 45–55. [Google Scholar] [CrossRef]
- Dillard, S.L.; Smith, S.R.; Hancock, D.W. Variability of ergovaline and total ergot alkaloid expression among endophytic tall fescue cultivars. Crop Sci. 2019, 59, 2866–2875. [Google Scholar] [CrossRef]
- Liebe, D.M.; White, R.R. Meta-analysis of endophyte-infected tall fescue effects on cattle growth rates. J Anim. Sci. 2018, 96, 1350–1361. [Google Scholar] [CrossRef]
- Rottinghaus, G.E.; Garner, G.B.; Cornell, C.N.; Ellis, J.L. HPLC method for quantitating ergovaline in endophyte-infested tall fescue: Seasonal variation of ergovaline levels in stems with leaf sheaths, leaf blades, and seed heads. J. Agric. Food Chem. 1991, 39, 112–115. [Google Scholar] [CrossRef]
- Klotz, J.L.; Aiken, G.E.; Bussard, J.R.; Foote, A.P.; Harmon, D.L.; Goff, B.M.; Schrick, F.N.; Strickland, J.R. Vasoactivity and Vasoconstriction Changes in Cattle Related to Time off Toxic Endophyte-Infected Tall Fescue. Toxins 2016, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.K.; Devine, T.L.; Mayberry, K.J.; Eisemann, J.H.; Poore, M.H.; Long, N.M.; Poole, D.H. Impact of slick hair trait on physiological and reproductive performance in beef heifers consuming ergot alkaloids from endophyte-infected tall fescue. J. Anim. Sci. 2019, 97, 1456–1467. [Google Scholar] [CrossRef]
- Schmidt, S.P.; Hoveland, C.S.; Clark, E.M.; Davis, N.D.; Smith, L.A.; Grimes, H.W.; Holliman, J.L. Association of an endophytic fungus with fescue toxicity in steers fed Kentucky 31 tall fescue seed or hay. J. Anim. Sci. 1982, 55, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Shoup, L.M.; Miller, L.M.; Srinivasan, M.; Ireland, F.A.; Shike, D.W. Effects of cows grazing toxic endophyte-infected tall fescue or novel endophyte-infected tall fescue in late gestation on cow performance, reproduction, and progeny growth performance and carcass characteristics. J. Anim. Sci. 2016, 94, 5105–5113. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Edwards, J.E.; Bazeley, K.J.; Brown, S.N.; Butterworth, A.; Warriss, P.D. Changes in the blood biochemical and haematological profile of neonatal calves with age. Vet. Rec. 2000, 147, 593–598. [Google Scholar] [CrossRef]
- Chinchilla-Vargas, J.; Kramer, L.M.; Tucker, J.D.; Hubbell, D.S., 3rd; Powell, J.G.; Lester, T.D.; Backes, E.A.; Anschutz, K.; Decker, J.E.; Stalder, K.J.; et al. Genetic Basis of Blood-Based Traits and Their Relationship With Performance and Environment in Beef Cattle at Weaning. Front. Genet. 2020, 11, 717. [Google Scholar] [CrossRef]
- Lakritz, J.; Leonard, M.J.; Eichen, P.A.; Rottinghaus, G.E.; Johnson, G.C.; Spiers, D.E. Whole-blood concentrations of glutathione in cattle exposed to heat stress or a combination of heat stress and endophyte-infected tall fescue toxins in controlled environmental conditions. Am. J. Vet. Res. 2002, 63, 799–803. [Google Scholar] [CrossRef]
- Galliou, J.M.; Khanal, P.; Mayberry, K.; Poore, M.H.; Poole, D.H.; Serao, N.V.L. Evaluation of a commercial genetic test for fescue toxicosis in pregnant Angus beef cattle. Transl. Anim. Sci. 2020, 4, txaa181. [Google Scholar] [CrossRef]
- Poole, D.H.; Lyons, S.E.; Poole, R.K.; Poore, M.H. Ergot alkaloids induce vasoconstriction of bovine uterine and ovarian blood vessels. J. Anim. Sci. 2018, 96, 4812–4822. [Google Scholar] [CrossRef]
- Klotz, J.L.; Kirch, B.H.; Aiken, G.E.; Bush, L.P.; Strickland, J.R. Effects of selected combinations of tall fescue alkaloids on the vasoconstrictive capacity of fescue-naive bovine lateral saphenous veins. J. Anim. Sci. 2008, 86, 1021–1028. [Google Scholar] [CrossRef]
- Porter, J.K.; Thompson, F.N., Jr. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 1992, 70, 1594–1603. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, T.J.; Wu, K.K.; Sturino, C.; Metters, K.; Gottesdiener, K.; Wright, S.D.; Wang, Z.; O’Neill, G.; Lai, E.; et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc. Natl. Acad. Sci. USA 2006, 103, 6682–6687. [Google Scholar] [CrossRef]
- Rungruang, S.; Collier, J.L.; Rhoads, R.P.; Baumgard, L.H.; de Veth, M.J.; Collier, R.J. A dose-response evaluation of rumen-protected niacin in thermoneutral or heat-stressed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5023–5034. [Google Scholar] [CrossRef]
- Holtcamp, A.J.; Sukumaran, A.T.; Schnedler, A.E.; McClenton, B.J.; Kunze, E.; Calkins, C.R.; Karisch, B.B.; Burnett, D.D.; Dinh, T.T.N. Effects of feeding endophyte-infected tall fescue seeds to stocker Angus steers on retail quality attributes of beef strip steaks. Meat. Sci. 2019, 149, 31–39. [Google Scholar] [CrossRef]
- Spiers, D.E.; Eichen, P.A.; Leonard, M.J.; Wax, L.E.; Rottinghaus, G.E.; Williams, J.E.; Colling, D.P. Benefit of dietary seaweed (Ascophyllum nodosum) extract in reducing heat strain and fescue toxicosis: A comparative evaluation. J. Therm. Biol. 2004, 29, 753–757. [Google Scholar] [CrossRef]
- Leach, R.J.; Chitko-McKown, C.G.; Bennett, G.L.; Jones, S.A.; Kachman, S.D.; Keele, J.W.; Leymaster, K.A.; Thallman, R.M.; Kuehn, L.A. The change in differing leukocyte populations during vaccination to bovine respiratory disease and their correlations with lung scores, health records, and average daily gain. J. Anim. Sci. 2013, 91, 3564–3573. [Google Scholar] [CrossRef]
- Coffey, K.P.; Moyer, J.L.; Lomas, L.W.; Smith, J.E.; La Rue, D.C.; Brazle, F.K. Implant and copper oxide needles for steers grazing Acremonium coenophialum-infected tall fescue pastures: Effects on grazing and subsequent feedlot performance and serum constituents. J. Anim. Sci. 1992, 70, 3203–3214. [Google Scholar] [CrossRef]
- Saker, K.E.; Allen, V.G.; Kalnitsky, J.; Thatcher, C.D.; Swecker, W.S., Jr.; Fontenot, J.P. Monocyte immune cell response and copper status in beef steers that grazed endophyte-infected tall fescue. J. Anim. Sci. 1998, 76, 2694–2700. [Google Scholar] [CrossRef] [PubMed]
- Stoszek, M.J.; Oldfield, J.E.; Carter, G.E.; Weswig, P.H. Effect of tall fescue and quackgrass on copper metabolism and weight gains of beef cattle. J. Anim. Sci. 1979, 48, 893–899. [Google Scholar] [CrossRef]
- Saker, K.E.; Allen, V.G.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Wester, D.B. Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J. Anim. Sci. 2001, 79, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper deficiency anemia: Review article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Joerling, J.; Doll, K. Monitoring of iron deficiency in calves by determination of serum ferritin in comparison with serum iron: A preliminary study. Open Vet. J. 2019, 9, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Al-Saif, F.A.; Refat, M.S. Ten metal complexes of vitamin B3/niacin: Spectroscopic, thermal, antibacterial, antifungal, cytotoxicity and antitumor studies of Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Pd(II), Cd(II), Pt(IV) and Au(III) complexes. J. Mol. Struct. 2012, 1021, 40–52. [Google Scholar] [CrossRef]
- Agte, V.V.; Paknikar, K.M.; Chiplonkar, S.A. Effect of nicotinic acid on zinc and iron metabolism. Biometals 1997, 10, 271–276. [Google Scholar] [CrossRef]
- Adebowale, T.O.; Liu, H.; Oso, A.O.; Oke, O.E.; Hussain, T.; Bamgbose, A.M.; Yao, K.; Yulong, Y. Effect of dietary niacin supplementation on performance, total tract nutrient retention, carcass yield and meat lipid profile of growing turkeys. Anim. Prod. Sci. 2019, 59, 1098–1107. [Google Scholar] [CrossRef]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef]
- Craig, T.M. Impact of internal parasites on beef cattle. J. Anim. Sci. 1988, 66, 1565–1569. [Google Scholar] [CrossRef]
- Costill, D.L.; Saltin, B. Changes in the ratio of venous to body hematocrit following dehydration. J. Appl. Physiol. 1974, 36, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Kishore, D.K. Effects of Heat Stress and Fescue Toxicosis on the Immune System and Other Physiological Parameters. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2010. [Google Scholar]
- Aristizábal, B.G.Á. Innate immune system. In Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Kostylina, G.; Simon, D.; Fey, M.F.; Yousefi, S.; Simon, H.U. Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A). Cell Death Differ. 2008, 15, 134–142. [Google Scholar] [CrossRef]
- Huntley, J.F. Mast cells and basophils: A review of their heterogeneity and function. J. Comp. Pathol. 1992, 107, 349–372. [Google Scholar] [CrossRef]
- Motomura, Y.; Morita, H.; Moro, K.; Nakae, S.; Artis, D.; Endo, T.A.; Kuroki, Y.; Ohara, O.; Koyasu, S.; Kubo, M. Basophil-Derived Interleukin-4 Controls the Function of Natural Helper Cells, a Member of ILC2s, in Lung Inflammation. Immunity 2014, 40, 758–771. [Google Scholar] [CrossRef]
- Oliver, J. Pathophysiologic response to endophyte toxins. In Neotyphodium in Cool-Season Grasse; Spiers, C.A.R.C.P.W.D.E., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 291–304. [Google Scholar]
- Piomelli, S. Chemical toxicity of red cells. Environ. Health Perspect. 1981, 39, 65–70. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Chirillo, R.; Zini, G.; Caenaro, G.; Tommasi, M.; Micciulli, G. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood 1995, 85, 818–823. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Turner, Z.B.; Sanders, Z.P.; Jones, D.P.; Suen, G.; Filipov, N.M. Response of Beef Cattle Fecal Microbiota to Grazing on Toxic Tall Fescue. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Nihsen, M.E.; Piper, E.L.; West, C.P.; Crawford, R.J., Jr.; Denard, T.M.; Johnson, Z.B.; Roberts, C.A.; Spiers, D.A.; Rosenkrans, C.F., Jr. Growth rate and physiology of steers grazing tall fescue inoculated with novel endophytes. J. Anim. Sci. 2004, 82, 878–883. [Google Scholar] [CrossRef]
- Melchior, E.A.; Smith, J.K.; Schneider, L.G.; Mulliniks, J.T.; Bates, G.E.; Flythe, M.D.; Klotz, J.L.; Ji, H.; Goodman, J.P.; Lee, A.R.; et al. Effects of endophyte-infected tall fescue seed and red clover isoflavones on rumen microbial populations and physiological parameters of beef cattle. Transl. Anim. Sci. 2019, 3, 315–328. [Google Scholar] [CrossRef]
- Schmidt, S.P.; Osborn, T.G. Effects of endophyte-infected tall fescue on animal performance. Agric. Ecosyst. Environ. 1993, 44, 233–262. [Google Scholar] [CrossRef]
- Greene, M.A.; Britt, J.L.; Bertrand, J.K.; Klotz, J.L.; Bridges, W., Jr.; Andrae, J.G.; Duckett, S.K. Feeding Tall Fescue Seed during Mid and Late Gestation Influences Subsequent Postnatal Growth, Puberty, and Carcass Quality of Offspring. Animals 2020, 10, 1859. [Google Scholar] [CrossRef]
- Flachowsky, G. Niacin in dairy and beef cattle nutrition. Arch. Tierernahr. 1993, 43, 195–213. [Google Scholar] [CrossRef]
- Thompson, F.N.; Stuedemann, J.A. Pathophysiology of fescue toxicosis. Agric. Ecosyst. Environ. 1993, 44, 263–281. [Google Scholar] [CrossRef]
- Rhodes, M.T.; Paterson, J.A.; Kerley, M.S.; Garner, H.E.; Laughlin, M.H. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. J. Anim. Sci. 1991, 69, 2033–2043. [Google Scholar] [CrossRef]
- Aiken, G.E.; Kirch, B.H.; Strickland, J.R.; Bush, L.P.; Looper, M.L.; Schrick, F.N. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers. J. Anim. Sci. 2007, 85, 2337–2345. [Google Scholar] [CrossRef]
- Aiken, G.E.; Strickland, J.R.; Looper, M.L.; Bush, L.P.; Schrick, F.N. Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations. J. Anim. Sci. 2009, 87, 2142–2150. [Google Scholar] [CrossRef]
- Al-Haidary, A.; Spiers, D.E.; Rottinghaus, G.E.; Garner, G.B.; Ellersieck, M.R. Thermoregulatory ability of beef heifers following intake of endophyte-infected tall fescue during controlled heat challenge. J. Anim. Sci. 2001, 79, 1780–1788. [Google Scholar] [CrossRef]
- Browning, R., Jr.; Leite-Browning, M.L. Effect of ergotamine and ergonovine on thermal regulation and cardiovascular function in cattle. J. Anim. Sci. 1997, 75, 176–181. [Google Scholar] [CrossRef]
- Browning, R., Jr. Effects of endophyte-infected tall fescue on indicators of thermal status and growth in Hereford and Senepol steers. J. Anim. Sci. 2004, 82, 634–643. [Google Scholar] [CrossRef][Green Version]
- Williams, A.F.; Boles, J.A.; Herrygers, M.R.; Berardinelli, J.G.; Meyers, M.C.; Thomson, J.M. Blood lactate and rectal temperature can predict exit velocity of beef feedlot steers. Transl. Anim. Sci. 2019, 3, 1530–1542. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Spain, J.N.; Spiers, D.E. Supplementation of nicotinic acid for lactating Holstein cows under heat stress conditions. J. Dairy Sci. 1997, 80, 1200–1206. [Google Scholar] [CrossRef]
- Zimbelman, R.B.; Baumgard, L.H.; Collier, R.J. Effects of encapsulated niacin on evaporative heat loss and body temperature in moderately heat-stressed lactating Holstein cows. J. Dairy Sci. 2010, 93, 2387–2394. [Google Scholar] [CrossRef]
- Aiken, G.E.; Klotz, J.L.; Looper, M.L.; Tabler, S.F.; Schrick, F.N. Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures. Prof. Anim. Sci. 2011, 27, 336–343. [Google Scholar] [CrossRef]
- Poole, R.K.; Poole, D.H. Impact of Ergot Alkaloids on Female Reproduction in Domestic Livestock Species. Toxins 2019, 11, 364. [Google Scholar] [CrossRef]
- Tudzynski, P.; Correia, T.; Keller, U. Biotechnology and genetics of ergot alkaloids. Appl. Microbiol. Biotechnol. 2001, 57, 593–605. [Google Scholar] [CrossRef]
- Littlejohn, M.D.; Henty, K.M.; Tiplady, K.; Johnson, T.; Harland, C.; Lopdell, T.; Sherlock, R.G.; Li, W.; Lukefahr, S.D.; Shanks, B.C.; et al. Functionally reciprocal mutations of the prolactin signalling pathway define hairy and slick cattle. Nat. Commun. 2014, 5, 5861. [Google Scholar] [CrossRef] [PubMed]
- Craven, A.J.; Nixon, A.J.; Ashby, M.G.; Ormandy, C.J.; Blazek, K.; Wilkins, R.J.; Pearson, A.J. Prolactin delays hair regrowth in mice. J. Endocrinol. 2006, 191, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.T.; Kojima, C.J.; Cooper, T.A.; Bastin, B.C.; Wojakiewicz, L.; Kallenbach, R.L.; Schrick, F.N.; Waller, J.C. A Single Nucleotide Polymorphism in the Dopamine Receptor D2 Gene May Be Informative for Resistance to Fescue Toxicosis in Angus-Based Cattle. Anim. Biotechnol. 2014, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hoveland, C.S.; Schmidt, S.P.; King, C.C.; Odom, J.W.; Clark, E.M.; McGuire, J.A.; Smith, L.A.; Grimes, H.W.; Holliman, J.L. Steer Performance and Association of Acremonium coenophialum Fungal Endophyte on Tall Fescue Pasture. Agron. J. 1983, 75, 821–824. [Google Scholar] [CrossRef]
- Coffey, K.P.; Coblentz, W.K.; Humphry, J.B.; Piper, E.L.; Rosenkrans, C.F.; Hubbell, D.S.; Harrison, K.F.; Denard, T.M.; Pohlman, F.W.; Hellwig, D.H.; et al. Growth Performance and Serum Prolactin Concentrations of Stocker Steers Implanted with Trenbolone Acetate While Grazing Endophyte-Infected Fescue in the Spring. Prof. Anim. Sci. 2001, 17, 166–173. [Google Scholar] [CrossRef]
- Gilbert, R.P.; Bailey, D.R. Hair coat characteristics and postweaning growth of Hereford and Angus cattle. J. Anim. Sci. 1991, 69, 498–506. [Google Scholar] [CrossRef]


| Ingredients 1 | % As-Fed | As-Fed, Kg | %DM | DM, Kg | %DM in Diet |
|---|---|---|---|---|---|
| Fescue Seeds 2 | 48.5 | 1.61 | 90.55 | 1.46 | 48.82 |
| Pellets 2,3 | 48.5 | 1.61 | 90.59 | 1.46 | 48.84 |
| Molasses 2 | 3 | 0.10 | 70 | 0.07 | 2.33 |
| Total | 100 | 3.32 | 251.14 | 2.98 | 100.00 |
| Ingredients 1 | DM | CP | NDF | ADF | TDN |
|---|---|---|---|---|---|
| Fescue Seeds 2 | 90.55 | 16.12 | 48.49 | 16.03 | 64.11 |
| Pellets 2,3 | 90.59 | 29.63 | 12.29 | 4.30 | 74.45 |
| Molasses 2 | 84.00 | 5.80 | - | 0.40 | 72.00 |
| Bermudagrass hay | 84.90 | 14.36 | 31.65 | 63.87 | 64.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro, G.F.; Rodriguez-Zas, S.L.; Southey, B.R.; Muntifering, R.B.; Rodning, S.P.; Pacheco, W.J.; Moisá, S.J. Complete Blood Count Analysis on Beef Cattle Exposed to Fescue Toxicity and Rumen-Protected Niacin Supplementation. Animals 2021, 11, 988. https://doi.org/10.3390/ani11040988
Alfaro GF, Rodriguez-Zas SL, Southey BR, Muntifering RB, Rodning SP, Pacheco WJ, Moisá SJ. Complete Blood Count Analysis on Beef Cattle Exposed to Fescue Toxicity and Rumen-Protected Niacin Supplementation. Animals. 2021; 11(4):988. https://doi.org/10.3390/ani11040988
Chicago/Turabian StyleAlfaro, Gaston F., Sandra L. Rodriguez-Zas, Bruce R. Southey, Russell B. Muntifering, Soren P. Rodning, Wilmer J. Pacheco, and Sonia J. Moisá. 2021. "Complete Blood Count Analysis on Beef Cattle Exposed to Fescue Toxicity and Rumen-Protected Niacin Supplementation" Animals 11, no. 4: 988. https://doi.org/10.3390/ani11040988
APA StyleAlfaro, G. F., Rodriguez-Zas, S. L., Southey, B. R., Muntifering, R. B., Rodning, S. P., Pacheco, W. J., & Moisá, S. J. (2021). Complete Blood Count Analysis on Beef Cattle Exposed to Fescue Toxicity and Rumen-Protected Niacin Supplementation. Animals, 11(4), 988. https://doi.org/10.3390/ani11040988






