Toll-Like Receptor Expression Profiles in Koala (Phascolarctos cinereus) Peripheral Blood Mononuclear Cells Infected with Multiple KoRV Subtypes
Abstract
Simple Summary
Abstract
1. Introduction
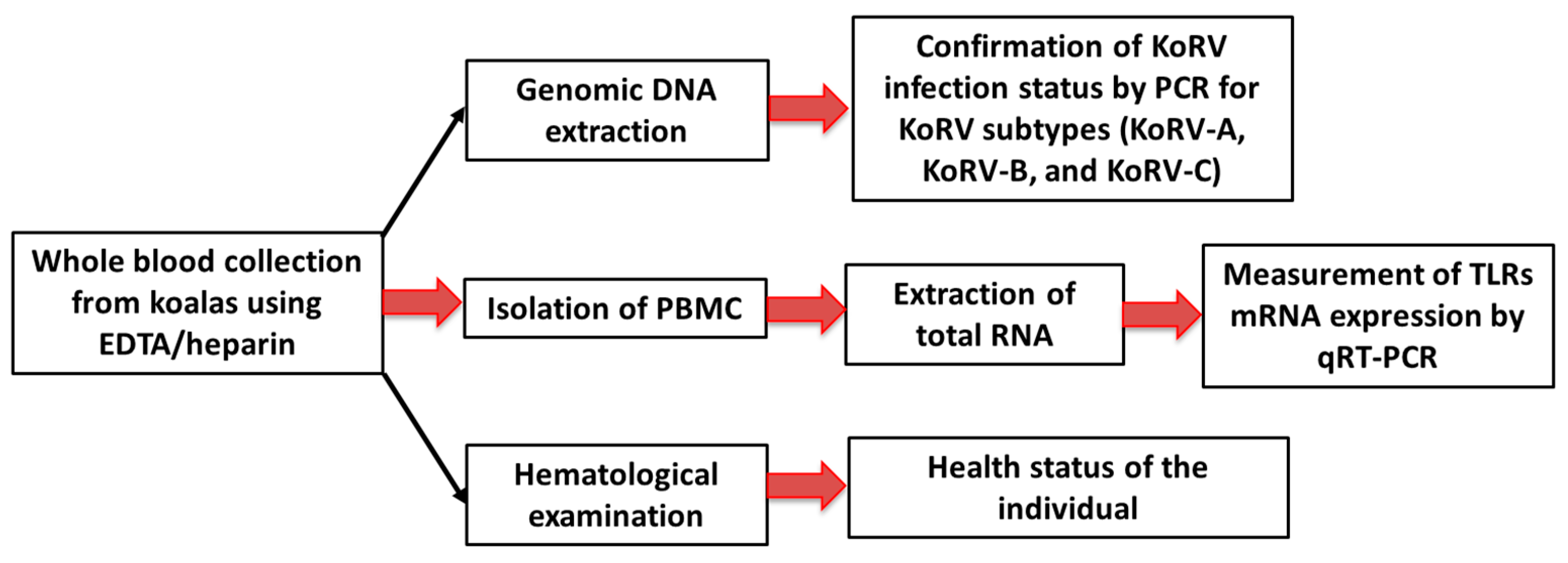
2. Materials and Methods
2.1. Sample Collection
2.2. Hematological Examination
2.3. Extraction of Genomic DNA from PBMCs
2.4. Extraction of RNA from PBMCs and Koala Tissues
2.5. Polymerase Chain Reaction (PCR)
2.6. Measurement of Gene Expression by qRT-PCR
2.7. Statistical Analysis
3. Results
3.1. Infection Status of Koalas
3.2. Hematological Examination
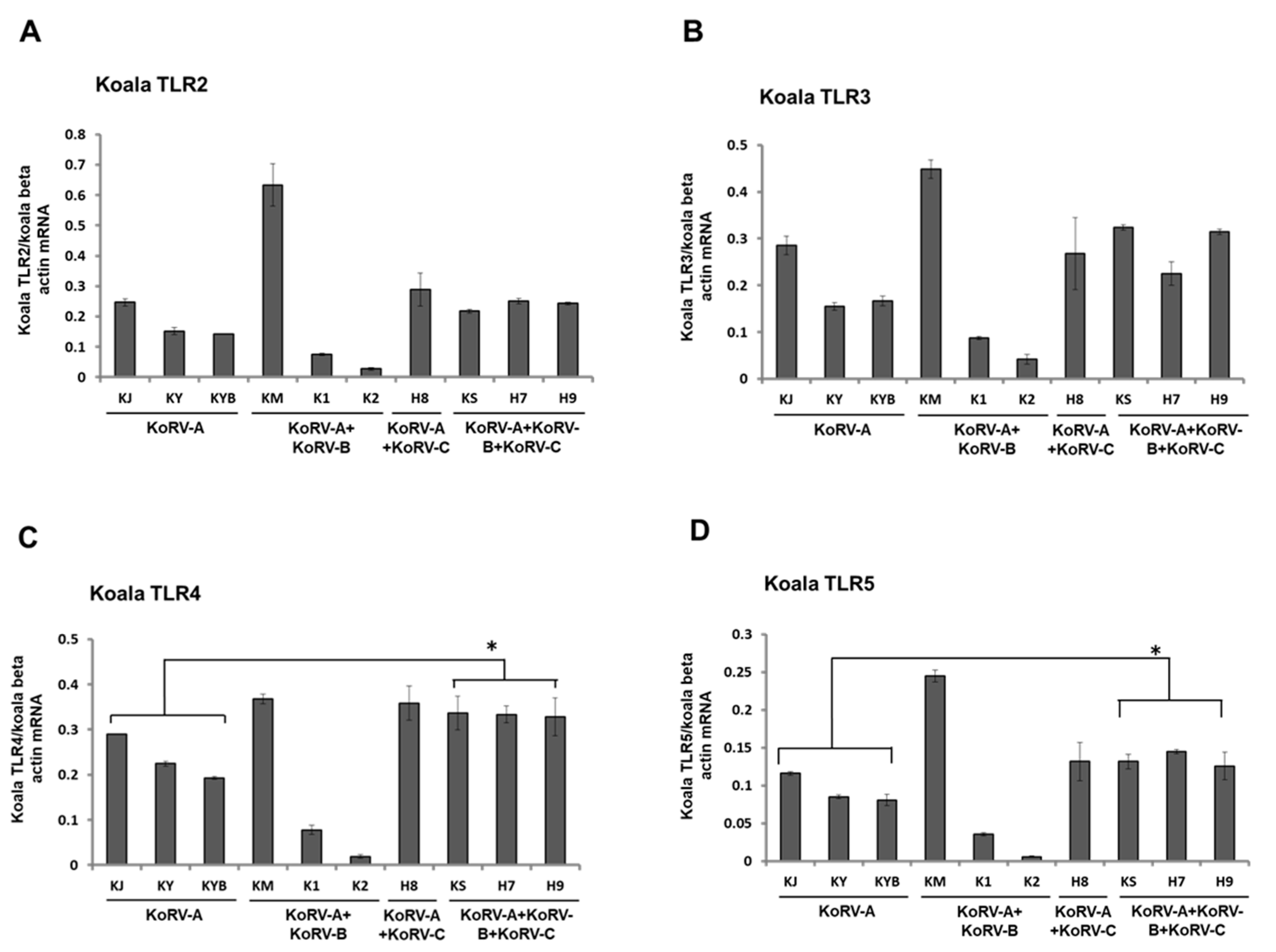
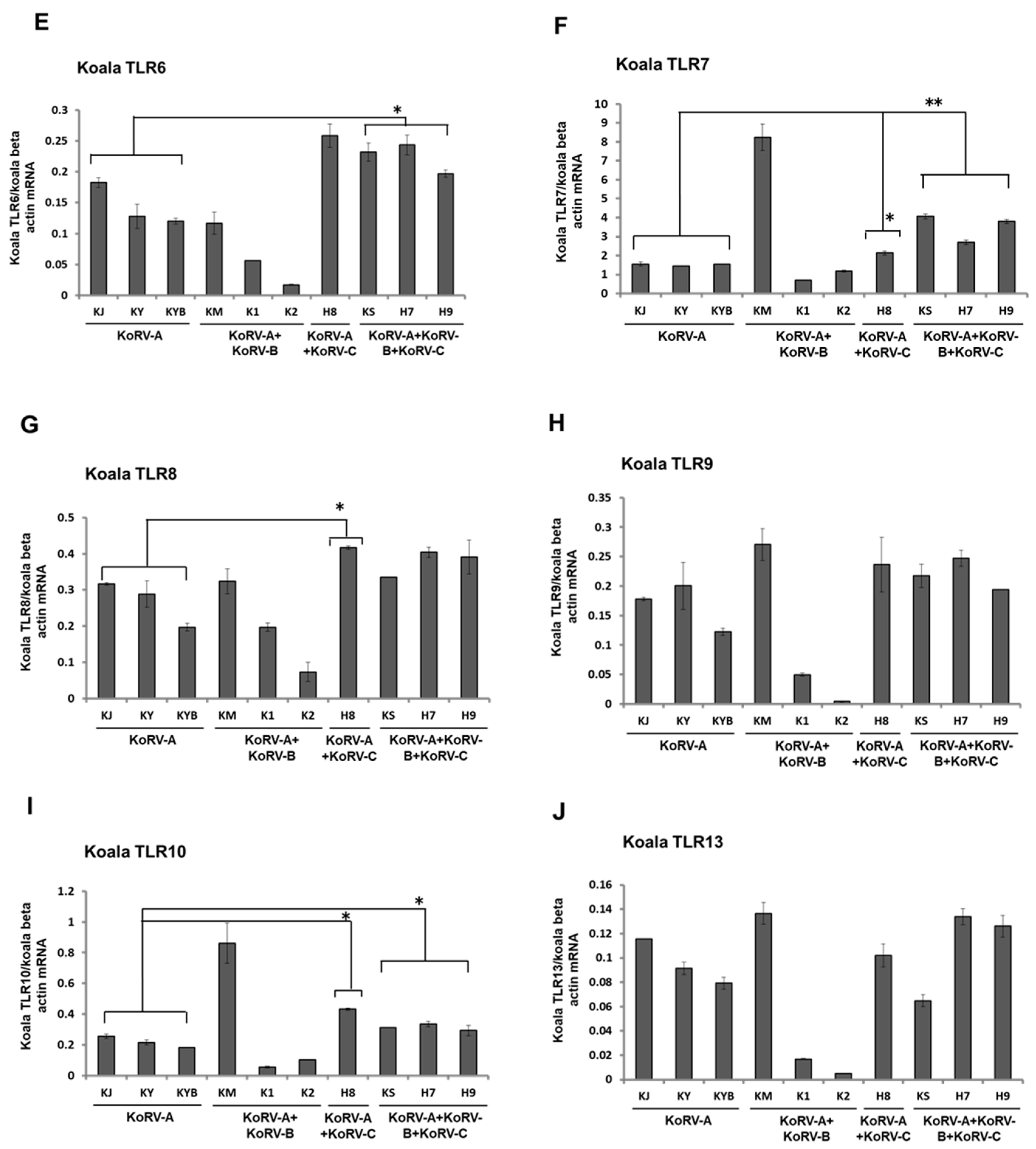
3.3. Expression Patterns of TLRs mRNA in Koala PBMCs
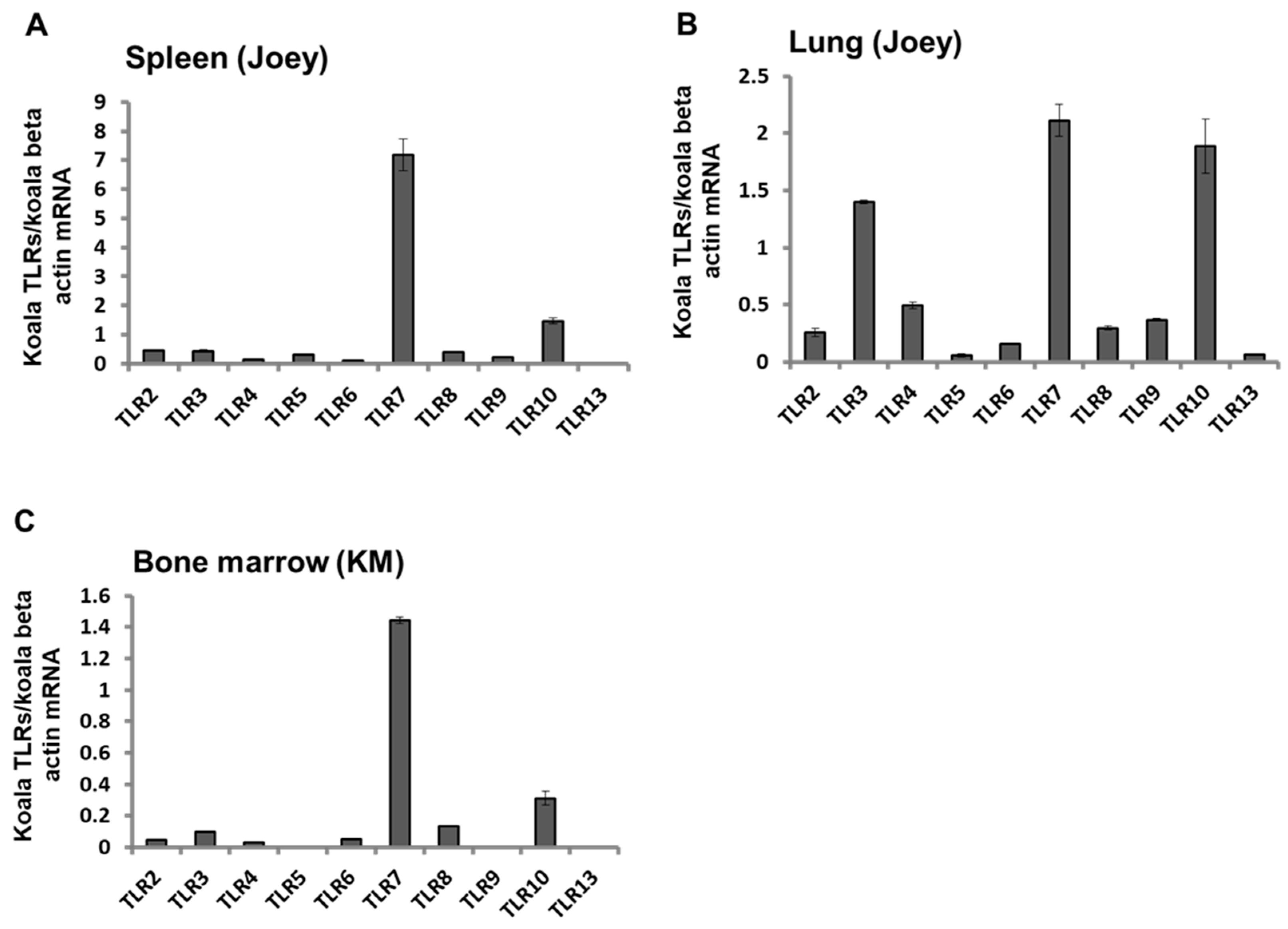
3.4. Expression Patterns of TLR mRNAs in Koala Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woinarski, J.; Burbidge, A.A. Phascolarctos Cinereus (Amended Version of 2016 Assessment). The IUCN Red List of Threatened Species 2020: E.T16892A166496779 (2020). IUCN. 2020. Available online: https://www.iucnredlist.org/species/16892/166496779 (accessed on 16 January 2021).
- Melzer, A.; Carrick, F.; Menkhorst, P.; Lunney, D.; John, B.S. Overview, critical assessment, and conservation implica-tions of koala distribution and abundance. Conserv. Biol. 2000, 14, 619–628. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Hashem, A.; Tsukiyama-Kohara, K. Koala retrovirus epidemiology, transmission mode, pathogenesis, and host immune response in koalas (Phascolarctos cinereus): A review. Arch. Virol. 2020, 165, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.; Canfield, P.; Hemsley, S.; Spencer, A. Lymphoid neoplasia in the koala. Aust. Veter. J. 1998, 76, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.; Meers, J.; Hanger, J.; Young, P. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. J. Gen. Virol. 2005, 86, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.; Meers, J.; Young, P. Biology and evolution of the endogenous koala retrovirus. Cell Mol. Life Sci. 2008, 65, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Stadler, C.K.; Gorman, K.; Jensen, N.; Kim, D.; Zheng, H.; Tang, S.; Switzer, W.M.; Pye, G.W.; Eiden, M.V. An exogenous retrovirus isolated from koalas with malignant neoplasias in a US zoo. Proc. Natl. Acad. Sci. USA 2013, 110, 11547–11552. [Google Scholar] [CrossRef] [PubMed]
- Denner, J.; Young, P.R. Koala retroviruses: Characterization and impact on the life of koalas. Retrovirology 2013, 10, 108. [Google Scholar] [CrossRef]
- Waugh, C.A.; Hanger, J.; Loader, J.; King, A.; Hobbs, M.; Johnson, R.; Timms, P. Infection with koala retrovirus subgroup B (KoRV-B), but not KoRV-A, is associated with chlamydial disease in free-ranging koalas (Phascolarctos cinereus). Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Fabijan, J.; Woolford, L.; Lathe, S.; Simmons, G.; Hemmatzadeh, F.; Trott, D.J.; Speight, K.N. Lymphoma, koala retrovi-rus infection and reproductive chlamydiosis in a koala (Phascolarctos cinereus). J. Comp. Pathol. 2017, 157, 188–192. [Google Scholar] [CrossRef]
- Quigley, B.L.; Phillips, S.; Olagoke, O.; Robbins, A.; Hanger, J.; Timms, P. Changes in Endogenous and Exogenous Koala Retrovirus Subtype Expression over Time Reflect Koala Health Outcomes. J. Virol. 2019, 93, 00849-19. [Google Scholar] [CrossRef]
- Hashem, A.; Kayesh, M.E.H.; Yamato, O.; Maetani, F.; Eiei, T.; Mochizuki, K.; Sakurai, H.; Ito, A.; Kannno, H.; Kasahara, T.; et al. Coinfection with koala retrovirus subtypes A and B and its impact on captive koalas in Japanese zoos. Arch. Virol. 2019, 164, 2735–2745. [Google Scholar] [CrossRef] [PubMed]
- Hanger, J.J.; Bromham, L.D.; McKee, J.J.; O’Brien, T.M.; Robinson, W.F. The Nucleotide Sequence of Koala (Phascolarctos cinereus) Retrovirus: A Novel Type C Endogenous Virus Related to Gibbon Ape Leukemia Virus. J. Virol. 2000, 74, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.E.; Meers, J.; Young, P.R. Retroviral invasion of the koala genome. Nat. Cell Biol. 2006, 442, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Denner, J. Transspecies Transmission of Gammaretroviruses and the Origin of the Gibbon Ape Leukaemia Virus (GaLV) and the Koala Retrovirus (KoRV). Viruses 2016, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Shojima, T.; Yoshikawa, R.; Hoshino, S.; Shimode, S.; Nakagawa, S.; Ohata, T.; Nakaoka, R.; Miyazawa, T. Identification of a Novel Subgroup of Koala Retrovirus from Koalas in Japanese Zoos. J. Virol. 2013, 87, 9943–9948. [Google Scholar] [CrossRef] [PubMed]
- Shimode, S.; Nakagawa, S.; Yoshikawa, R.; Shojima, T.; Miyazawa, T. Heterogeneity of koala retrovirus isolates. FEBS Lett. 2013, 588, 41–46. [Google Scholar] [CrossRef]
- Hashem, M.A.; Maetani, F.; Kayesh, M.E.H.; Eiei, T.; Mochizuki, K.; Ito, A.; Sakurai, H.; Asai, T.; Kohara, K.T. Transmis-sion of koala retrovirus from parent koalas to a joey in a Japanese zoo. J. Virol. 2020, 94, e00019-20. [Google Scholar] [CrossRef]
- Ishida, Y.; Zhao, K.; Greenwood, A.D.; Roca, A.L. Proliferation of Endogenous Retroviruses in the Early Stages of a Host Germ Line Invasion. Mol. Biol. Evol. 2015, 32, 109–120. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, Y.; Tang, S.; Pye, G.W.; Stadler, C.K.; Vogelnest, L.; Herrin, K.V.; Rideout, B.A.; Switzer, W.M. Koala retrovirus diversity, transmissibility, and disease associations. Retrovirology 2020, 17, 1–23. [Google Scholar] [CrossRef]
- Quigley, B.L.; Timms, P. Helping koalas battle disease—Recent advances in Chlamydia and koala retrovirus (KoRV) disease understanding and treatment in koalas. FEMS Microbiol. Rev. 2020, 44, 583–605. [Google Scholar] [CrossRef]
- Zuniga, E.I.; Macal, M.; Lewis, G.M.; Harker, J.A. Innate and adaptive immune regulation during chronic viral infec-tions. Annu. Rev. Virol. 2015, 2, 573–597. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.C.; Glusman, G.; Rowen, L.; Kaur, A.; Purcell, M.K.; Smith, K.D.; Hood, L.E.; Aderem, A. The evolution of ver-tebrate toll-like receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 9577–9582. [Google Scholar] [CrossRef]
- Cui, J.; Cheng, Y.; Belov, K. Diversity in the Toll-like receptor genes of the Tasmanian devil (Sarcophilus harrisii). Immunogenetics 2015, 67, 195–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, J.; Frankham, G.J.; Johnson, R.N.; Polkinghorne, A.; Timms, P.; O’Meally, D.; Cheng, Y.; Belov, K. SNP Marker Discovery in Koala TLR Genes. PLoS ONE 2015, 10, e0121068. [Google Scholar] [CrossRef] [PubMed]
- Young, G.R.; Eksmond, U.; Salcedo, R.; Alexopoulou, L.; Stoye, J.P.; Kassiotis, G. Resurrection of endogenous retrovi-ruses in antibody-deficient mice. Nature 2012, 491, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lübben, W.; Slomka, H.; Gebler, J.; Konert, M.; Cai, C.; Neubrandt, L.; Da Costa, O.P.; Paul, S.; Dehnert, S.; et al. Nucleic Acid-Sensing Toll-like Receptors Are Essential for the Control of Endogenous Retrovirus Viremia and ERV-Induced Tumors. Immunity 2012, 37, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Denner, J.; Norley, S.; Kurth, R. The immunosuppressive peptide of HIV-1: Functional domains and immune response in AIDS patients. AIDS 1994, 8, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; McCallister, C.; Nikolaidis, N.; Tsangaras, K.; Helgen, K.M.; Greenwood, A.D.; Roca, A.L. Sequence variation of koala retrovirus transmembrane protein p15E among koalas from different geographic regions. Virology 2015, 475, 28–36. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine Adjuvants: Mode of Action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, U.; Keller, M.; Möller, A.; Timms, P.; Denner, J. Lack of antiviral antibody response in koalas infected with koala retroviruses (KoRV). Virus Res. 2015, 198, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Hashem, A.; Maetani, F.; Eiei, T.; Mochizuki, K.; Ochiai, S.; Ito, A.; Ito, N.; Sakurai, H.; Asai, T.; et al. CD4, CD8b, and Cytokines Expression Profiles in Peripheral Blood Mononuclear Cells Infected with Different Subtypes of KoRV from Koalas (Phascolarctos cinereus) in a Japanese Zoo. Viruses 2020, 12, 1415. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Shojima, T.; Yoshikawa, R.; Ohata, T. Isolation of Koala Retroviruses from Koalas in Japan. J. Veter Med. Sci. 2011, 73, 65–70. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Yamato, O.; Rahman, M.M.; Hashem, A.; Maetani, F.; Eiei, T.; Mochizuki, K.; Sakurai, H.; Tsukiyama-Kohara, K. Molecular dynamics of koala retrovirus infection in captive koalas in Japan. Arch. Virol. 2019, 164, 757–765. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 2009, 21, 185–193. [Google Scholar] [CrossRef]
- Waugh, C.; Gillett, A.; Polkinghorne, A.; Timms, P. Serum Antibody Response to Koala Retrovirus Antigens Varies in Free-Ranging Koalas ( Phascolarctos cinereus ) in Australia: Implications for Vaccine Design. J. Wildl. Dis. 2016, 52, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Olagoke, O.; Miller, D.; Hemmatzadeh, F.; Stephenson, T.; Fabijan, J.; Hutt, P.; Finch, S.; Speight, N.; Timms, P. Induc-tion of neutralizing antibody response against koala retrovirus (KoRV) and reduction in viral load in koalas following vaccination with recombinant KoRV envelope protein. NPJ Vaccines 2018, 3, 30. [Google Scholar] [CrossRef]
- Olagoke, O.; Quigley, B.L.; Eiden, M.V.; Timms, P. Antibody response against koala retrovirus (KoRV) in koalas harboring KoRV-A in the presence or absence of KoRV-B. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Olagoke, O.; Quigley, B.L.; Hemmatzadeh, F.; Tzipori, G.; Timms, P. Therapeutic vaccination of koalas harbouring endogenous koala retrovirus (KoRV) improves antibody responses and reduces circulating viral load. NPJ Vaccines 2020, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olagoke, O.; Quigley, B.L.; Timms, P. Koalas vaccinated against Koala retrovirus respond by producing increased levels of interferon-gamma. Virol. J. 2020, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Bryant, C.E.; Doyle, S.L. Therapeutic Targeting of Toll-Like Receptors for Infectious and Inflammatory Diseases and Cancer. Pharmacol. Rev. 2009, 61, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.C.; Bildstein, L.S.; Gach, J.S. Recent Insights into the HIV/AIDS Pandemic. Microb. Cell 2016, 3, 450–474. [Google Scholar] [CrossRef] [PubMed]
- Herniou, E.; Martin, J.; Miller, K.; Cook, J.; Wilkinson, M.; Tristem, M. Retroviral diversity and distribution in verte-brates. J. Virol. 1998, 72, 5955–5966. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P. The Role of Toll-like Receptors in Retroviral Infection. Microorganisms 2020, 8, 1787. [Google Scholar] [CrossRef]
- Salaun, B.; Romero, P.; Lebecque, S. Toll-like receptors’ two-edged sword: When immunity meets apoptosis. Eur. J. Immunol. 2007, 37, 3311–3318. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Unkeless, J.C.; Feng, Z.H.; Xiong, H. TLR signaling by tumor and immune cells: A double-edged sword. Oncogene 2008, 27, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Manavalan, B.; Yoo, T.H.; Kim, S.G.; Choi, S. Roles of toll-like receptors in Cancer: A double-edged sword for defense and offense. Arch. Pharmacal Res. 2012, 35, 1297–1316. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) | Product Size | Reference or GenBank Accession Numbers |
|---|---|---|---|---|
| Pol (KoRV) | CCTTGGACCACCAAGAGACTTTTGA | TCAAATCTTGGACTGGCCGA | 523 | [14] |
| Env (KoRV-A) | TCCTGGGAACTGGAAAAGAC | GGGTTCCCCAAGTGATCTG | 321 | [9] |
| Env (KoRV-B) | TCCTGGGAACTGGAAAAGAC | GGCGCAGACTGTTGAGATTC | 271 | [7] |
| Env (KoRV-C) | TCCTGGGAACTGGAAAAGAC | AAGGCTGGTCCCGCGAAGGT | 290 | [18] |
| Beta actin (Koala) | AGATCATTGCCCCACCT | TGGAAGGCCCAGATTC | 123 | [14] |
| TLR2 (Koala) | CCATTCCAAGTGAGGGGCAA | ACTCCAGTCAGCAAGGCAAG | 122 | KP792545.1 |
| TLR3 (Koala) | GGAATGGCTTGGGTTGGAGT | AGCCACTGGAAAGAAAAATCATCT | 162 | KP792547.1 |
| TLR4 (Koala) | TCCACAAGAGCCGGAAAGTC | GAGTTCCACCTGTTGCCGTA | 176 | KP792551.1 |
| TLR5 (Koala) | CCTTAGCCTGGATGGCAACA | GGTAGGGTCAGGGGATAGCA | 109 | KP792550.1 |
| TLR6 (Koala) | TTCAGTTTCCCGCCCAACTA | ATGTGGCCATCCACTTACCA | 157 | KP792540.1 |
| TLR7 (Koala) | TTGCCTTGTAACGTCACCCA | GTGAGGGTCAGGTTGGTTGT | 119 | KP792558.1 |
| TLR8 (Koala) | CCTCTTCGTTTACCACCCTCC | CTTCAAAGGCCCCGTCATCT | 178 | KP792567.1 |
| TLR9 (Koala) | ATCTTCAGCCACTTCCGCTC | AGGCTCTCTCCAGCCCTAAA | 133 | KP792555.1 |
| TLR10 (Koala) | GCCCTAAAGGTGGAGCATGT | TATATGTGGCATCCCCGCAC | 123 | KP792539.1 |
| TLR13 (Koala) | AGCCTACTGGTGGCTATGGA | TGGCCAGGTACAGGGACTTA | 172 | KP334161.1 |
| Koala | Age (at the Time of Sampling) | Sex | KoRV Subtypes | ||
|---|---|---|---|---|---|
| KoRV-A | KoRV-B | KoRV-C | |||
| KJ | 6 y | Female | Positive | Negative | Negative |
| KY | 2 y 4 m | Female | Positive | Negative | Negative |
| KYB | 1 y | Female | Positive | Negative | Negative |
| KM | 12 y | Female | Positive | Positive | Negative |
| K1 | 4 y 5 m | Male | Positive | Positive | Negative |
| K2 | 4 y 5 m | Female | Positive | Positive | Negative |
| H8 | 5 y 5 m | Female | Positive | Negative | Positive |
| KS | 1 y 6 m | Male | Positive | Positive | Positive |
| H7 | 4 y 11 m | Female | Positive | Positive | Positive |
| H9 | 10 y 3 m | Female | Positive | Positive | Positive |
| Joey | 6 m | Male | Positive | Negative | Positive |
| Koala | Blood Parameters | Clinical Determination | ||||||
|---|---|---|---|---|---|---|---|---|
| WBC (102/μL) | RBC (104/μL) | HGB (g/dL) | PCV (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | ||
| KJ | 125 | 359 | 13.2 | 37.8 | 105.3 | 36.8 | 34.9 | Healthy |
| KY | 62 | 342 | 12.4 | 37.6 | 109.9 | 36.3 | 33 | Healthy |
| KYB | 52 | 308 | 12 | 35.9 | 116.6 | 39 | 33.4 | Healthy |
| KM | 2275 | 241 | 8.8 | 25.4 | 105.4 | 36.5 | 34.6 | Leukemic (subsequently died) |
| K1 | 1007.5 | 149 | 6 | 17 | 114.1 | 40.3 | 35.3 | Leukemic |
| K2 | 77 | 256 | 7.9 | 38 | 148.4 | 30.9 | 20.8 | Healthy |
| H8 | 82 | 342 | 13 | 38 | 111.1 | 38 | 34.2 | Healthy |
| KS | 4000 | 23.4 | 8.8 | 26 | 111.1 | 37.6 | 33.8 | Leukemic |
| H7 | 97 | 330 | 12.2 | 35.3 | 107 | 37 | 34.6 | Healthy |
| H9 | 52 | 319 | 11 | 33.8 | 106 | 34.5 | 32.5 | Healthy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayesh, M.E.H.; Hashem, M.A.; Tsukiyama-Kohara, K. Toll-Like Receptor Expression Profiles in Koala (Phascolarctos cinereus) Peripheral Blood Mononuclear Cells Infected with Multiple KoRV Subtypes. Animals 2021, 11, 983. https://doi.org/10.3390/ani11040983
Kayesh MEH, Hashem MA, Tsukiyama-Kohara K. Toll-Like Receptor Expression Profiles in Koala (Phascolarctos cinereus) Peripheral Blood Mononuclear Cells Infected with Multiple KoRV Subtypes. Animals. 2021; 11(4):983. https://doi.org/10.3390/ani11040983
Chicago/Turabian StyleKayesh, Mohammad Enamul Hoque, Md Abul Hashem, and Kyoko Tsukiyama-Kohara. 2021. "Toll-Like Receptor Expression Profiles in Koala (Phascolarctos cinereus) Peripheral Blood Mononuclear Cells Infected with Multiple KoRV Subtypes" Animals 11, no. 4: 983. https://doi.org/10.3390/ani11040983
APA StyleKayesh, M. E. H., Hashem, M. A., & Tsukiyama-Kohara, K. (2021). Toll-Like Receptor Expression Profiles in Koala (Phascolarctos cinereus) Peripheral Blood Mononuclear Cells Infected with Multiple KoRV Subtypes. Animals, 11(4), 983. https://doi.org/10.3390/ani11040983






