Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
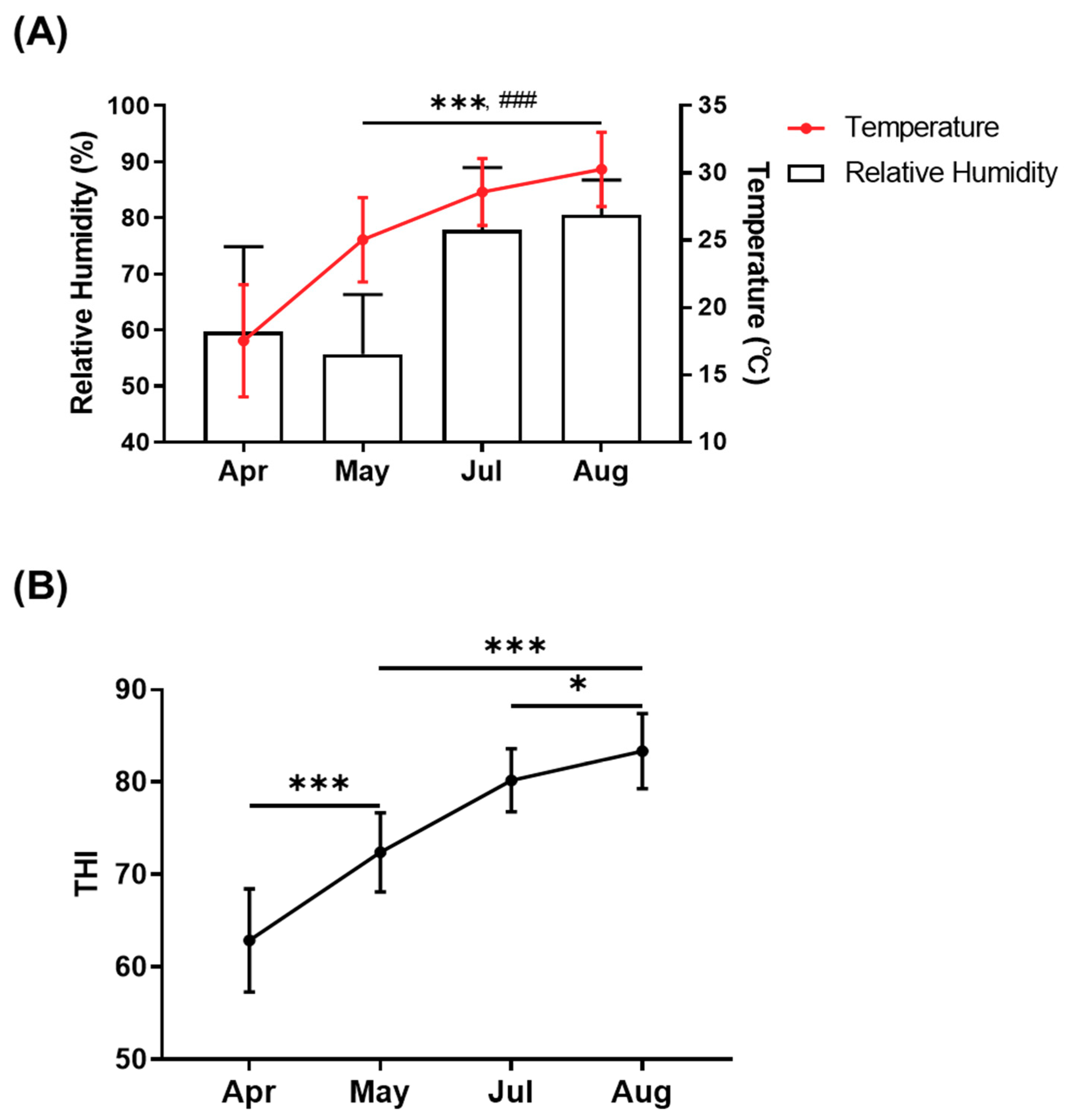
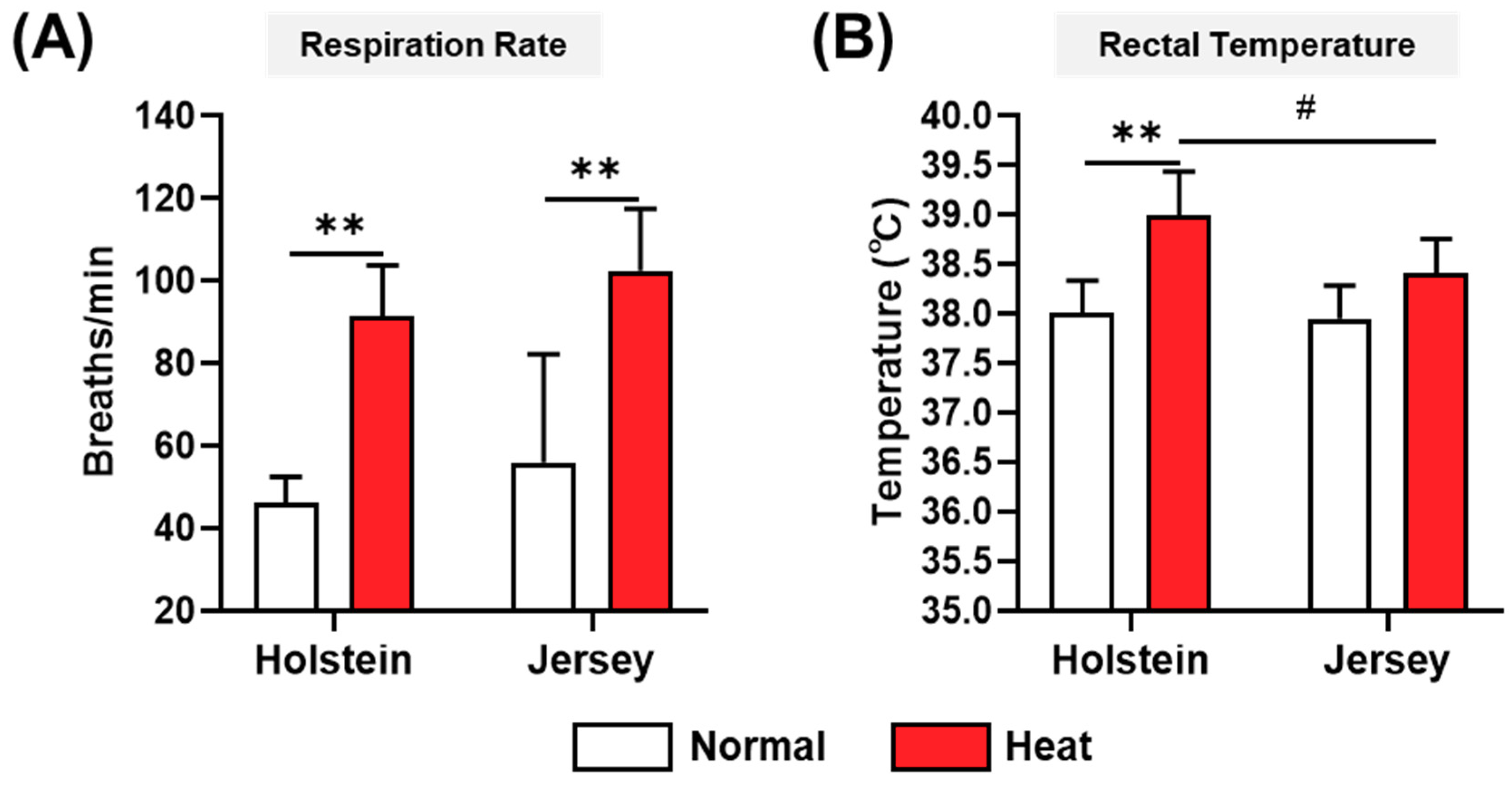
2.2. Measurement of Temperature–Humidity Index, Respiration, and Rectal Temperature
2.3. Blood Collection and Sample Preparation
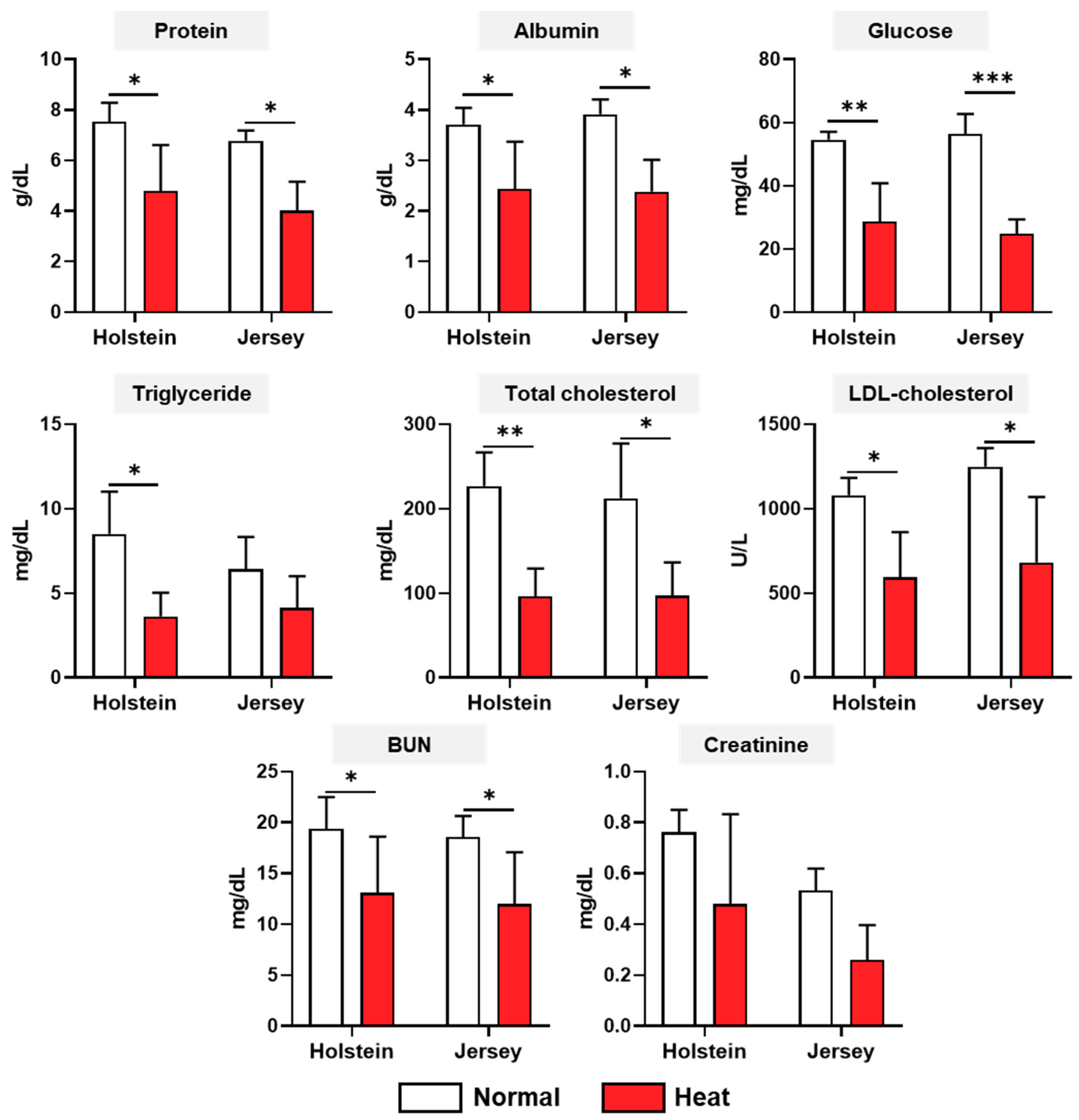
2.4. Biochemistry Analysis
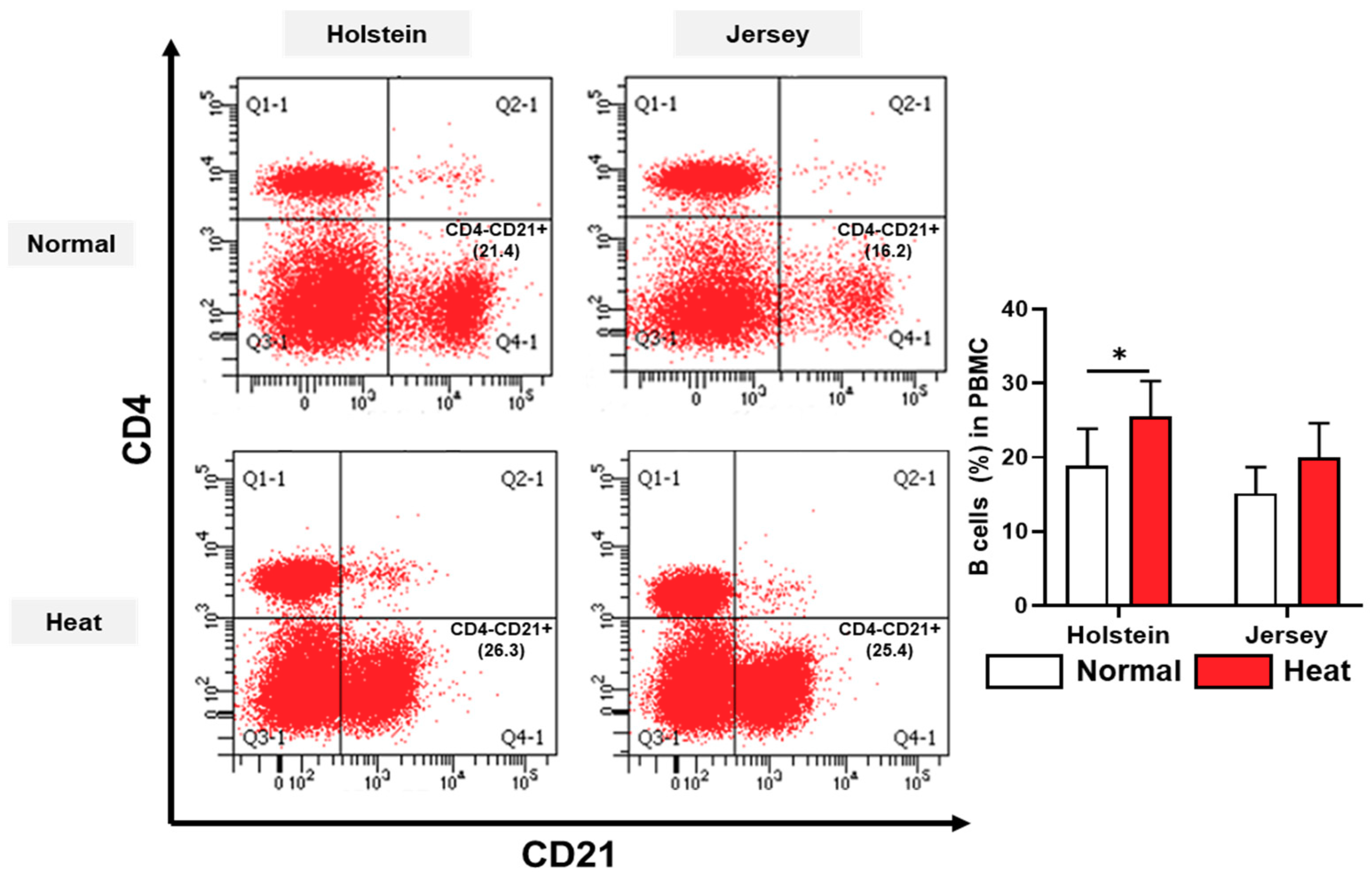
2.5. Flow Cytometry Analysis of Dairy Cow PBMCs
2.6. Statistical Analysis
3. Results
3.1. Changes in Environmental Conditions Based on Temperature–Humidity Index
3.2. Measurement of Respiration Rate and Rectal Temperature of Dairy Cows
3.3. Changes in the Biochemistry Analysis of Two Breeds of Dairy Cows under Different Enviromental Conditions
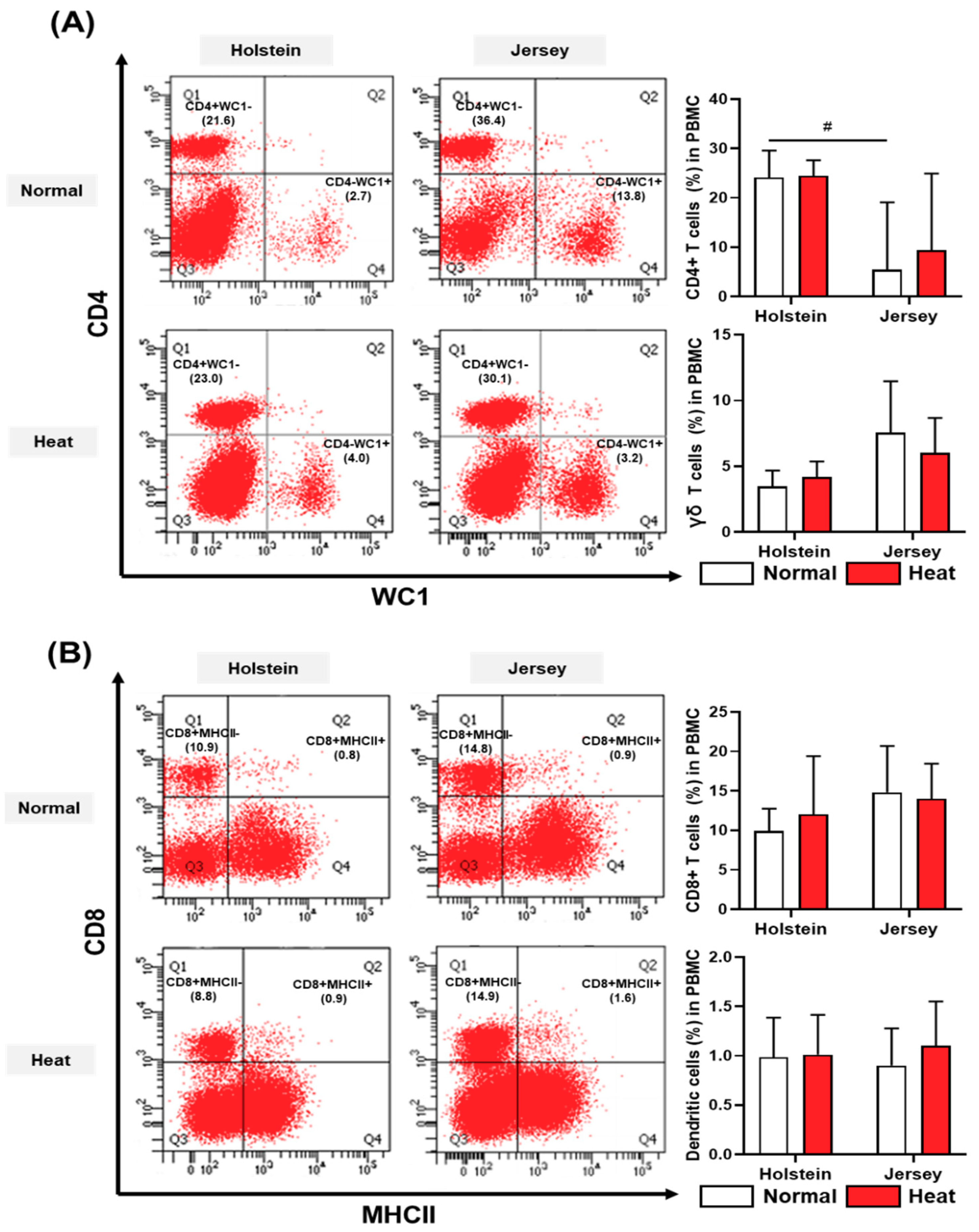
3.4. Alteration in the Population of Peripheral Blood Mononuclear Cells in Dairy Cows by Heat Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fuquay, J.W. Heat stress as it affects animal production. J. Anim. Sci. 1981, 52, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M. From molecular and cellular to integrative heat defense during exposure to chronic heat. Comp. Biochem. Physiol. Part A Mol. Intergr. Physiol. 2002, 131, 475–483. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Garner, J.B.; Douglas, M.; Williams, S.R.O.; Wales, W.J.; Marett, L.C.; DiGiacomo, K.; Leury, B.J.; Hayes, B.J. Responses of dairy cows to short-term heat stress in controlled-climate chambers. Anim. Prod. Sci. 2017, 57, 1233–1241. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Ronchi, B.; Kuzminsky, G.; Nardone, A. Lymphocyte functions in dairy cows in hot environment. Int. J. Biometeoral 2005, 50, 105–110. [Google Scholar] [CrossRef]
- Strong, R.A.; Silva, E.B.; Cheng, H.W.; Eicher, S.D. Acute brief heat stress in late gestation alters neonatal calf innate immune functions. J. Dairy Sci. 2015, 98, 7771–7783. [Google Scholar] [CrossRef]
- Bhan, C.; Singh, S.V.; Hooda, O.K.; Upadhyay, R.C.; Beenam, V.M.; Mangesh, V. Influence of temperature variability on physiological, hematological and biochemical profile of growing and adult sahiwal cattle. J. Environ. Dev. 2012, 7, 986–994. [Google Scholar]
- Seath, D.M.; Miller, G.D. Heat tolerance comparisons between Jersey and Holstein cows. J. Anim. Sci. 1947, 6, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.L.; Shrode, R.R.; Rupel, I.W.; Leighton, R.E. A study of solar radiation as related to physiological and production responses of lactating Holstein and Jersey cows. J. Dairy Sci. 1960, 43, 1255–1262. [Google Scholar] [CrossRef]
- Collier, R.J.; Eley, R.M.; Sharma, A.K.; Pereira, R.M.; Buffington, D.E. Shade management in subtropical environment for milk yield and composition in Holstein and Jersey cows. J. Dairy Sci. 1981, 64, 844–849. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Smith, D.L.; Smith, T.; Rude, B.J.; Ward, S.H. Comparison of the effects of heat stress on milk and component yields and somatic cell score in Holstein and Jersey cows. J. Dairy Sci. 2013, 96, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.; Green, M. Use and interpretation of somatic cell count data in dairy cows. In Practice 2005, 27, 310–315. [Google Scholar] [CrossRef]
- Korea Research Council. Korean Feeding Standard for Dairy cattle, 3rd ed.; The National Institute of Animal Science, Rural Development Administration Press: Wanju, Jeolabuk-do, Korea, 2017. [Google Scholar]
- Council, N.R. A Guide to Environmental Research on Animals; National Academies: Washington, DC, USA, 1971. [Google Scholar]
- Armstrong, D. Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.H.; Kim, S.B.; Son, J.K.; Lee, J.H.; Joo, S.S.; Gu, B.-H.; Kim, E.T. Differential dynamics of the ruminal microbiome of Jersey Cows in a heat stress environment. Animals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Ouellet, V.; Cabrera, V.E.; Fadul-Pacheco, L.; Charbonneau, É. The relationship between the number of consecutive days with heat stress and milk production of Holstein dairy cows raised in a humid continental climate. J. Dairy Sci. 2019, 102, 8537–8545. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Monteiro, A.P.A. Effects of late-gestation heat stress on immunity and performance of calves. J. Dairy Sci. 2016, 99, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Peng, D.; Li, G.; Chen, J.; Gu, X. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M.A. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Thom, E.C. The discomfort index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Kibler, H.H. Thermal effects of various temperature-humidity combinations on Holstein cattle as measured by eight physiological responses. Res. Bull. Mo. Agric. Exp. Stn. 1964, 862, 1–42. [Google Scholar]
- Yousef, M.K. Stress Physiology in Livestock; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Dairy Sci. 2006, 84, 712–719. [Google Scholar]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier Health Sciences: London, UK, 2006. [Google Scholar]
- Wenz, J.R.; Moore, D.A.; Kasimanickam, R. Factors associated with the rectal temperature of Holstein dairy cows during the first 10 days in milk. J. Dairy Sci. 2011, 94, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Igono, M.O.; Steevens, B.J.; Shanklin, M.D.; Johnson, H.D. Spray cooling effects on milk production, milk, and rectal temperatures of cows during a moderate temperate summer season. J. Dairy Sci. 1985, 68, 979–985. [Google Scholar] [CrossRef]
- Kabuga, J.D. The influence of thermal conditions on rectal temperature, respiration rate and pulse rate of lactating Holstein-Friesian cows in the humid tropics. Int. J. Biometeorol. 1992, 36, 146–150. [Google Scholar] [CrossRef]
- Debnath, T.; Bera, S.; Deb, S.; Pal, P.; Debbarma, N.; Haldar, A. Application of radio frequency based digital thermometer for real-time monitoring of dairy cattle rectal temperature. Vet. World 2017, 10, 1052. [Google Scholar] [CrossRef][Green Version]
- West, J.W.; Mullinix, B.G.; Bernard, J.K. Effects of hot, humid weather on milk temperature, dry matter intake, and milk yield of lactating dairy cows. J. Dairy Sci. 2003, 86, 232–242. [Google Scholar] [CrossRef]
- Bianca, W. Section A. Physiology. Cattle in a hot environment. J. Dairy Res. 1965, 32, 291–345. [Google Scholar] [CrossRef]
- Abeni, F.; Calamari, L.; Stefanini, L. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int. J. Biometeorol. 2007, 52, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Li, D.; Tong, X.; Nan, X.; Ding, D.; Xu, B.; Wang, G. Nutritional strategies for alleviating the detrimental effects of heat stress in dairy cows: A review. Int. J. Biometeorol. 2019, 63, 1283–1302. [Google Scholar] [CrossRef]
- Garcia-Herreros, M.; Aparicio, I.M.; Rath, D.; Fair, T.; Lonergan, P. Differential glycolytic and glycogenogenic transduction pathways in male and female bovine embryos produced in vitro. Reprod. Fertil. Dev. 2012, 24, 344–352. [Google Scholar] [CrossRef]
- Thiangtum, W.; Yawongsa, A.; Schonewille, J.T.; Rukkwamsuk, T.; Yuangklang, C.; Verstegen, M.W.; Hendriks, W.H. An attempt to define the sodium requirement of lactating dairy cows in a tropical environment. J. Sci. Food Agric. 2011, 91, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Mahen, P.J.; Williams, H.J.; Smith, R.F.; Grove-White, D. Effect of blood ionised calcium concentration at calving on fertility outcomes in dairy cattle. Vet. Rec. 2018, 183, 263. [Google Scholar] [CrossRef]
- Sejersen, H.; Sørensen, M.T.; Larsen, T.; Bendixen, E.; Ingvartsen, K.L. Liver protein expression in dairy cows with high liver triglycerides in early lactation. J. Dairy Sci. 2012, 95, 2409–2421. [Google Scholar] [CrossRef]
- Katoh, N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002, 64, 293–307. [Google Scholar] [CrossRef]
- Emery, R.S.; Liesman, J.S.; Herdt, T.H. Metabolism of long chain fatty acids by ruminant liver. J. Nutr. 1992, 122 (Suppl. 3), 832–837. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, L.; Huang, D.; Peng, Z.; Zhao, C.; Zhang, Y.; Zhu, Y.; Wang, Z.; Li, X.; Liu, G. Elevated apoptosis in the liver of dairy cows with ketosis. Cell. Physiol. Biochem. 2017, 43, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Srikandakumar, A.; Johnson, E.H. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu cows. Trop. Anim. Health Prod. 2004, 36, 685–692. [Google Scholar] [CrossRef]
- Garcia, A.B.; Angeli, N.; Machado, L.; de Cardoso, F.C.; Gonzalez, F. Relationships between heat stress and metabolic and milk parameters in dairy cows in southern Brazil. Trop. Anim. Health Prod. 2015, 47, 889–894. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef]
- Naik, B.R.; Kumar, A.V.N.S.; Ravi, A.; Bramhaiah, K.V.; Chakravarthi, V.P. Effect of seasons on physiological and hematological values in Punganur cattle. Int. J. Pharma Bio Sci. 2013, 4, 40–49. [Google Scholar]
- Elvinger, F.; Hansen, P.J.; Natzke, R.P. Modulation of function of bovine polymorphonuclear leukocytes and lymphocytes by high temperature in vitro and in vivo. Am. J. Vet. Res. 1991, 52, 1692–1698. [Google Scholar] [PubMed]
- Kamwanja, L.A.; Chase, C.C., Jr.; Gutierrez, J.A.; Guerriero, V., Jr.; Olson, T.A.; Hammond, A.C.; Hansen, P.J. Responses of bovine lymphocytes to heat shock as modified by breed and antioxidant status. J. Anim. Sci. 1994, 72, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Basiricò, L.; Morera, P.; Nardone, A. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J. Dairy Sci. 2006, 89, 4606–4612. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T cells: Differentiation and functions. Dev. Comp. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Oukka, M.; Kuchroo, V.K. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007, 8, 345–350. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int. Rev. Immunol. 2005, 24, 211–226. [Google Scholar] [CrossRef]
- Ceciliani, F.; Morales, G.Á.; De Matteis, G.; Grandoni, F.; Ferreira, R.F.; Roccabianca, P.; Lecchi, C. Methods in isolation and characterization of bovine monocytes and macrophages. Methods 2020, 186, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Essentials of Veterinary Hematology, 1st ed.; Lea & Febiger: Philadelphia, PA, USA, 1993. [Google Scholar]
- Logan, L.; Suarez-Trujillo, A.; Plaut, K.; Casey, T. Analysis of the relationship of blood metabolites with white blood cells in periparturient dairy cattle. J. Stud. Res. 2019, 8, 24–29. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, 1–50. [Google Scholar] [CrossRef]
- Srikanth, K.; Kwon, A.; Lee, E.; Chung, H. Characterization of genes and pathways that respond to heat stress in Holstein calves through transcriptome analysis. Cell Stress Chaperones 2017, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaisi, M.; Mayorga, E.J.; Horst, E.A.; Kvidera, S.K.; McCarthy, C.S.; Abeyta, M.A.; Goetz, B.M.; Ramiere-Ramirez, H.A.; Timms, L.L.; Baumgard, L.H. Validating a heat stress model: The effects of an electric heat blanket and nutritional plane on lactating dairy cows. J. Dairy Sci. 2020, 103, 5550–5560. [Google Scholar] [CrossRef]



| Item | Amount |
|---|---|
| Ingredients composition, % of DM | - |
| Concentrate | 15.3 |
| Soybean meal | 2.4 |
| Corn silage | 47.2 |
| Alfalfa hay | 7.1 |
| Tall fescue | 9.4 |
| Timothy | 5.9 |
| Energy booster † | 7.1 |
| Cash Gold † | 4.5 |
| Lyzin-Plus ‡ | 0.2 |
| Limestone | 0.2 |
| Zin Care † | 0.1 |
| Supex-F † | 0.5 |
| Trace minerals § | 0.05 |
| Vitamin premix¶ | 0.05 |
| Chemical composition | - |
| Dry matter (DM), % | 53.2 |
| Crude protein, % of DM | 16.6 |
| Neutral detergent fiber, % of DM | 37.0 |
| Acid detergent fiber, % of DM | 25.6 |
| Calcium, % of DM | 0.4 |
| Phosphorus, % of DM | 0.15 |
| Total digestible nutrient, % | 69.6 |
| Digestible energy, Mcal/kg | 3.069 |
| Metabolizable energy, Mcal/kg | 2.650 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.S.; Lee, S.J.; Park, D.S.; Kim, D.H.; Gu, B.-H.; Park, Y.J.; Rim, C.Y.; Kim, M.; Kim, E.T. Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress. Animals 2021, 11, 974. https://doi.org/10.3390/ani11040974
Joo SS, Lee SJ, Park DS, Kim DH, Gu B-H, Park YJ, Rim CY, Kim M, Kim ET. Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress. Animals. 2021; 11(4):974. https://doi.org/10.3390/ani11040974
Chicago/Turabian StyleJoo, Sang Seok, Sang Jin Lee, Da Som Park, Dong Hyeon Kim, Bon-Hee Gu, Yei Ju Park, Chae Yun Rim, Myunghoo Kim, and Eun Tae Kim. 2021. "Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress" Animals 11, no. 4: 974. https://doi.org/10.3390/ani11040974
APA StyleJoo, S. S., Lee, S. J., Park, D. S., Kim, D. H., Gu, B.-H., Park, Y. J., Rim, C. Y., Kim, M., & Kim, E. T. (2021). Changes in Blood Metabolites and Immune Cells in Holstein and Jersey Dairy Cows by Heat Stress. Animals, 11(4), 974. https://doi.org/10.3390/ani11040974






