Puma (Puma concolor) in the Neighborhood? Records Near Human Settlements and Insights into Human-Carnivore Coexistence in Central Chile
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
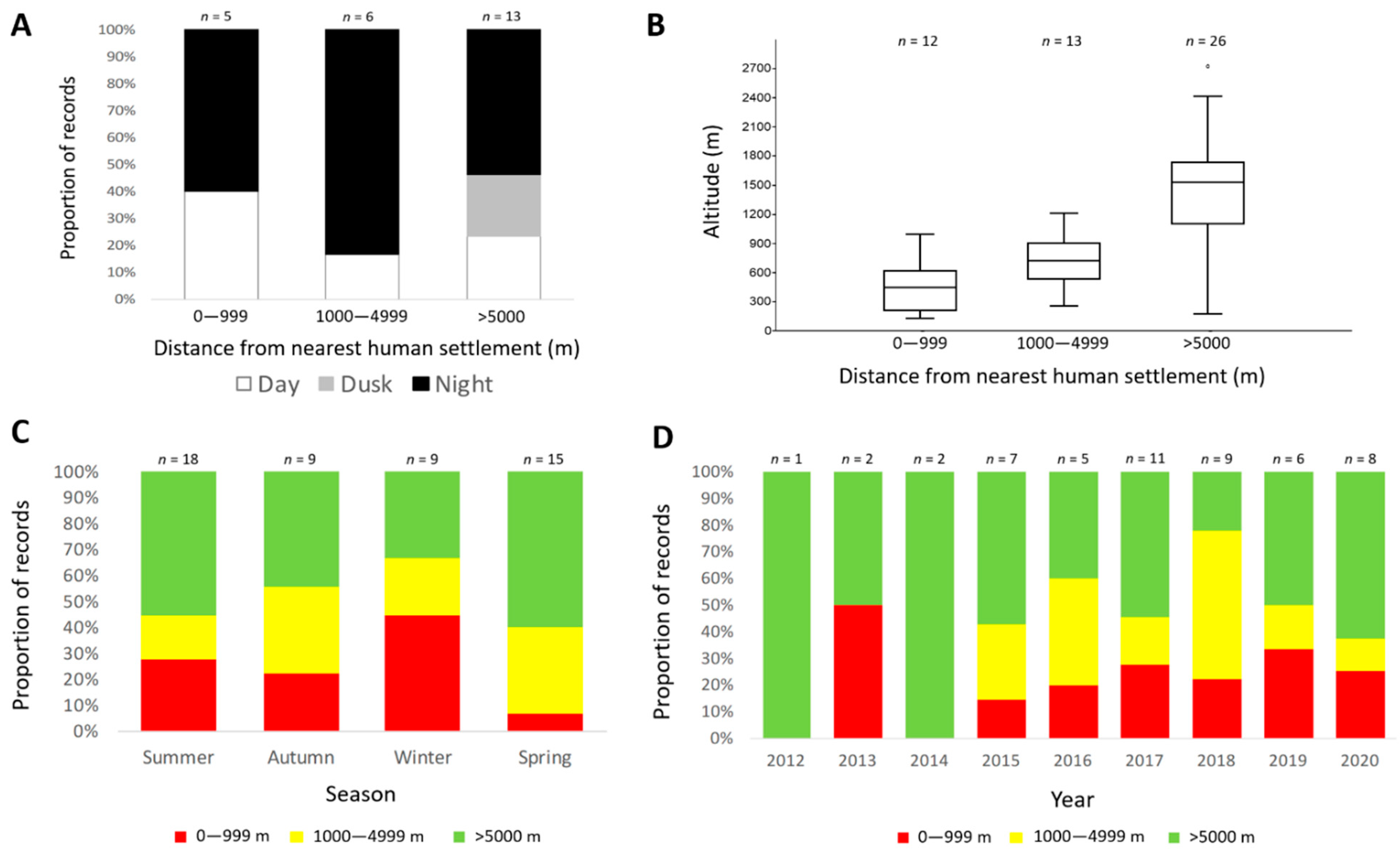
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linnaeus, C. Mantissa Plantarum, Altera. Regni Animalis, Appendix; Impensis Direct: Stockholm, Sweden, 1771; pp. 1–584. [Google Scholar]
- Chapman, J.A.; Feldhamer, G.A. (Eds.) Wild Mammals of North America; The Johns Hopkins University Press: Baltimore, MD, USA, 1982; p. 1147. [Google Scholar]
- Culver, M.; Jhonson, M.; Peccon-Slattery, J.; O’Brien, S. Genomic Ancestry of the American Puma (Puma concolor). J. Hered. 2000, 91, 186–197. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.O.; Cook, J.A. Mammals and amphibians of Southeast Alaska. In The Museum of Southwestern Biology; Special Publication: Albuquerque, NM, USA, 2007; Volume 8, pp. 1–191. [Google Scholar]
- Parera, A. Los mamíferos de la Argentina y la Región Austral de Sudamérica. Lista Comentada de Órdenes, Familias y Géneros de la Fauna de Mamíferos Silvestres del Cono Sur, con Mención a los Géneros y Especies de la Argentina; El Ateneo: Buenos Aires, Argentina, 2002; p. 453. [Google Scholar]
- Iriarte, A. Mamíferos de Chile; Lynx Edicions: Barcelona, Spain, 2008. [Google Scholar]
- Robinson, H.S.; Wielgus, R.B.; Cooley, H.S.; Cooley, S.W. Sink populations in carnivore management: Cougar demography and immigration in a hunted population. Ecol. Appl. 2008, 18, 1028–1037. [Google Scholar] [CrossRef]
- Stoner, D.C.; Rieth, W.R.; Wolfe, M.L.; Mecham, M.B.; Neville, A. Long-distance dispersal of a female cougar in a basin and range landscape. J. Wildl. Manag. 2008, 72, 933–939. [Google Scholar] [CrossRef]
- Elbroch, M.; Wittmer, H.U.; Saucedo, C.; Corti, P. Long-distance dispersal of a male puma (Puma concolor puma) in Patagonia. Rev. Chil. Hist. Nat. 2009, 82, 459–461. [Google Scholar] [CrossRef]
- Elbroch, L.M.; Wittmer, H.U. Puma spatial ecology in open habitats with aggregate prey. Mamm. Biol. 2012, 77, 377–384. [Google Scholar] [CrossRef]
- Hawley, J.E.; Rego, P.W.; Wydeven, A.P.; Schwartz, M.K.; Viner, T.C.; Kays, R.; Pilgrim, K.L.; Jenks, J.A. Long-distance dispersal of a subadult male cougar from South Dakota to Connecticut documented with DNA evidence. J. Mammal. 2016, 97, 1435–1440. [Google Scholar] [CrossRef]
- Seidensticker, J.C.; Hornocker, M.G.; Wiles, W.V.; Messick, J.P. Mountain lion social organization in the Idaho Primitive Area. Wildl. Monogr. 1973, 35, 3–60. [Google Scholar]
- Hansen, K. Cougar: The American Lion; Northland Publishing: Flagstaff, AZ, USA, 1992; p. 129. [Google Scholar]
- Laundré, J.; Loxterman, J. Impact of Edge Habitat on Summer Home Range Size in Female Pumas. Am. Midl. Nat. 2007, 157, 221–229. [Google Scholar] [CrossRef]
- Gelin, M.L.; Branch, L.C.; Thornton, D.H.; Novaro, A.J.; Gould, M.J.; Caragiulo, A. Response of pumas (Puma concolor) to migration of their primary prey in Patagonia. PLoS ONE 2017, 12, e0188877. [Google Scholar] [CrossRef]
- Maehr, D.S. Florida panther: Felis concolor coryi. In Rare and Endangered Biota of Florida; Humphrey, S.R., Ed.; Florida Game and Fresh Water Fish Commission: Naples, FL, USA, 1992; Volume 1, pp. 176–189. [Google Scholar]
- Tesky, J.L. Puma concolor. In Fire Effects Information System; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO, USA, 1995. Available online: www.fs.fed.us/database/feis/animals/mammal/puco/all.html (accessed on 21 October 2020).
- Molina, G.I. Saggia Sulla Storia Naturale del Chili; Stamperia di S. Tommaso d’ Aquino: Bologna, Italy, 1782. [Google Scholar]
- Péfaur, J.; Hermosilla, W.; Di Castri, F.; González, R.; Salinas, F. Estudio preliminar de mamíferos silvestres chilenos: Su distribución, valor económico e importancia. Rev. Soc. Med. Vet. 1968, 18, 1–15. [Google Scholar]
- Franklin, W.L.; Johnson, W.E.; Sarno, R.J.; Iriarte, A. Ecology of the Patagonia puma Felis concolor patagonica in southern Chile. Biol. Conserv. 1999, 90, 33–40. [Google Scholar] [CrossRef]
- Bank, M.; Sarno, R.; Campbell, N.; Franklin, W. Predation of guanacos (Lama guanicoe) by southernomost mountain lions (Puma concolor) during a historically severe winter in Torres del Paine National Park, Chile. J. Zool. 2002, 258, 215–222. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Pino, R.; Donoso, D.; Iriarte, A. El Puma en la Región de Coquimbo: Develando su Ecología e Interacción con la Ganadería, Segunda Edición ed; Ediciones SAG Región de Coquimbo y Flora & Fauna Chile: Santiago, Chile, 2017; p. 62.
- Quintana, V.; Yáñez, J.; Valdebenito, M. Orden Carnívora. In Mamíferos de Chile; Muñoz-Pedreros, A., Yáñez, J., Eds.; CEA Ediciones: Valdivia, Chile, 2000; pp. 155–187. [Google Scholar]
- MMA. Ficha de Clasificación de la Especie Puma concolor en el Marco del Séptimo Proceso de Clasificación de Especies. Ministerio del Medio Ambiente de Chile. 2010; p. 8. Available online: http://www.mma.gob.cl/clasificacionespecies/fichas7proceso/fichas_pac/Puma_concolor_P07.pdf (accessed on 4 September 2020).
- Iriarte, J.; Johnson, W.; Franklin, W. Feeding Ecology of the Patagonia Puma in southermost Chile. Rev. Chil. Hist. Nat. 1991, 64, 145–156. [Google Scholar]
- Redford, K.; Eisenberg, J. Mammals of the Neotropics Vol 2; The University of Chicago Press: Chicago, IL, USA, 1992; p. 430. [Google Scholar]
- Nowell, K.; Jackson, P. Wild Cats, Status Survey and Conservation Action Plan; IUCN/SSC Cat Specialist Group: Gland, Switzerland, 1996. [Google Scholar]
- Castillo, O. Antecedentes Biogeográficos y Ecológicos del Puma en el Territorio Chileno; Congreso de Geografía y V Jornada de Cartografía Temática: Valparaíso, Chile, 1992. [Google Scholar]
- Castillo, O. Sobrevivencia del Puma o León andino (Felis concolor) en el semiárido y en la región central de Chile Sudamericano. Nadir Rev. Electron. Geogr. Austral 2009, 1, 60–80. [Google Scholar]
- Grimberg, M. (Ed.) Plan Nacional de Conservación del Guanaco (Lama Guanicoe) en Chile. 2010–2015; CONAF: Santiago, Chile, 2010. [Google Scholar]
- Nielsen, C.; Thompson, D.; Kelly, M.; Lopez-Gonzalez, C.A. Puma concolor (errata version published in 2016). IUCN Red List Threat. Species 2015, e.T18868A97216466. [Google Scholar] [CrossRef]
- MMA. Decreto Supremo N° 42 2011. Séptimo Proceso de Clasificación de Especies del Reglamento de Clasificación de Especies del Ministerio del Medio Ambiente de Chile; MMA: Santiago, Chile, 2011. [Google Scholar]
- Tala, C.H.; Guerrero, S.; Aviles, R.; Stutzin, M. Especies Amenazadas de Chile: Protejámoslas y Evitemos su Extinción; Comisión Nacional del Medio Ambiente; Serie divulgativa sobre Biodiversidad: Santiago, Chile, 2009; Volume I, p. 122. [Google Scholar]
- Amar, F.; Melo, O.; Bonacic, C. Efectos Económicos de la Coexistencia Entre Pumas y Ganaderos en la Comuna de San José de Maipo, Región Metropolitana; Reunión Binacional de Ecología: La Serena, Chile, 2007.
- Pavez, E.; Guarda, N. Puma y Arriero en el Alto Cachapoal, Hacia la Resolución de un Conflicto; Editorial Kactus: Santiago, Chile, 2017; p. 96. [Google Scholar]
- Pauchard, A.; Aguayo, M.; Peña, E.; Urrutia, R. Multiple effects of urbanization on the biodiversity of developing countries: The case of a fast-growing metropolitan area (Concepción, Chile). Biol. Conserv. 2006, 127, 272–281. [Google Scholar] [CrossRef]
- Dobbs, C.; Escobedo, F.J.; Clerici, N.; De la Barrera, F.; Eleuterio, A.A.; MacGregor-Fors, I.; Reyes-Paecke, S.; Vásquez, A.; Camaño, J.D.; Hernández, H.J. Urban ecosystem Services in Latin America: Mismatch between global concepts and regional realities? Urban Ecosyst. 2019, 22, 173–187. [Google Scholar] [CrossRef]
- Bonacic, C.; Galvez, N.; Ibarra, J.; Amar, M.; Sanhueza, D.; Murphy, T.; Guarda, N. Evaluación del Conflicto entre Carnívoros Silvestres y Ganadería; Laboratorio de Vida Silvestre Fauna Australis, Facultad de Agronomía e Ingeniería Forestal; Pontificia Universidad Católica de Chile: Santiago, Chile, 2007; p. 94. [Google Scholar]
- Guarda, N.; Gálvez, N.; Hernández, F.; Rubio, A.; Ohrens, O.; Bonacic, C. Manual de Verificación: Denuncias de Depredación en Ganado Doméstico; Serie Fauna Australis, Facultad de Agronomía e Ingeniería Forestal, Pontificia Universidad Católica de Chile; Servicio Agrícola y Ganadero: Santiago, Chile, 2010; p. 80.
- Guarda, N.; Bonacic, C. Informe Técnico Final: Ecología y Relación con el ser Humano de Puma Concolor en la Reserva Nacional Río los Cipreses y Zonas Aledañas; Laboratorio Fauna Australis, Pontificia Universidad Católica de Chile, Fondo de Investigación Científica Alto Cachapoal, Pacific Hydro Chile: Santiago, Chile, 2012. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; B da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Guarda, N.; Gálvez, N.; Leichtle, J.; Osorio, C.; Bonacic, C. Puma Puma concolor density estimation in the Mediterranean Andes of Chile. Oryx 2017, 51, 263–267. [Google Scholar] [CrossRef]
- Kelly, M.; Noss, A.; Di Bitetti, M.; Maffei, L.; Arispe, R.; Paviolo, A.; Angelo, C.; Di Blanco, Y. Estimating Puma Densities from Camera Trapping across Three Study Sites: Bolivia, Argentina, and Belize. J. Mammal. 2008, 89, 408–418. [Google Scholar] [CrossRef]
- Negroes, N.; Sarmento, P.; Cruz, J.; Eira, C.; Revilla, E.; Fonseca, C.; Sollmann, R.; Torres, N.; Furtado, M.; Jacomo, A.; et al. Use of Camera-Trapping to Estimate Puma Density and Influencing Factors in Central Brazil. J. Wildl. Manag. 2010, 74, 1195–1203. [Google Scholar] [CrossRef]
- Guarda, N.; Aguilar, A.; López, J.; Carrasco, J.M.; Orellana NDurán, H.; Lobos, P.; Bonacic, P. Sobrevivencia de crías de puma (Puma concolor) hasta edad de dispersión en la Reserva Nacional Río los Cipreses, Región del Libertador Bernardo O’Higgins. Biodiversidata 2014, 1, 39–41. [Google Scholar]
- SAG. Sistema de Atención de Denuncias de Ataques de Carnívoros Silvestres Sobre Ganado Doméstico; Servicio Agrícola y Ganadero: O’Higgins, Chile, 2020.
- McRae, B.H.; Beier, P.; Dewald, L.E.; Huynh, L.Y.; Keim, P. Habitat barriers limit gene flow and illuminate historical events in a wide-ranging carnivore, the American puma. Mol. Ecol. 2005, 14, 1965–1977. [Google Scholar] [CrossRef]
- Zuñiga, A.; Muñoz-Pedreros, A.; Fierro, A. Habitat use of four terrestrial carnivores in southern Chile. Gayana 2009, 73, 200–210. [Google Scholar]
- Banfield, J.E.; Ciuti, S.; Nielsen, C.C.; Boyce, M.S. Cougar roadside habitat selection: Incorporating topography and traffic. Glob. Ecol. Conserv. 2020, 23, e01186. [Google Scholar] [CrossRef]
- INE. Censo de Población y Vivienda año 2017 del Instituto Nacional de Estadísticas de Chile; INE: Santiago, Chile, 2017. [Google Scholar]
- Vickers, T.W.; Sanchez, J.N.; Johnson, C.K.; Morrison, S.A.; Botta, R.; Smith, T.; Cohen, B.S.; Huber, P.R.; Ernest, H.B.; Boyce, W.M. Survival and Mortality of Pumas (Puma concolor) in a Fragmented, Urbanizing Landscape. PLoS ONE 2015, 10, e0131490. [Google Scholar] [CrossRef] [PubMed]
- García, C.B.; Svensson, G.L.; Bravo, C.; Undurraga, M.I.; Díaz-Forestier, J.; Godoy, K.; Neaman, A.; Barbosa, O.; Abades, S.; Celis-Diez, J. Remnants of native forests support carnivore diversity in the vineyard landscapes of central Chile. Oryx 2020, 1–8. [Google Scholar] [CrossRef]
- Stoner, D.C.; Wolfe, M.L.; Mecham, C.; Mecham, M.B.; Durham, S.L.; Choate, D.M. Dispersal behaviour of a polygynous carnivore: Do cougars Puma concolor follow source-sink predictions? Wildl. Biol. 2013, 19, 289–301. [Google Scholar] [CrossRef]
- Kertson, B.N.; Spencer, R.D.; Marzluff, J.M.; Hepinstall-Cymerman, J.; Grue, C.E. Cougar space use and movements in the wildland–urban landscape of western Washington. Ecol. Appl. 2011, 21, 2866–2881. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Wang, Y.; Nickel, B.; Houghtaling, P.; Shakeri, Y.; Allen, M.L.; Kermish-Wells, J.; Yovovich, V.; Williams, T. Scale Dependent Behavioral Responses to Human Development by a Large Predator, the Puma. PLoS ONE 2013, 8, e60590. [Google Scholar] [CrossRef]
- Sweanor, L.L.; Logan, K.A.; Hornocker, M.G. Cougar dispersal patterns, metapopulation dynamics, and conservation. Conserv. Biol. 2000, 14, 798–808. [Google Scholar] [CrossRef]
- Matte, E.; Castilho, C.; Miotto, R.; Sana, D.; Johnson, W.; O’Brien, S.; Freitas, T.; Eizirik, E. Molecular evidence for a recent demographic expansion in the puma (Puma concolor) (Mammalia, Felidae). Genet. Mol. Biol. 2013, 36, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Cote, J.; Clobert, J.; Brodin, T.; Fogerty, S.; Sih, A. Personality-dependent dispersal: Characterization, ontogeny, and consequences for spatially-structured populations. Philos. Trans. R. Soc. B. 2010, 365, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.L.; Elbroch, L.M.; Casady, D.S.; Wittmer, H.U. Seasonal variation in the feeding ecology of pumas (Puma concolor) in northern California. Cannadian J. Zool. 2014, 92, 397–403. [Google Scholar] [CrossRef]
- Odden, M.; Athreya, V.; Rattan, S.; Linnell, J.D.C. Adaptable Neighbours: Movement Patterns of GPS-Collared Leopards in Human Dominated Landscapes in India. PLoS ONE 2014, 9, e112044. [Google Scholar] [CrossRef] [PubMed]
- Santini, L.; González-Suárez, M.; Russo, D.; Gonzalez-Voyer, A.; von Hardenberg, A.; Ancillotto, L. One strategy does not fit all: Determinants of urban adaptation in mammals. Ecol. Lett. 2019, 22, 365–376. [Google Scholar] [CrossRef]
- Zellmer, A.J.; Wood, E.M.; Surasinghe, T.; Putman, B.J.; Pauly, G.B.; Magle, S.B.; Lewis, J.S.; Kay, C.A.M.; Fidino, M. What can we learn from wildlife sightings during the COVID-19 global shutdown? Ecosphere 2020, 11, e03215. [Google Scholar] [CrossRef]
- Silva-Rodríguez, E.A.; Gálvez, N.; Swan, G.J.F.; Cusack, J.J.; Moreira-Arce, D. Urban wildlife in times of COVID-19: What can we infer from novel carnivore records in urban areas? Sci. Total Environ. 2020. [Google Scholar] [CrossRef]
- Rutz, C.; Loretto, M.C.; Bates, A.E.; Davidson, S.C.; Duarte, C.M.; Jetz, W.; Johnson, M.; Kato, A.; Kays, R.; Mueller, T.; et al. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 2020, 4, 1156–1159. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Šálek, M.; Drahníková, L.; Tkadlec, E. Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mammal Rev. 2015, 45, 1–14. [Google Scholar]
- Castañeda, I.; Bellard, C.; Jarić, I.; Pisanu, B.; Chapuis, J.L.; Bonnaud, E. Trophic patterns and home-range size of two generalist urban carnivores: A review. J. Zool. 2019, 307, 79–92. [Google Scholar] [CrossRef]
- Magle, S.B.; Hunt, V.M.; Vernon, M.; Crooks, K.R. Urban wildlife research: Past, present and future. Biol. Conserv. 2012, 155, 23–32. [Google Scholar] [CrossRef]
- Mora, M.; Napolitano, C.; Ortega, R.; Poulin E y Pizarro-Lucero, J. Feline immunodeficiency virus and feline leukemia virus infection in free-ranging guignas (Leopardus guigna) and sympatric domestic cats in human perturbed landscapes on Chiloé Island, Chile. J. Wildl. Dis. 2015, 51, 199–208. [Google Scholar] [CrossRef]
- Napolitano, C.; Gálvez, N.; Bennett, M.; Acosta-Jamett, G.; Sanderson, J. Leopardus guigna. IUCN Red List Threat. Species 2015, e.T15311A50657245. [Google Scholar] [CrossRef]
- Ortega, R.; Mena, J.; Grecco, S.; Perez, R.; Panzera, Y.; Napolitano, C.; Sandoval, A.Z.N.-A.; Sandoval, D.; Gonzalez-Acuña, D.; Cofré, S.; et al. Domestic dog origin of Carnivore Protoparvovirus 1 infection in a rescued free-ranging guiña (Leopardus guigna). Chile. Transbound. Emerg. Dis. 2020, 1–7. [Google Scholar] [CrossRef]
- Sacristán, I.; Esperon, F.; Pérez, R.; Acuña, F.; Aguilar, E.; Garcia, S.; López, M.J.; Neves, E.; Cabello, J.; Hidalgo-Hermoso, E.; et al. Epidemiology and molecular characterization of Carnivore protoparvovirus-1 infection in the wild felid Leopardus guigna. Chile. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef]
- Sacristán, I.; Acuña, F.; Aguilar, E.; Garcia, S.; López, M.J.; Cabello, J.; Hidalgo-Hermoso, E.; Sanderson, J.; Terio, K.A.; Barrs, V.; et al. Cross-species transmission of retroviruses among domestic and wild felids in human-occupied landscapes in Chile. Evol. Appl. 2021, 1–13. [Google Scholar] [CrossRef]
- Cunningham, M.W.; Brown, M.A.; Shindle, D.B.; Terrell, S.P.; Hayes, K.A.; Ferree, B.C.; McBride, R.T.; Blankenship, E.L.; Jansen, D.; Citino, S.B. Epizootiology and management of feline leukemia virus in the Florida puma. J. Wildl. Dis. 2008, 44, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.E.; Swift, P.; Fleer, K.A.; Torres, S.; Girard, Y.A.; Johnson, C.K. Risk factors for exposure to feline pathogens in California mountain lions (Puma concolor). J. Wildl. Dis. 2013, 49, 279–293. [Google Scholar] [CrossRef]
- Lee, J.S.; Bevins, S.N.; Serieys, L.E.K.; Vickers, W.; Logan, K.A.; Aldredge, M.; Boydston, E.E.; Lyren, L.M.; McBride, R.; Roelke-Parker, M.; et al. Evolution of Puma Lentivirus in Bobcats (Lynx rufus) and Mountain Lions (Puma concolor). North America. J. Virol. 2014, 88, 7727–7737. [Google Scholar] [CrossRef]
- Antunes, A.; Troyer, J.L.; Roelke, M.E.; Pecon-Slattery, J.; Packer, C.; Winterbach, C.; Winterbach, H.; Hemson, G.; Frank, L.; Stander, P. The evolutionary dynamics of the lion Panthera leo revealed by host and viral population genomics. Plos Genet. 2008, 4, e1000251. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.L.; Cattori, V.; Martínez, F.; López, G.; Vargas, A.; Simón, M.A.; Zorrilla, I.; Muñoz, A.; Palomares, F.; López-Bao, J.V.; et al. Feline Leukemia Virus and Other Pathogens as Important Threats to the Survival of the Critically Endangered Iberian Lynx (Lynx pardinus). PLoS ONE 2009, 4, e4744. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.L.; Cattori, V.; Martínez, F.; López, G.; Vargas, A.; Palomares, F.; López-Bao, J.V.; Hofmann-Lehmann, R.; Lutz, H. Feline leukemia virus infection: A threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Vet. Immunol. Immunopathol. 2010, 134, 61–67. [Google Scholar] [CrossRef]
- Chapron, G.; Kaczensky, P.; Linell, J.D.C.; von Arx, M.; Huber, D.; Andrén, H.; Lopéz-Bao, J.V.; Adamec, M.; Álvarez, F. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 1517–1519. [Google Scholar] [CrossRef]
- Gilroy, J.J.; Ordiz, A.; Bischof, R. Carnivore coexistence: Value the wilderness. Science 2015, 347, 382. [Google Scholar] [CrossRef] [PubMed]
- Gompper, M.E.; Belant, J.L.; Kays, R. Carnivore coexistence: America’s recovery. Science 2015, 347, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.H. Human emotions toward wildlife. Hum. Dimens. Wildl. 2012, 17, 1–3. [Google Scholar] [CrossRef]
- Jacobs, M.; Vaske, J. Understanding emotions as opportunities for and barriers to coexistence with wildlife. In Human–Wildlife Interactions: Turning Conflict into Coexistence; Frank, B., Glikman, J., Marchini, S., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 65–84. [Google Scholar] [CrossRef]
- Kansky, R.; Kidd, M.; Knight, A.T. A wildlife tolerance model and case study for understanding human wildlife conflicts. Biol. Conserv. 2016, 201, 137–145. [Google Scholar] [CrossRef]
- Marino, F.; Kansky, R.; Shivji, I.; Di Croce, A.; Ciucci, P.; Knight, A.T. Understanding drivers of human tolerance to gray wolves and brown bears as a strategy to improve landholder-carnivore coexistence. Conserv. Sci. Pract. 2020. [Google Scholar] [CrossRef]
- Laundré, J.W.; Papouchis, C. The Elephant in the room: What can we learn from California regarding the use of sport hunting of pumas (Puma concolor) as a management tool? PLoS ONE 2020, 15, e0224638. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, E.R.; Chimento, N.R. El puma (Puma concolor) en las pampas de la provincia de Buenos Aires: Una actualización sobre distribución geográfica y conflicto con el hombre. Hist. Nat. 2020, 10, 53–79. [Google Scholar]
- Lamb, C.T.; Ford, A.T.; McLellan, B.N.; Proctor, M.F.; Mowat, G.; Ciarniello, L.; Nielsen, S.E.; Boutin, S. The ecology of human–carnivore coexistence. Proc. Natl. Acad. Sci. USA 2020, 117, 17876–17883. [Google Scholar] [CrossRef] [PubMed]


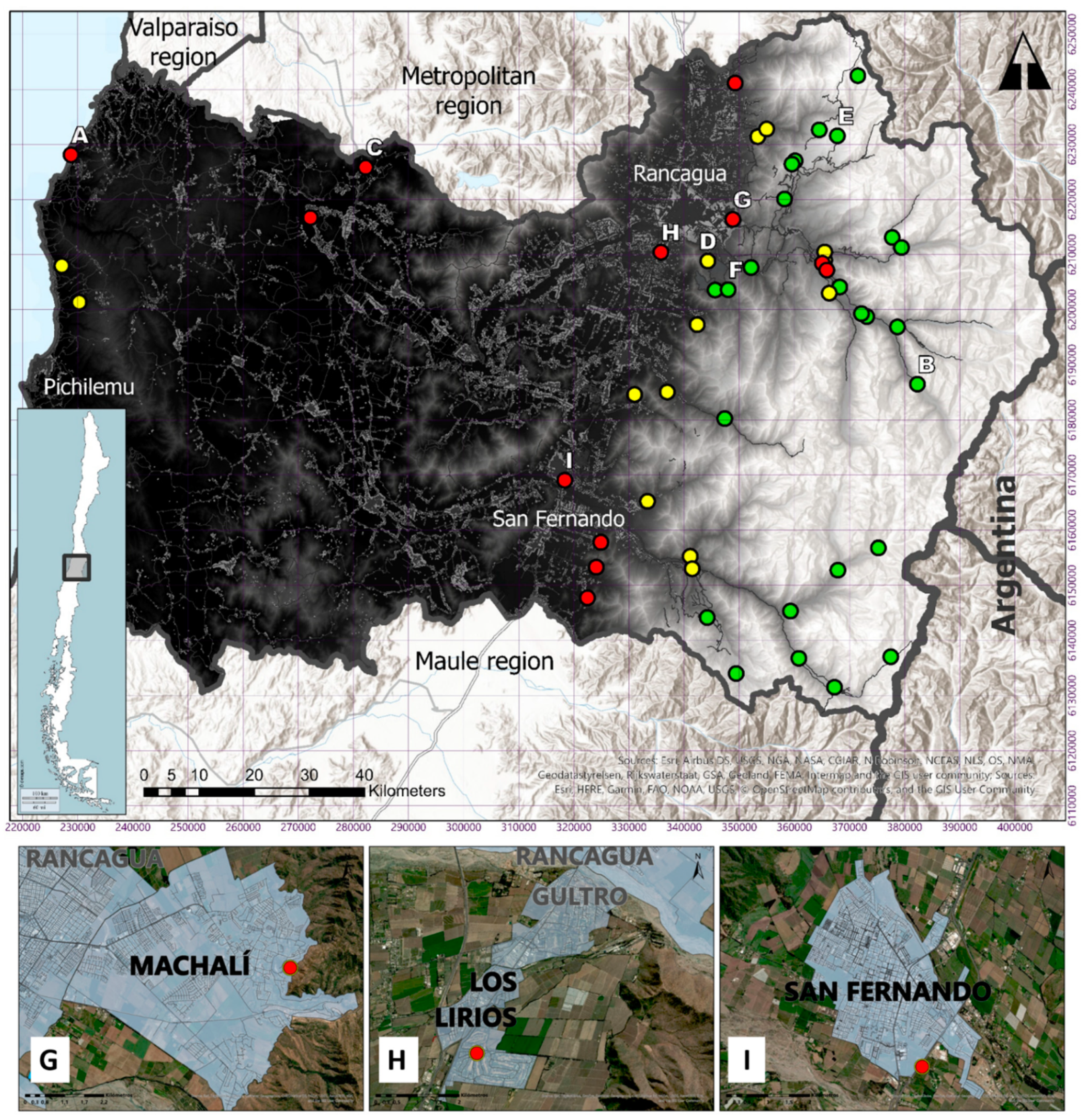
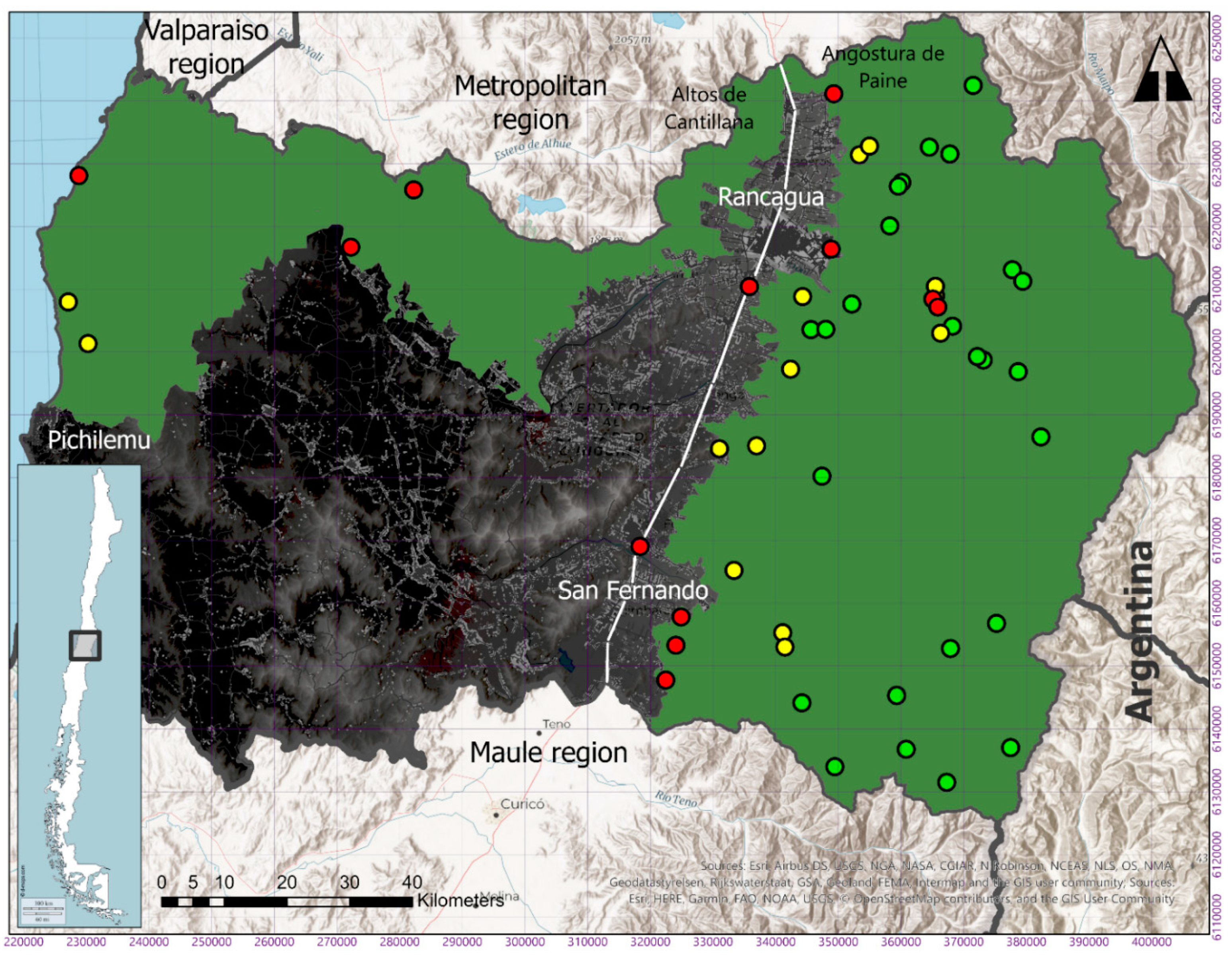
| N° | Location | Date (m/d/y) | H19 E | H19 N | Elevation (m.a.s.l.) | Source of Record | Type of Record |
|---|---|---|---|---|---|---|---|
| 1 | Central Chacayes, Machalí | 05-08-2012 | 373132 | 6198750 | 1116 | SAG forensic analysis | Drowned puma corpse finding |
| 2 | Los Lirios, Rancagua | 01-21-2013 | 335803 | 6210431 | 482 | Citizen report | Video camera |
| 3 | Cortaderal, Machalí | 02-14-2013 | 382327 | 6186473 | 1701 | SAG field work | Live capture |
| 4 | Casa Piedra, Codegua | 10-27-2014 | 364506 | 6232646 | 1897 | SAG forensic analysis | Livestock attack |
| 5 | La Confluencia, San Fernando | 12-12-2014 | 359295 | 6145326 | 1171 | SAG forensic analysis | Livestock attack |
| 6 | Cerro Agujereado, Machalí | 06-09-2015 | 365510 | 6208951 | 1040 | Citizen report | Camera trap |
| 7 | Alto Huemul, San Fernando | 09-25-2015 | 344185 | 6144160 | 1352 | SAG field work | Sighting |
| 8 | Codegua, Chimbarongo | 10-26-2015 | 322469 | 6147747 | 498 | SAG forensic analysis | Livestock attack |
| 9 | El Baluarte, Rengo | 11-24-2015 | 336950 | 6185055 | 535 | SAG field work | Sighting |
| 10 | Glaciar Universidad, San Fernando | 11-29-2015 | 375233 | 6156787 | 2414 | Citizen report | Sighting |
| 11 | Central Chacayes, Machalí | 12-07-2015 | 368219 | 6204112 | 1104 | SAG forensic analysis | Drowned puma corpse finding |
| 12 | Puma Lodge, Machalí | 12-30-2015 | 378698 | 6196868 | 1330 | Citizen report | Sighting |
| 13 | Chapa Verde, Machalí | 01-19-2016 | 367799 | 6231552 | 2324 | SAG field work | Corpse finding |
| 14 | Cajón Río Blanco, Machalí | 02-17-2016 | 377758 | 6213110 | 1685 | SAG field work | Sighting |
| 15 | Cerrito San Juan, Machalí | 07-26-2016 | 348847 | 6216399 | 623 | SAG field work | Sighting |
| 16 | Los Peumos, RN Cipreses, Machalí | 09-13-2016 | 366325 | 6202997 | 1210 | Citizen report | Camera trap |
| 17 | Sierra Nevada, Machalí | 12-22-2016 | 365496 | 6210477 | 903 | SAG forensic analysis | Livestock attack |
| 18 | Alto Huemul, San Fernando | 01-05-2017 | 349447 | 6133998 | 1807 | SAG field work | Corpse finding |
| 19 | Chacayes, Machalí | 01-22-2017 | 365077 | 6208430 | 885 | SAG forensic analysis | Livestock attack |
| 20 | Central Chacayes, Machalí | 02-21-2017 | 372176 | 6199283 | 1090 | Citizen report | Sighting |
| 21 | La Correana, San Fernando | 04-09-2017 | 360809 | 6136760 | 1409 | SAG forensic analysis | Livestock attack |
| 22 | La Leonera, Codegua | 06-01-2017 | 353359 | 6231408 | 724 | Citizen report | Photograph |
| 23 | Los Petriles, Chimbarongo | 09-02-2017 | 324072 | 6153298 | 436 | SAG forensic analysis | Livestock attack |
| 24 | Cajón Portillo, San Fernando | 10-28-2017 | 367890 | 6152761 | 1532 | SAG field work | Sighting |
| 25 | La Rufina, San Fernando | 10-29-2017 | 341114 | 6155275 | 794 | SAG forensic analysis | Livestock attack |
| 26 | Haras Sauzal, Machalí | 11-27-2017 | 352144 | 6207659 | 711 | Police report | Sighting |
| 27 | Las Cayanas, Machalí | 12-24-2017 | 379438 | 6211289 | 1639 | SAG forensic analysis | Livestock attack |
| 28 | Cruce Alhué, Las Cabras | 12-26-2017 | 282227 | 6225842 | 132 | Citizen report | Corpse finding |
| 29 | Hotel La Leonera, Codegua | 03-24-2018 | 354969 | 6232797 | 753 | SAG forensic analysis | Livestock attack |
| 30 | La Polcura, Navidad | 03-28-2018 | 228788 | 6228110 | 210 | Citizen report | Photograph |
| 31 | Fundo Las Nieves, Rengo | 04-04-2018 | 347370 | 6180227 | 851 | SAG field work | Sighting |
| 32 | Embalse Cauquenes, Requinoa | 08-06-2018 | 345620 | 6203567 | 797 | Citizen report | Camera trap |
| 33 | Panilonco, Pichilemu | 08-07-2018 | 230263 | 6201355 | 260 | Citizen report | Photograph |
| 34 | Los Maquis, Pelequen | 10-23-2018 | 331012 | 6184593 | 387 | Citizen report | Sighting |
| 35 | Agua Buena, San Fernando | 11-29-2018 | 333353 | 6165216 | 608 | SAG forensic analysis | Livestock attack |
| 36 | La Pimpinela, Requinoa | 12-11-2018 | 342374 | 6197274 | 490 | Citizen report | Sighting |
| 37 | San Juan de Sierra, Chimbarongo | 12-28-2018 | 324903 | 6157789 | 448 | Citizen report | Sighting |
| 38 | Quebrada Santa Clara, Machalí | 01-24-2019 | 371512 | 6242481 | 2725 | SAG field work | Camera trap |
| 39 | Picarquín, Mostazal | 02-13-2020 | 349266 | 6241144 | 586 | SAG forensic analysis | Livestock attack |
| 40 | Tranque Barahona, Machalí | 02-27-2019 | 360132 | 6227026 | 1582 | Citizen report | Sighting |
| 41 | La Matancilla, San Fernando | 03-04-2019 | 341469 | 6153015 | 962 | SAG forensic analysis | Livestock attack |
| 42 | Camino Central Chacayes, Machalí | 07-19-2019 | 365874 | 6207190 | 992 | Citizen report | Photograph |
| 43 | Río Damas, San Fernando | 10-02-2019 | 377486 | 6137038 | 2401 | SAG field work | Camera trap |
| 44 | Maitenes, Machalí | 12-17-2019 | 358206 | 6220093 | 1152 | Citizen report | Video camera |
| 45 | Embalse Colihues, Requinoa | 02-12-2020 | 344301 | 6208833 | 655 | Citizen report | Camera trap |
| 46 | Termas del Flaco, San Fernando | 03-02-2020 | 367279 | 6131495 | 1735 | SAG forensic analysis | Livestock attack |
| 47 | Estero Los Leones, Requinoa | 04-10-2020 | 347988 | 6203626 | 724 | Citizen report | Camera trap |
| 48 | San Fernando | 04-26-2020 | 318357 | 6169040 | 356 | Citizen report | Video camera |
| 49 | Lago Rapel, Las Cabras | 06-26-2020 | 272202 | 6216714 | 167 | Citizen report | Sighting |
| 50 | Tranque Barahona, Machalí | 07-06-2020 | 359558 | 6226429 | 1598 | Citizen report | Camera trap |
| 51 | Panilonco, Pichilemu | 07-29-2020 | 227101 | 6207978 | 180 | Citizen report | Video camera |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Álvarez, D.; Napolitano, C.; Salgado, I. Puma (Puma concolor) in the Neighborhood? Records Near Human Settlements and Insights into Human-Carnivore Coexistence in Central Chile. Animals 2021, 11, 965. https://doi.org/10.3390/ani11040965
Ramírez-Álvarez D, Napolitano C, Salgado I. Puma (Puma concolor) in the Neighborhood? Records Near Human Settlements and Insights into Human-Carnivore Coexistence in Central Chile. Animals. 2021; 11(4):965. https://doi.org/10.3390/ani11040965
Chicago/Turabian StyleRamírez-Álvarez, Diego, Constanza Napolitano, and Iván Salgado. 2021. "Puma (Puma concolor) in the Neighborhood? Records Near Human Settlements and Insights into Human-Carnivore Coexistence in Central Chile" Animals 11, no. 4: 965. https://doi.org/10.3390/ani11040965
APA StyleRamírez-Álvarez, D., Napolitano, C., & Salgado, I. (2021). Puma (Puma concolor) in the Neighborhood? Records Near Human Settlements and Insights into Human-Carnivore Coexistence in Central Chile. Animals, 11(4), 965. https://doi.org/10.3390/ani11040965






