Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
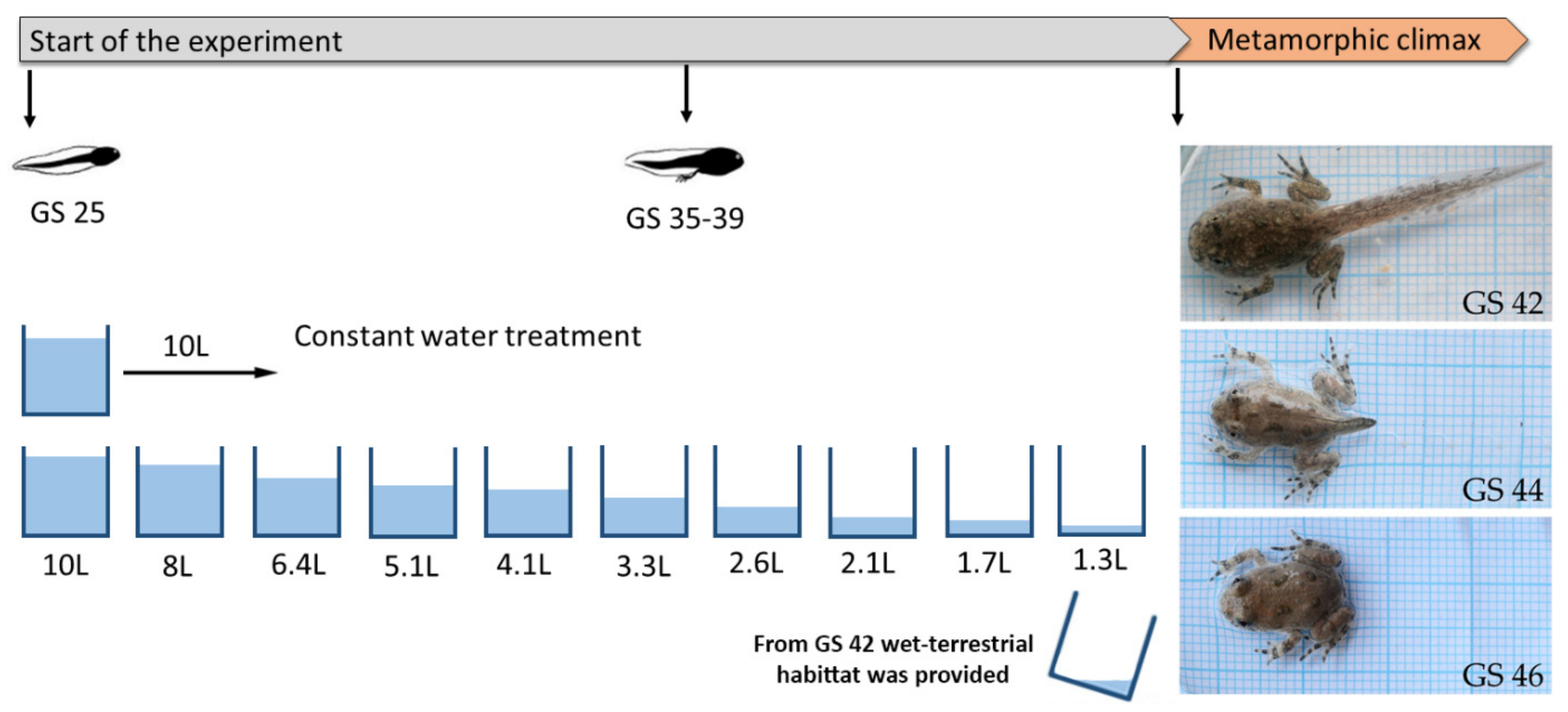
2.1. Experimental Design
2.2. Sample Processing
2.3. Biochemical Parameters
2.4. Statistical Analyses
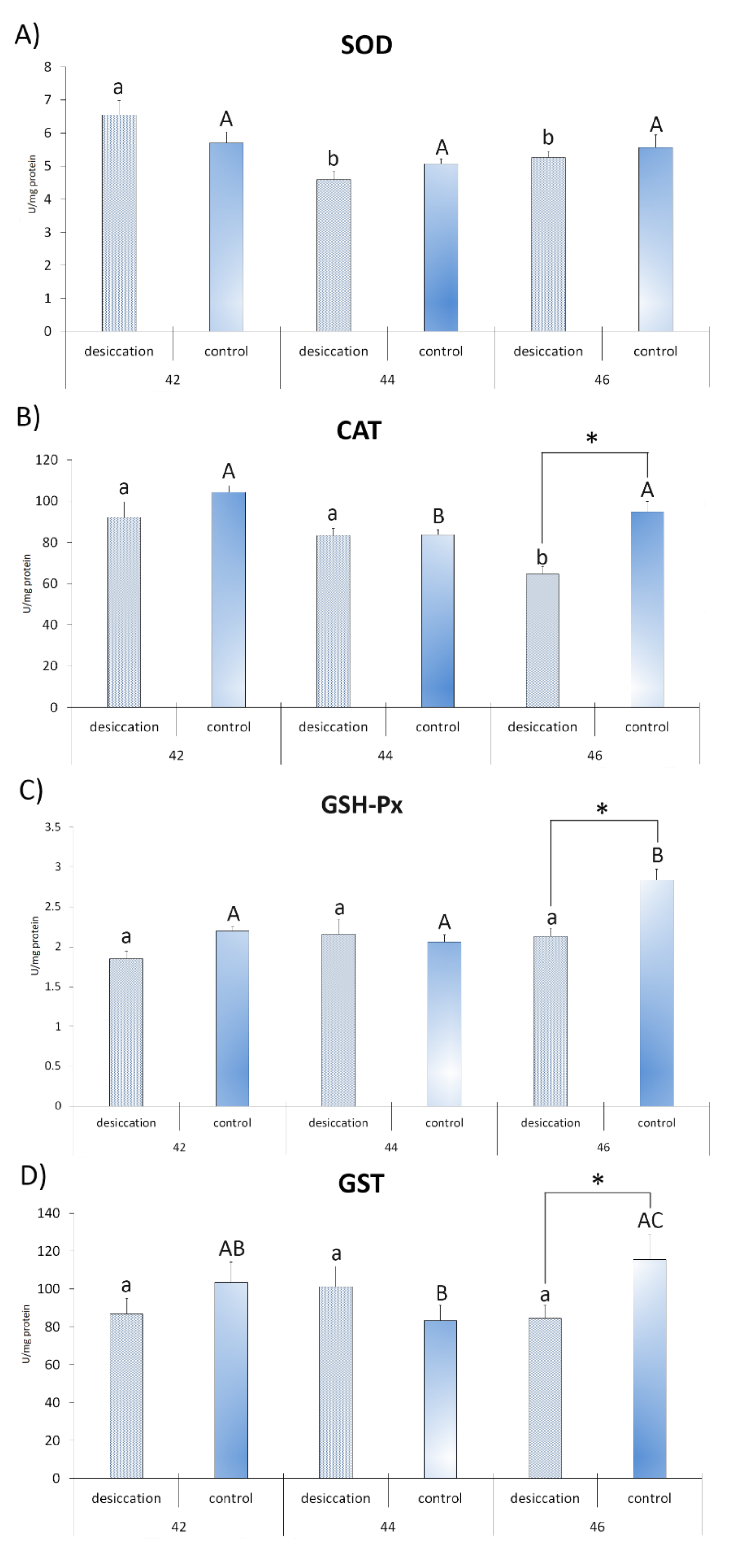
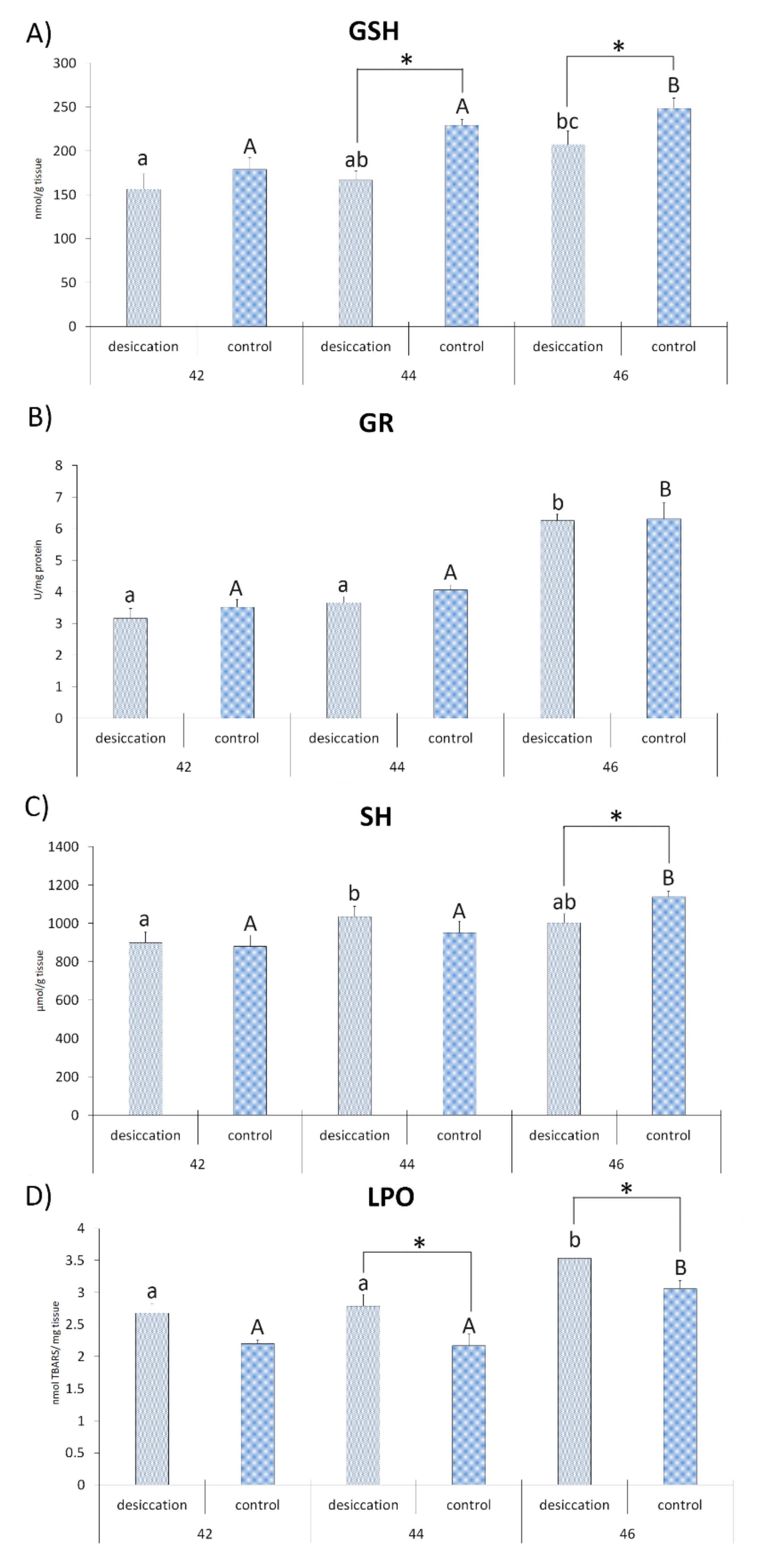
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, B.; Fletcher, Q.E.; Boonstra, R.; Sheriff, M.J. Measures of physiological stress: A transparent or opaque window into the status, management and conservation of species? Conserv. Physiol. 2014, 2, cou023. [Google Scholar] [CrossRef]
- Sparling, D.W.; Linder, G.; Bishop, C.A.; Krest, S. Ecotoxicology of Amphibians and Reptiles; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gavrilović, B.R.; Petrović, T.G.; Radovanović, T.B.; Despotović, S.G.; Gavrić, J.P.; Krizmanić, I.I.; Ćirić, M.D.; Prokić, M.D. Hepatic oxidative stress and neurotoxicity in Pelophylax kl. esculentus frogs: Influence of long-term exposure to a cyanobacterial bloom. Sci. Total Environ. 2021, 750, 141569. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Székely, D.; Cogălniceanu, D.; Székely, P.; Armijos-Ojeda, D.; Espinosa-Mogrovejo, V.; Denoël, M. How to recover from a bad start: Size at metamorphosis affects growth and survival in a tropical amphibian. BMC Ecol. 2020, 20, 24. [Google Scholar] [CrossRef]
- Sinsch, U.; Leus, F.; Sonntag, M.; Hantzschmann, A.M. Carry-over effects of the larval environment on the post-metamorphic performance of Bombina variegata (Amphibia, Anura). Herpetol. J. 2020, 30, 126–134. [Google Scholar] [CrossRef]
- Kohli, A.K.; Lindauer, A.L.; Brannelly, L.A.; Ohmer, M.E.; Richards-Zawacki, C.; Rollins-Smith, L.; Voyles, J. Disease and the drying pond: Examining possible links among drought, immune function, and disease development in amphibians. Physiol. Biochem. Zool. 2019, 92, 339–348. [Google Scholar] [CrossRef]
- Gervasi, S.S.; Foufopoulos, J. Costs of plasticity: Responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct. Ecol. 2008, 22, 100–108. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Saccoccio, V.L.; Iijima, T.; Collins, E.M.; Rosenthal, G.G.; Warkentin, K.M. The shape of things to come: Linking developmental plasticity to post-metamorphic morphology in anurans. J. Evol. Biol. 2010, 23, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mestre, I.; Kulkarni, S.; Buchholz, D.R. Mechanisms and consequences of developmental acceleration in tadpoles responding to pond drying. PLoS ONE 2013, 8, e84266. [Google Scholar] [CrossRef]
- Burraco, P.; Díaz-Paniagua, C.; Gomez-Mestre, I. Different effects of accelerated development and enhanced growth on oxidative stress and telomere shortening in amphibian larvae. Sci. Rep. 2017, 7, 7494. [Google Scholar] [CrossRef] [PubMed]
- Ruthsatz, K.; Dausmann, K.H.; Paesler, K.; Babos, P.; Sabatino, N.M.; Peck, M.A.; Glos, J. Shifts in sensitivity of amphibian metamorphosis to endocrine disruption: The common frog (Rana temporaria) as a case study. Conser. Physiol. 2020, 8, coaa100. [Google Scholar] [CrossRef]
- Van Buskirk, J.O.S.H.; McCollum, S.A. Influence of tail shape on tadpole swimming performance. J. Exp. Biol. 2000, 203, 2149–2158. [Google Scholar] [PubMed]
- Prokić, M.D.; Gavrić, J.P.; Petrović, T.G.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Krizmanić, I.I.; Pavlović, S.Z. Oxidative stress in Pelophylax esculentus complex frogs in the wild during transition from aquatic to terrestrial life. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 234, 98–105. [Google Scholar] [CrossRef]
- Gosner, K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Shi, Y.B. Amphibian Metamorphosis: From Morphology to Molecular Biology; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Zhu, W.; Chang, L.; Zhao, T.; Wang, B.; Jiang, J. Remarkable metabolic reorganization and altered metabolic requirements in frog metamorphic climax. Front. Zool. 2020, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Damjanovski, S.; Amano, T.; Li, Q.; Ueda, S.; Shi, Y.B.; Ishizuya-Oka, A. Role of ECM remodeling in thyroid hormone-dependent apoptosis during anuran metamorphosis. Ann. N. Y. Acad. Sci. 2000, 926, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Burraco, P.; Valdés, A.E.; Johansson, F.; Gomez-Mestre, I. Physiological mechanisms of adaptive developmental plasticity in Rana temporaria island populations. BMC Evol. Biol. 2017, 17, 164. [Google Scholar] [CrossRef]
- Johnson, J.; Manzo, W.; Gardner, E.; Menon, J. Reactive oxygen species and anti-oxidant defenses in tail of tadpoles, Xenopus laevis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 101–108. [Google Scholar] [CrossRef]
- Menon, J.; Rozman, R. Oxidative stress, tissue remodeling and regression during amphibian metamorphosis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 625–631. [Google Scholar] [CrossRef]
- Hanada, H.; Kashiwagi, A.; Takehara, Y.; Kanno, T.; Yabuki, M.; Sasaki, J.; Inoue, M.; Utsumi, K. Do reactive oxygen species underlie the mechanism of apoptosis in the tadpole tail? Free Radic. Biol. Med. 1997, 23, 294–301. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Hanada, H.; Yabuki, M.; Kanno, T.; Ishisaka, R.; Sasaki, J.; Inoue, M.; Utsumi, K. Thyroxine enhancement and the role of reactive oxygen species in tadpole tail apoptosis. Free Radic. Biol. Med. 1999, 26, 1001–1009. [Google Scholar] [CrossRef]
- Melvin, S.D. Oxidative stress, energy storage, and swimming performance of Limnodynastes peronii tadpoles exposed to a sub-lethal pharmaceutical mixture throughout development. Chemosphere 2016, 150, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Pinya, S.; Tejada, S.; Capó, X.; Sureda, A. Invasive predator snake induces oxidative stress responses in insular amphibian species. Sci. Total Environ. 2016, 566, 57–62. [Google Scholar] [CrossRef]
- Prokić, M.D.; Petrović, T.G.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Faggio, C.; Saičić, Z.S. Comparative assessment of the antioxidative defense system in subadult and adult anurans: A lesson from the Bufotes viridis toad. Zoology 2018, 130, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Johansson, F.; Richter-Boix, A.; Gomez-Mestre, I. Morphological consequences of developmental plasticity in Rana temporaria are not accommodated into among-population or among-species variation. Evol. Biol. 2016, 43, 242–256. [Google Scholar] [CrossRef]
- Rohlf, F.J. TPS Dig 1.31 and TPS Relative Wards Software; State University of New York: Stony Brook, NY, USA, 2001. [Google Scholar]
- Lillywhite, H.B.; Shine, R.; Jacobson, E.; Denardo, D.; Gordon, M.S.; Navas, C.A.; Wang, T.; Seymour, R.S.; Storey, K.B.; Heatwole, H.; et al. Anesthesia and euthanasia of amphibians and reptiles used in scientific research: Should hypothermia and freezing be prohibited? BioScience 2017, 67, 53–61. [Google Scholar] [CrossRef]
- Takada, Y.; Noguchit, T.; Kayiyama, M. Superoxide dismutase in various tissues from rabbits bearing the Vx-2 carcinoma in the maxillary sinus. Cancer Res. 1982, 42, 4233–4235. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Tamura, M.; Oshino, N.; Chance, B. Some characteristics of hydrogen- and alkylhydroperoxides metabolizing systems in cardiac tissue. J. Biochem. 1982, 92, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974, 30, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Rehncrona, S.; Smith, D.S.; Akesson, B.; Westerberg, E.; Siesjö, B.K. Peroxidative changes in brain cortical fatty acids and phospholipids, as characterized during Fe2+ and ascorbic acid stimulated lipid peroxidation in vitro. J. Neurochem. 1980, 34, 1630–1638. [Google Scholar] [CrossRef]
- STATISTICA (Data Analysis Software System); Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007; Available online: www.statsoft.de (accessed on 22 December 2020).
- Addinsoft, XLSTAT. Data Analysis and Statistics Software for Microsoft Excel; Addinsoft: Paris, France, 2015. [Google Scholar]
- Richter-Boix, A.; Tejedo, M.; Rezende, E.L. Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecol. Evol. 2011, 1, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Böll, S. Ephemere Laichgewässer: Anpassungsstrategien und Physiologische Zwänge der Gelbbauchunke (Bombina Variegata) in Einem Lebensraum mit Unvorhersehbarem Austrocknungsrisiko. Ph.D. Thesis, Bayerische Julius-Maximilians-Universität Würzburg, Würzburg, Germany, 2002. (In German). [Google Scholar]
- Hourdry, J.; L’Hermite, A.; Ferrand, R. Changes in the digestive tract and feeding behavior of anuran amphibians during metamorphosis. Physiol. Zool. 1996, 69, 219–251. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Cai, L.; Brown, D.D. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc. Natl. Acad. Sci. USA 2005, 102, 3720–3725. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Dausmann, K.H.; Reinhardt, S.; Robinson, T.; Sabatino, N.M.; Peck, M.A.; Glos, J. Endocrine disruption alters developmental energy allocation and performance in Rana temporaria. Integr. Comp. Biol. 2019, 59, 70–88. [Google Scholar] [CrossRef]
- Petrović, T.G.; Vučić, T.Z.; Nikolić, S.Z.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Faggio, C.; Prokić, M.D. The effect of shelter on oxidative stress and aggressive behavior in crested newt larvae (Triturus spp.). Animals 2020, 10, 603. [Google Scholar] [CrossRef]
- Prokić, M.D.; Petrović, T.G.; Despotović, S.G.; Vučić, T.; Gavrić, J.P.; Radovanović, T.B.; Gavrilović, B.R. The effect of short-term fasting on the oxidative status of larvae of crested newt species and their hybrids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 251, 110819. [Google Scholar] [CrossRef] [PubMed]
- Crespi, E.J.; Warne, R.W. Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 843–863. [Google Scholar] [CrossRef]
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Morita, S. Effects of feeding and fasting on hepatolobular distribution of glutathione and cadmium-induced hepatotoxicity. Toxicology 1992, 75, 97–107. [Google Scholar] [CrossRef]
- Orlofske, S.A.; Hopkins, W.A. Energetics of metamorphic climax in the pickerl frog (Lithobates palustris). Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2009, 154, 191–196. [Google Scholar] [CrossRef] [PubMed]
- De Block, M.; Stoks, R. Compensatory growth and oxidative stress in a damselfly. Proc. R. Soc. Lond. B Biol. Sci. 2008, 275, 781–785. [Google Scholar] [CrossRef]
- Paterson, P.G.; Lyon, A.W.; Kamencic, H.; Andersen, L.B.; Juurlink, B.H. Sulfur amino acid deficiency depresses brain glutathione concentration. Nutr. Neurosci. 2001, 4, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Eikenaar, C.; Isaksson, C.; Hegemann, A. A hidden cost of migration? Innate immune function versus antioxidant defense. Ecol. Evol. 2018, 8, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; Stoks, R. Rapid larval development under time stress reduces adult lifespan through increasing oxidative damage. Funct. Ecol. 2018, 32, 1036–1046. [Google Scholar] [CrossRef]
- Mahapatra, P.K.; Mohanty-Hejmadi, P.; Chainy, G.B. Changes in oxidative stress parameters and acid phosphatase activity in the pre-regressing and regressing tail of Indian jumping frog Polypedates maculatus (Anura, Rhacophoridae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 281–288. [Google Scholar] [CrossRef]
- Dalton, T.P.; Chen, Y.; Schneider, S.N.; Nebert, D.W.; Shertzer, H.G. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004, 37, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
| Parameter | Effects | df | F | p |
|---|---|---|---|---|
| SOD | treatment | 1 | 0.004 | 0.94833 |
| - | stage | 2 | 9.007 | 0.00036 |
| - | treatment × stage | 2 | 2.573 | 0.08444 |
| CAT | treatment | 1 | 15.944 | 0.00017 |
| - | stage | 2 | 8.975 | 0.00037 |
| - | treatment × stage | 2 | 6.210 | 0.00348 |
| GSH-Px | treatment | 1 | 8.953 | 0.00406 |
| - | stage | 2 | 8.113 | 0.00078 |
| - | treatment × stage | 2 | 7.127 | 0.00170 |
| GSH | treatment | 1 | 18.382 | 0.00006 |
| - | stage | 2 | 11.369 | 0.00006 |
| - | treatment × stage | 2 | 1.410 | 0.25197 |
| GST | treatment | 1 | 1.421 | 0.23781 |
| - | stage | 2 | 0.323 | 0.72505 |
| - | treatment × stage | 2 | 3.410 | 0.03944 |
| GR | treatment | 1 | 0.919 | 0.34136 |
| - | stage | 2 | 41.668 | <0.00001 |
| - | treatment × stage | 2 | 0.171 | 0.84298 |
| SH | treatment | 1 | 0.091 | 0.76290 |
| - | stage | 2 | 7.638 | 0.00109 |
| - | treatment × stage | 2 | 3.392 | 0.04009 |
| LPO | treatment | 1 | 14.118 | 0.00054 |
| - | stage | 2 | 20.676 | <0.00001 |
| - | treatment × stage | 2 | 1.113 | 0.33852 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, T.G.; Kijanović, A.; Kolarov Tomašević, N.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Vukov, T.; Faggio, C.; Prokić, M.D. Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters. Animals 2021, 11, 953. https://doi.org/10.3390/ani11040953
Petrović TG, Kijanović A, Kolarov Tomašević N, Gavrić JP, Despotović SG, Gavrilović BR, Radovanović TB, Vukov T, Faggio C, Prokić MD. Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters. Animals. 2021; 11(4):953. https://doi.org/10.3390/ani11040953
Chicago/Turabian StylePetrović, Tamara G., Ana Kijanović, Nataša Kolarov Tomašević, Jelena P. Gavrić, Svetlana G. Despotović, Branka R. Gavrilović, Tijana B. Radovanović, Tanja Vukov, Caterina Faggio, and Marko D. Prokić. 2021. "Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters" Animals 11, no. 4: 953. https://doi.org/10.3390/ani11040953
APA StylePetrović, T. G., Kijanović, A., Kolarov Tomašević, N., Gavrić, J. P., Despotović, S. G., Gavrilović, B. R., Radovanović, T. B., Vukov, T., Faggio, C., & Prokić, M. D. (2021). Effects of Desiccation on Metamorphic Climax in Bombina variegata: Changes in Levels and Patterns of Oxidative Stress Parameters. Animals, 11(4), 953. https://doi.org/10.3390/ani11040953










