Application of Ultrasound Images Texture Analysis for the Estimation of Intramuscular Fat Content in the Longissimus Thoracis Muscle of Beef Cattle after Slaughter: A Methodological Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Ultrasonographic and Chemical Evaluation of the Meat Samples
2.3. Ultrasonographic Features Extraction and Selection
2.4. Statistical Analysis
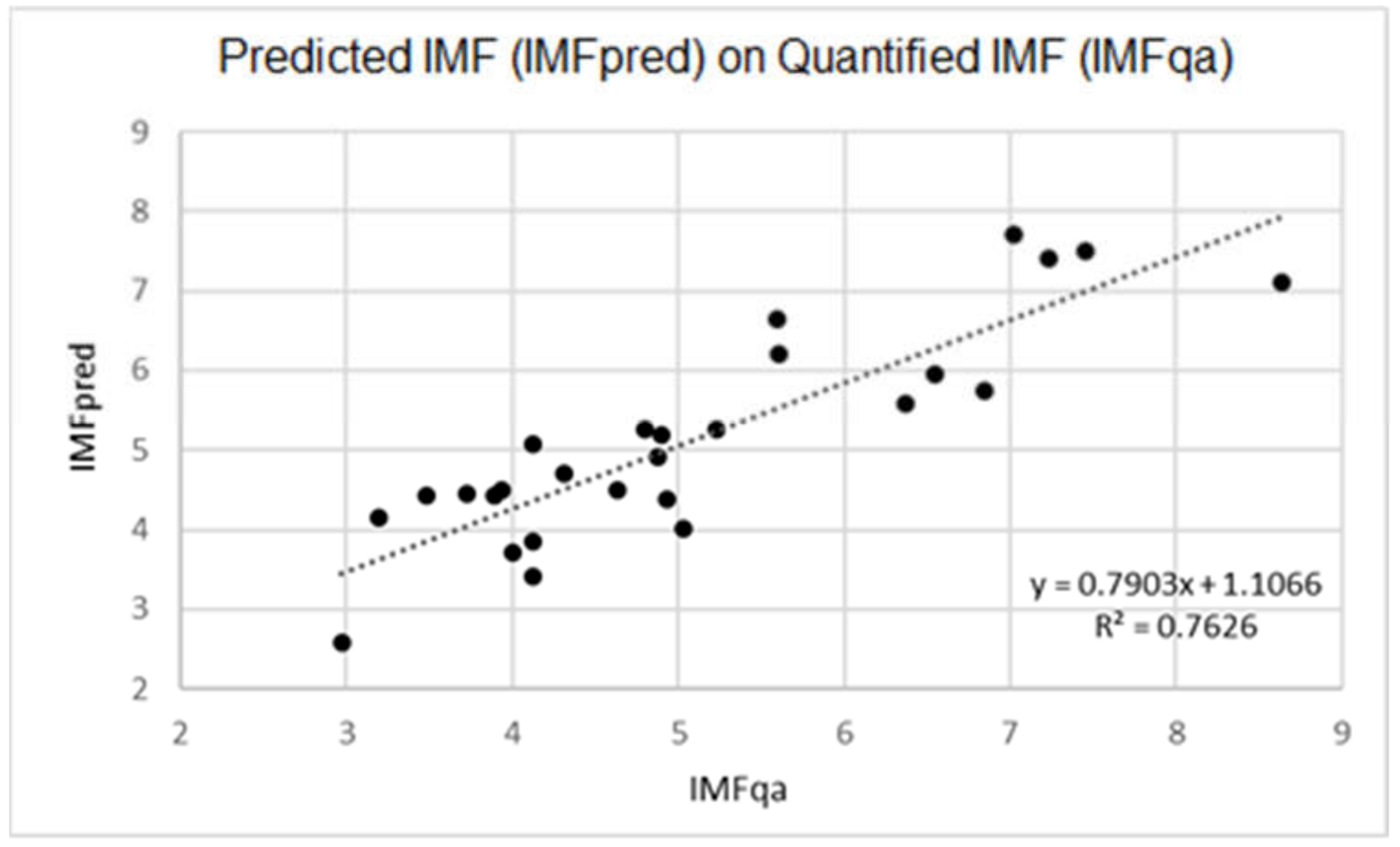
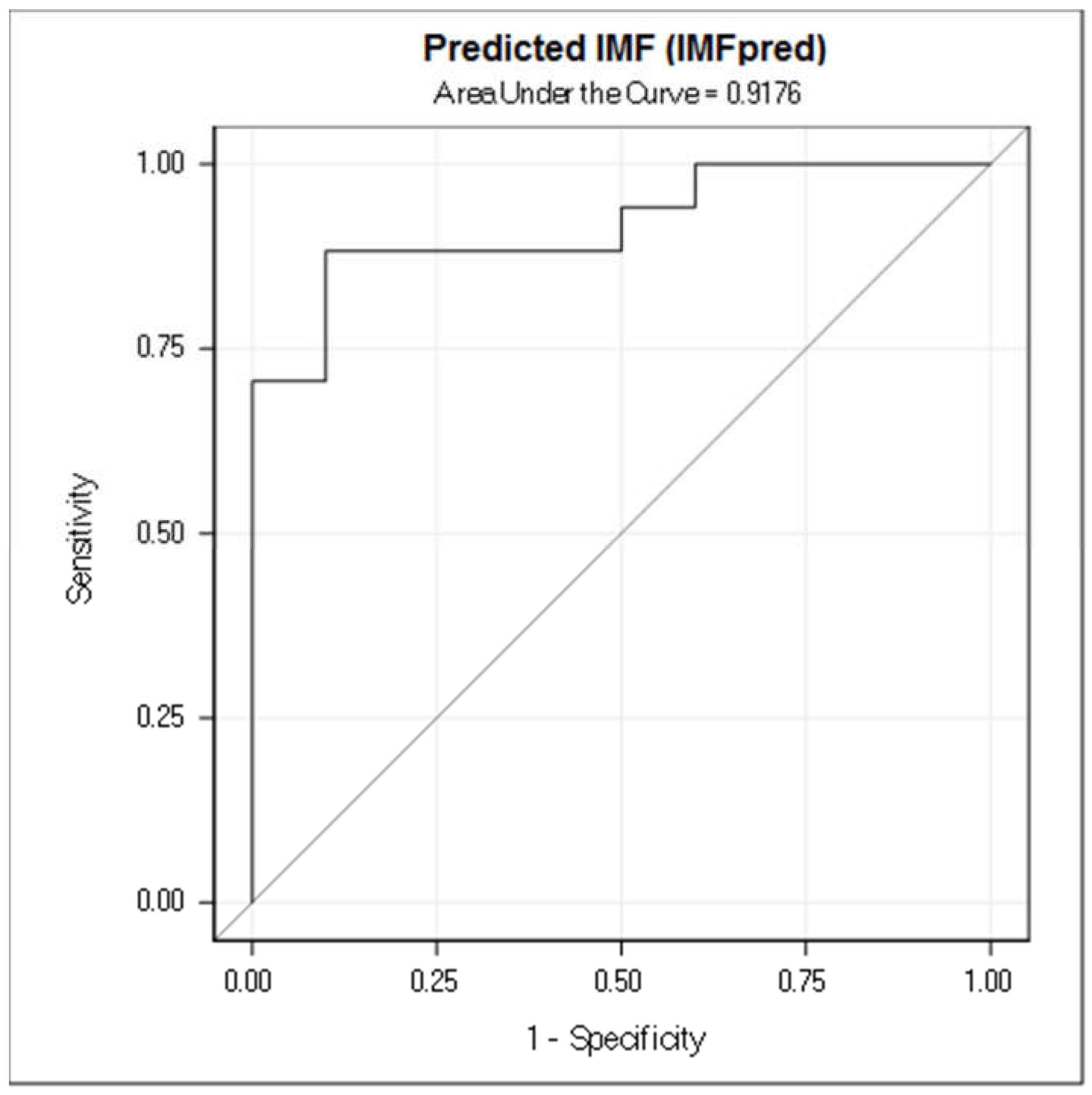
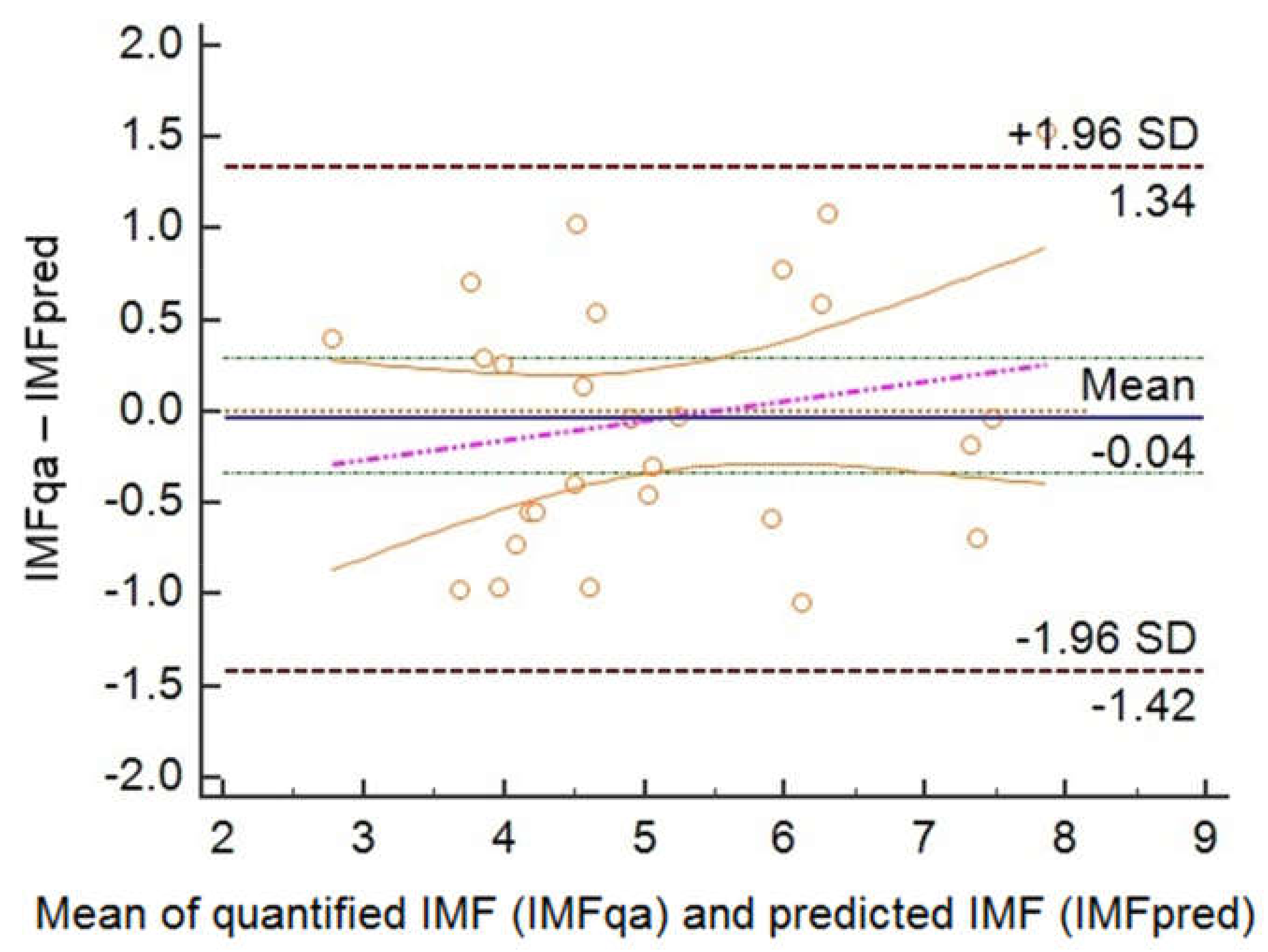
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; McCarthy, M.; Resconi, V.C.; Troy, D. Meat consumption: Trends and quality matters. Meat Sci. 2014, 98, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.C.; Carpenter, Z.L.; Cross, H.R.; Murphey, C.E.; Abraham, H.C.; Savell, J.W.; Davis, G.W.; Berry, B.W.; Parrish, F.C. Relationship of Usda Marbling Groups to Palatability of Cooked Beef. J. Food Qual. 1985, 7, 289–308. [Google Scholar] [CrossRef]
- Savell, J.W.; Cross, H.R.; Smith, G.C. Percentage Ether Extractable Fat and Moisture Content of Beef Longissimus Muscle as Related to USDA Marbling Score. J. Food Sci. 1986, 51, 838–839. [Google Scholar] [CrossRef]
- Cheng, W.; Cheng, J.-H.; Sun, D.-W.; Pu, H. Marbling Analysis for Evaluating Meat Quality: Methods and Techniques. Compr. Rev. Food Sci. Food Saf. 2015, 14, 523–535. [Google Scholar] [CrossRef]
- Dow, D.L.; Wiegand, B.R.; Ellersieck, M.R.; Lorenzen, C.L. Prediction of fat percentage within marbling score on beef longissimus muscle using 3 different fat determination methods1. J. Anim. Sci. 2011, 89, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Indurain, G.; Carr, T.; Goñi, M.; Insausti, K.; Beriain, M. The relationship of carcass measurements to carcass composition and intramuscular fat in Spanish beef. Meat Sci. 2009, 82, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Allen, P. New methods for grading beef and sheep carcasses. In Evaluation of Carcass and Meat Quality in Cattle and Sheep; Lazzaroni, C., Gigli, S., Gabiña, D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2007; pp. 39–48. [Google Scholar]
- Fukuda, O.; Nabeoka, N.; Miyajima, T. Estimation of marbling score in live cattle based on dynamic ultrasound image using a neural network. In Proceedings of the 19th International Conference on Systems, Signals and Image Processing IWSSIP 2012, Vienna, Austria, 11–13 April 2012. [Google Scholar]
- Emerson, M.R.; Woerner, D.R.; Belk, K.E.; Tatum, J.D. Effectiveness of USDA instrument-based marbling measurements for categorizing beef carcasses according to differences in longissimus muscle sensory attributes1. J. Anim. Sci. 2013, 91, 1024–1034. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, D.M. Objective on-line assessment of marbling: A brief review. Aust. J. Exp. Agric. 2004, 44, 681–685. [Google Scholar] [CrossRef]
- Maeda, S.; Grose, J.; Kato, K.; Kuchida, K. Comparing AUS-MEAT marbling scores using image analysis traits to estimate genetic parameters for marbling of Japanese Black cattle in Australia. Anim. Prod. Sci. 2014, 54, 557. [Google Scholar] [CrossRef]
- Buczinski, S. Cardiovascular Ultrasonography in Cattle. Veter Clin. N. Am. Food Anim. Pract. 2009, 25, 611–632. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Gianesella, M.; Morgante, M.; Armato, L.; Bonato, O.; Fiore, E. Ultrasonographic alterations of bovine claws sole soft tissues associated with claw horn disruption lesions, body condition score and locomotion score in Holstein dairy cows. Res. Veter. Sci. 2020, 131, 146–152. [Google Scholar] [CrossRef]
- Fiore, E.; Perillo, L.; Morgante, M.; Giudice, E.; Contiero, B.; Curone, G.; Manuali, E.; Pavone, S.; Piccione, G.; Gianesella, M. Ultrasonographic measurement of liver, portal vein, hepatic vein and perivisceral adipose tissue in high-yielding dairy cows with fatty liver during the transition period. J. Dairy Res. 2018, 85, 431–438. [Google Scholar] [CrossRef]
- Brethour, J.R. Relationship of ultrasound speckle to marbling score in cattle. J. Anim. Sci. 1990, 68, 2603–2613. [Google Scholar] [CrossRef]
- Whittaker, A.D.; Park, B.; Thane, B.R.; Miller, R.K.; Savell, J.W. Principles of ultrasound and measurement of intramuscular fat. J. Anim. Sci. 1992, 70, 942–952. [Google Scholar] [CrossRef] [Green Version]
- Brethour, J.R. Estimating marbling score in live cattle from ultrasound images using pattern recognition and neural network procedures1. J. Anim. Sci. 1994, 72, 1425–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, E.; Fabbri, G.; Gallo, L.; Morgante, M.; Muraro, M.; Boso, M.; Gianesella, M. Application of texture analysis of b-mode ultrasound images for the quantification and prediction of intramuscular fat in living beef cattle: A methodological study. Res. Veter. Sci. 2020, 131, 254–258. [Google Scholar] [CrossRef]
- Boccard, R.; Buchter, L.; Casteels, E.; Cosentino, E.; Dransfield, E.; Hood, D.; Joseph, R.; MacDougall, D.; Rhodes, D.; Schön, I.; et al. Procedures for measuring meat quality characteristics in beef production experiments. Report of a working group in the commission of the European communities’ (CEC) beef production research programme. Livest. Prod. Sci. 1981, 8, 385–397. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Wu, Z.; Matsui, O.; Kitao, A.; Kozaka, K.; Koda, W.; Kobayashi, S.; Ryu, Y.; Minami, T.; Sanada, J.; Gabata, T. Hepatitis C Related Chronic Liver Cirrhosis: Feasibility of Texture Analysis of MR Images for Classification of Fibrosis Stage and Necroinflammatory Activity Grade. PLoS ONE 2015, 10, e0118297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banzato, T.; Fiore, E.; Morgante, M.; Manuali, E.; Zotti, A. Texture analysis of B-mode ultra-sound images to stage hepatic lipidosis in the dairy cow: A methodological study. Res. Vet. Sci. 2016, 108, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Materka, A.; Strzelecki, M.; Szczypinski, P.M. MaZda Manual. 2006. Available online: http://www.eletel.p.lodz.pl/programy/mazda/download/mazda_manual.pdf (accesed on 12 April 2012).
- Liu, Y.; Aneshansley, D.J.; Stouffer, J.R. Autocorrelation of Ultrasound Speckle and Its Relationship to Beef Marbling. Trans. ASAE 1993, 36, 971–977. [Google Scholar] [CrossRef]
- Izquierdo, M.M.; Amin, V.; Wilson, D.E.; Rouse, G.H. Models to predict intramuscular fat percentage in live beef animals using real time ultrasound and image parameters: Report on data from 1991−1994. Beef Research Report, Iowa State Univ. Ames. A. S. Leaflet R1324. Beef Res. Rep. 1996, 1, 3–6. [Google Scholar]
- Herring, W.O.; Kriese, L.A.; Bertrand, J.K.; Crouch, J. Comparison of four real-time ultrasound systems that predict intramuscular fat in beef cattle. J. Anim. Sci. 1998, 76, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Hassen, A.; Wilson, D.E.; Amin, V.R.; Rouse, G.H.; Hays, C.L. Predicting percentage of intramuscular fat using two types of real-time ultrasound equipment. J. Anim. Sci. 2001, 79, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Aass, L.; Gresham, J.; Klemetsdal, G. Prediction of intramuscular fat by ultrasound in lean cattle. Livest. Sci. 2006, 101, 228–241. [Google Scholar] [CrossRef]
- Aass, L.; Fristedt, C.-G.; Gresham, J. Ultrasound prediction of intramuscular fat content in lean cattle. Livest. Sci. 2009, 125, 177–186. [Google Scholar] [CrossRef]
- Chambaz, M.R.L.; Scheeder, M.; Kreuzer, P.; Dufey, A. Meat quality of Angus, Simmental, Charolais and Limousin steers compared at the same intramuscular fat content. Meat Sci. 2003, 63, 491–500. [Google Scholar] [CrossRef]
- Cafferky, J.; Hamill, R.M.; Allen, P.; O’Doherty, J.V.; Cromie, A.; Sweeney, T. Effect of Breed and Gender on Meat Quality of M. longissimus thoracis et lumborum Muscle from Crossbred Beef Bulls and Steers. Foods 2019, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Beak, S.-H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [Green Version]

| Feed Ingredients | Total Dry Matter (%) |
|---|---|
| Maize silage | 33.2 |
| Corn mash | 13.63 |
| Corn gluten feed | 9.14 |
| Maize meal | 10.78 |
| Soybean meal 44 | 2.76 |
| Sugar beet pulps | 9.29 |
| Wheat straw | 9.2 |
| Protein, vitamin, and mineral premix 1 | 12 |
| Chemical Composition | |
| Dry matter (%) | 54.02 |
| Crude protein | 13.85 |
| Ether extract | 3.29 |
| Ash | 5.84 |
| Neutral detergent fiber | 37.22 |
| Non-fiber carbohydrates | 31.15 |
| Texture Parameter | Texture Category | Group 1 | Group 2 | Group 3 |
|---|---|---|---|---|
| GrKurtosis | Gradient | 2.14 ± 0.35 a | 1.38 ± 0.83 b | 3.44 ± 1.43 a |
| Teta2 | Autoregressive model | −0.43 ± 0.03 a | −0.47 ± 0.03 b | −0.47 ± 0.04 b |
| Teta4 | Autoregressive model | 0.09 ± 0.02 | 0.10 ± 0.02 | 0.09 ± 0.03 |
| S(2,2)InvDfMom | Co-occurrence matrix | 0.22 ± 0.02 | 0.23 ± 0.02 | 0.21 ± 0.02 |
| S(3,−3)Contrast | Co-occurrence matrix | 42.13 ± 9.72 | 40.47 ± 16.46 | 53.29 ± 20.54 |
| S(4,−4)DifEntrp | Co-occurrence matrix | 1.17 ± 0.05 | 1.15 ± 0.08 | 1.21 ± 0.08 |
| 45dgr_ShrtREmp | Run-length matrix | 0.91 ± 0.01 | 0.90 ± 0.01 | 0.91 ± 0.01 |
| Group 1 | Group 2 | Group 3 | ||||
|---|---|---|---|---|---|---|
| IMFqa | IMFpred | IMFqa | IMFpred | IMFqa | IMFpred | |
| Mean | 3.76 | 4.06 | 4.99 | 5.10 | 7.16 | 6.71 |
| SD | 0.41 | 0.71 | 0.40 | 0.81 | 0.75 | 0.91 |
| Maximum value | 4.12 | 5.08 | 5.61 | 6.63 | 8.64 | 7.71 |
| Minimum value | 2.97 | 2.57 | 4.31 | 4.00 | 6.37 | 5.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, G.; Gianesella, M.; Gallo, L.; Morgante, M.; Contiero, B.; Muraro, M.; Boso, M.; Fiore, E. Application of Ultrasound Images Texture Analysis for the Estimation of Intramuscular Fat Content in the Longissimus Thoracis Muscle of Beef Cattle after Slaughter: A Methodological Study. Animals 2021, 11, 1117. https://doi.org/10.3390/ani11041117
Fabbri G, Gianesella M, Gallo L, Morgante M, Contiero B, Muraro M, Boso M, Fiore E. Application of Ultrasound Images Texture Analysis for the Estimation of Intramuscular Fat Content in the Longissimus Thoracis Muscle of Beef Cattle after Slaughter: A Methodological Study. Animals. 2021; 11(4):1117. https://doi.org/10.3390/ani11041117
Chicago/Turabian StyleFabbri, Giorgia, Matteo Gianesella, Luigi Gallo, Massimo Morgante, Barbara Contiero, Michele Muraro, Matteo Boso, and Enrico Fiore. 2021. "Application of Ultrasound Images Texture Analysis for the Estimation of Intramuscular Fat Content in the Longissimus Thoracis Muscle of Beef Cattle after Slaughter: A Methodological Study" Animals 11, no. 4: 1117. https://doi.org/10.3390/ani11041117
APA StyleFabbri, G., Gianesella, M., Gallo, L., Morgante, M., Contiero, B., Muraro, M., Boso, M., & Fiore, E. (2021). Application of Ultrasound Images Texture Analysis for the Estimation of Intramuscular Fat Content in the Longissimus Thoracis Muscle of Beef Cattle after Slaughter: A Methodological Study. Animals, 11(4), 1117. https://doi.org/10.3390/ani11041117







