Simple Summary
Leptospirosis is caused by pathogenic spirochaetes of the genus Leptospira. Humans can become infected with these bacteria through direct contact with urine from infected animals or indirectly through interaction with a urine contaminated environment. Among wildlife species, rodents are considered the primary reservoir hosts for leptospirosis in rural and urban environments. Epidemiological data, regarding leptospirosis in various wild species in Europe, suggest that these animals play a different role in leptospiral persistence. Unfortunately, studies on the presence and typing of Leptospira species in wild mammals are lacking in Sardinia. The aim of the present study was to investigate the prevalence of Leptospira species in wild mammals. Kidneys collected from carcasses were analyzed by culture and molecular testing. Greater positivity was found in hedgehogs, followed by weasels and rodents. The results obtained suggest that Sardinian fauna may play a possible sentinel role in the transmission cycle of leptospirosis to humans. Gathering this information in different wildlife species is crucial for better understanding of the epidemiology of leptospirosis and for the development of appropriate prevention measures.
Abstract
Leptospirosis is a global zoonosis caused by pathogenic species of Leptospira that infect a large spectrum of domestic and wild animals. This study is the first molecular identification, characterization, and phylogeny of Leptospira strains with veterinary and zoonotic impact in Sardinian wild hosts. All samples collected were cultured and analyzed by multiplex real time polymerase chain reaction (qPCR). Sequencing, phylogenetic analyses (based on rrs and secY sequences), and Multilocus Sequence Typing (MLST) based on the analysis of seven concatenated loci were also performed. Results revealed the detection of Leptospira DNA and cultured isolates in 21% and 4% of the samples examined, respectively. Sequence analysis of Leptospira positive samples highlighted the presence of the interrogans and borgpetersenii genospecies that grouped in strongly supported monophyletic clades. MLST analyses identified six different Sequence Types (ST) that clustered in two monophyletic groups specific for Leptospira interrogans, and L. borgpetersenii. This study provided about the prevalence of leptospires in wild mammals in Sardinia, and increased our knowledge of this pathogen on the island. Monitoring Leptospira strains circulating in Sardinia will help clinicians and veterinarians develop strategic plans for the prevention and control of leptospiral infections.
1. Introduction
Leptospirosis, which is caused by pathogenic spirochaetes of the genus Leptospira, is a re-emerging zoonotic disease with veterinary and public health importance due to its cross-over between humans, domestic animals, and wildlife. Leptospirosis cases have been reported worldwide, in particular in the tropical regions of South and Southeast Asia [1], Africa [2], Western Pacific [3], and Central and North America [4]. It has been estimated that more than one million cases occur each year, including about 60,000 deaths [5].
The most recent advances in the description of Leptospira phylogeny have resulted from the study of Vincent et al. [6] in which new species of Leptospira belonging to subclades P1 and P2 have been classified on the basis of their pathogenicity. To date, at least 64 different Leptospira species have been validated worldwide, based on the average nucleotide identity (ANI) values of their genomes.
The main reservoirs of pathogenic Leptospira species are rodents; however, more recently, an increasing number of vertebrate and invertebrate hosts have also been reported to shed this pathogen in their urine. Among rodents, rats represent the main reservoir of pathogenic Leptospira, while wild and domestic mammals [7,8], livestock [9,10], amphibians [11], reptiles [12], ticks [13], and bats [14] also appear to play an important role in the spread of the leptospires. Most human infections occur following exposure to soil or water contaminated with the urine of reservoir animals [15].
In Italy, molecular studies have highlighted the presence of pathogenic Leptospira in wild boars [16] and pigs [17], porcupines [18], rodents [19,20,21], horses [22], dogs [23], and humans [24]. In Sardinia, recent isolations of Leptospira Bratislava and Pomona from wild boars [25] and marine mammals [26] suggest that these animals could potentially act as reservoirs of these serovars. To date, the current literature lacks information on the presence and spread of Leptospira species in Sardinian wildlife or the possible role that wild mammals play as maintenance or accidental hosts for these bacteria. The purpose of the present study was to (i) investigate the potential presence of Leptospira genomospecies and their sequence types (ST) in wild vertebrates, (ii) identify and genotype leptospires by 16S rRNA, secY, and Multilocus Sequence Typing (MLST), and (iii) reconstruct the phylogeny of the obtained sequences.
2. Materials and Methods
2.1. Sample Collection
Carcasses of small mammals, whose cause of death was related to traffic accidents or attacks from wildlife or domestic animals, were collected between January 2011 to December 2019 from 6 collection sites of North Sardinia (Sassarese, Angola, Gallura, Mantacuto, Nurra, and Goceano). Sardinia, the second largest island in the Mediterranean Sea with an area of 23,821 km2, is a region with a great naturalistic importance. The island is characterized by a wide diversity in geology, vegetation and landscape features, surrounded by mountains, forests, and green valleys covered by the typical Mediterranean maquis with Cistus, lentisk, myrtle, and rosemary shrubs. The landscape is also characterized by cultivated coastal plains, watercourses and rocky sheer coasts. Many areas are dedicated to rearing and grazing of sheep, goats, bovines, swine, and horses. Sardinia is also an extraordinary habitat for wild animals such as mouflons, Sardinian deer, wild pigs and foxes, and many birds. The field researchers were trained to sample the dead animals following predetermined guidelines. The carcasses were trans-ported at 4 °C to the laboratory where the general body condition of each carcass was evaluated, and only those animals that did not show obvious signs of deterioration were analyzed. Additionally, nutritional conditions, size, age, and sex of each animal was evaluated before the necropsy. Decomposed carcasses were not included in this study. A total of 387 carcasses were identified phenotypically by an expert veterinarian and included in the study [27]. Dead animals were necropsied and the kidneys were collected under sterile conditions from each animal. Specifically, 25 mg of tissue extracted between the cortical and medullary areas was immediately used for culture, the other was frozen at −20 °C for molecular investigation and characterization of leptospires.
2.2. Leptospira spp. Isolation
In order to evaluate the presence or the potential growth of leptospires, collected kidneys were homogenized by using a Stomacher bag (bag filter Avantor®, Lutterworth, UK) and inoculated into a sterile tube (Corning—Falcon®, Corning, NY, USA) (by using a sterile pipette) containing the commercial semi-solid Ellinghausen-McCullough-Johnson-Harris (EMJH) medium with EMJH enrichment (DifcoTM, BD, Franklin Lakes, NJ, USA), supplemented with 5-Fluorouracil (5-FU; 2 g/L). The media was incubated aerobically at 28 °C and the growth of leptospires was examined under a dark-field microscope weekly, over a period of 3 months for the presence or potential growth of leptospires. Samples that failed to show any evidence of growth after 3 months were considered negative and were discarded [28].
Positive cultures were subjected to purification. Briefly, exceeding nucleotides and primers were inactivated by using the Applied Biosystems™ CleanSweep™ PCR Purification Reagent (Life Technologies Europe BV, Monza MB, Italy), according to vendor’s recommendations. Pure isolates, free of contaminants, were used for serological and molecular identification.
2.3. Genomic DNA Extraction
DNA from the kidney was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Due to validate the extraction processes and all downstream steps, nuclease-free water and 10 fg of DNA extracted from Leptospira interrogans serovar Copenhageni (Fiocruz L1-130) were used as positive and negative controls, respectively. DNA extracted from each sample was stored at −20 °C until use.
2.4. Molecular Detection of Leptospira spp. by Multiplex qPCR, and Amplification of rrs and secY Genes
To discriminate between both pathogenic and non-pathogenic leptospires, all DNA samples were tested by multiplex qPCR using lipL32 and 16S rRNA partial target genes. More specifically, primers LipL32-45F (5′-AAGCATTACCGCTTGTGGTG-3′), LipL32-286R (5′-GAA CTCCCA TTT CAG CGA TT-3′), and the probeLipL32-189P (FAM-5′-AA AGC CAG GAC AAG CGCCG-3′-BHQ1) [29], were combined with primers 16S-P1 forward (5′-TAGTGAACGGGATTAGATAC-3′), and 16S-P2 reverse (5′-GGTCTACTTAATCCGTTAGG-3′) and probe 16S-Prob (Cy5-5′-AATCCACGCCCTAAACGTTGTCTAC-3′-BHQ2) that amplify 242 and 104 bp of the lipL32 and 16S rRNA genes, respectively. An internal control consisting of exogenous DNA added to the sample before the extraction phase. The qPCR was performed in a total volume of 20 μL containing 50 ng of Leptospira spp. genomic DNA, 250 nM of each of the forward and reverse primers, and 10 μL of 5× Master Mix QuantiFast Pathogen PCR + IC Kit (Qiagen, Milan, Italy). All the reactions were performed in duplicates on a 7500 Fast Real-Time PCR System (Applied Biosystems) under the following conditions: 95 °C for 5 min, followed by 45 cycles of denaturation at 95 °C for 15 s, and annealing and elongation for 30 s at 60 °C. A negative control (DNA extracted from water) and a positive control (DNA extracted from the reference strain of L. interrogans ATCC® BAA1198D5TM) were included in each PCR test.
Among all positive samples obtained by qPCR, only samples with a threshold cycle (Ct) values lower than or equal to 32 were tested for further analyses. Specifically, 6 kidney samples and 17 Leptospira isolates (Table 1) were analyzed with a set of primers that amplified a fragment of 541 bp of the 16S rRNA gene, and of 549 bp of the secY partial gene [30]. Negative and positive controls were included in each test, with a negative and a positive for every 20 samples tested. The PCR reactions were performed by using a T100 Thermal Cycler (Bio-Rad apparatus). PCR products were visualized by electrophoresis in 1.5% agarose gel stained with SYBR-Safe DNA Gel Stain (Invitrogen, Carlsbad, CA, USA), and examined under UV transillumination.

Table 1.
Origin of samples investigated in this study.
2.5. Sequencing and Phylogenetic Analyses
All rrs (16S rRNA gene) and secY positive amplicons were purified and directly sequenced by using an BigDye terminator cycle sequencing ready reaction kit (Life Technologies, UK). Sequences were edited with Chromas 2.2 (Technelysium, Helensvale, Australia), then aligned with Clustal X [31] in order to assign them to unique sequence types, and checked against the GenBank database with nucleotide blast (BLASTn) [32]. Multiple sequence alignments and sequence similarities were calculated using the Clustal W [33] and the identity matrix options of BioEdit [34], respectively. For phylogenetic analyses, the sequence types obtained in this study were aligned with a set of 22 sequences representing rrs and secY variability of the different species belonging to the genus Leptospira.
2.6. MLST Analysis of Leptospira Isolated Strains
In order to reveal Sequence Types (ST) of Leptospira isolates, MLST was performed using 7 housekeeping genes: pntA, sucA, tpiA, pfkB, mreA, glmU, and caiB [35]. Each allele and the allelic profiles (glmU-pntA-sucA-tpiA-pfkB-mreA-caiB) were submitted to the Leptospira database (http://pubmlst.org/leptospira, accessed on January 2021) to define the STs. Phylogenetic analysis was performed using MEGA6 software [36].
3. Results
3.1. Detection of Leptospira Exposure and Infection in Wild Mammals
A total of 387 carcasses of 15 different animal species belonging to Rodentia (n = 177 46%; 95% CI: 41–51%), Erinaceomorpha (n = 37 10%; 95% CI: 7–13%), Carnivora (n = 162 42%; 95% CI: 37–47%), and Lagomorpha (n = 11 3%; 95% CI: 1–5%) orders, were collected in this study (Table 2). The majority of the samples analyzed were adults (n = 340 88%; 95% CI: 85–91%). All samples did not show any macroscopic lesions compatible with Leptospira infection after pathologic examination post mortem. The 387 kidneys tested, 80 (21%; 95% CI 17–25%) samples belonging to 7 animal species, were positive for pathogenic Leptospira species upon amplification by using multiplex qPCR. Bacterial cultures revealed that 4% (n = 17/387; 95% CI: 2–6%) of the kidney samples were positive for Leptospira approximately after 60 days of incubation. All kidney cultures isolates exhibited Leptospira positivity after qPCR analyses. The results of qPCR and cultures are presented in Table 2.

Table 2.
Percentages of Leptospira spp. isolated from kidney samples from multiplex qPCR and culture from different host species in Sardinia.
3.2. Characterization of Leptospira Isolates
Sequencing results performed on the rrs and secY amplicons obtained from the 17 kidney cultures and the 6 samples (23 positive samples in total) from qPCR analyses produced clear sequencing signals, with an identity superior to 99% (Table 3). Among the rrs positive samples, the resulting BLASTn analysis revealed that 9 sequences were members of L. borgpetersenii group, 13 belonged to the L. interrogans group, and 1 sequence exhibited the highest homology with the intermediate L. johnsonii (100% identity). Samples positive for the Leptospira species by using 16S rRNA target gene were also positive when tested with the set of primers targeting the protein translocase secY subunit present in Leptospira species. Sequencing of the 23 secY amplicons revealed that 14 (61%; 95% CI: 41–81%) and 9 (39%; 95% CI: 19–59%) sequences were 99–100% similar to L. interrogans and L. borgpetersenii strains, respectively. The rrs and secY sequence types, the host origin of all sequences and BLASTn identity are shown in Table 3.

Table 3.
Designation, sequence type, and maximum identities of the rrs and secY gene sequence types identified in this study.
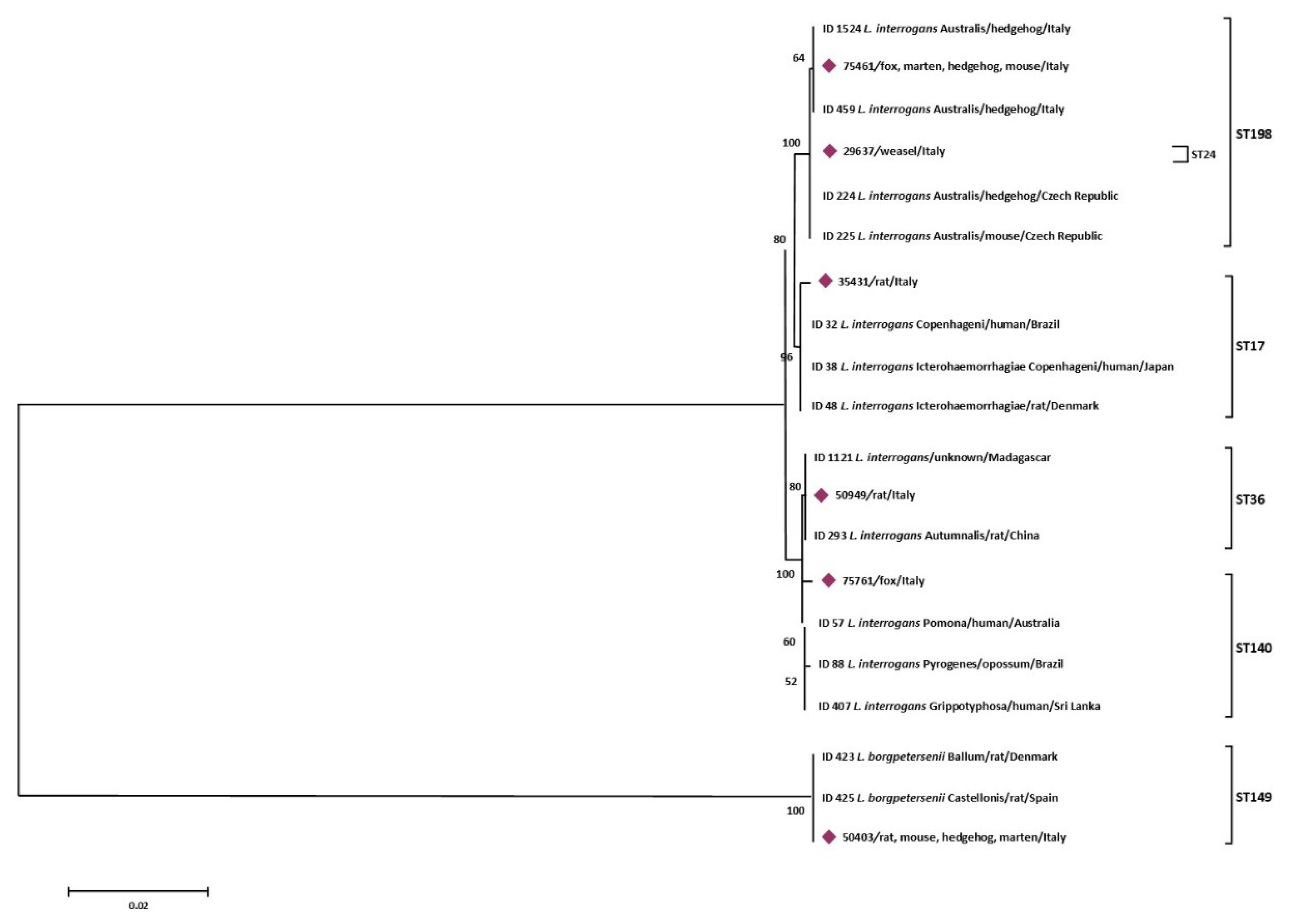
The MLST analysis of the 23 Leptospira strains, allowed to obtain 6 different sequence types (ST), belonging to ST149 (derived from 3 Apodemus sylvaticus, 1 Rattus rattus, 4 Erinaceus europaeus, and 1 Martes martes), ST198 (found in 1 Apodemus sylvaticus, 4 Erinaceus europaeus, 1 Martes martes, and 1 Vulpes vulpes). ST36 (from 1 Rattus rattus and 1 Rattus norvegicus), ST24 (from 1 Mustela nivalis), ST17 (from 1 Rattus rattus), and ST140 (from 1 Vulpes vulpes). MLST based on 7-loci scheme results obtained from the 23 Leptospira isolates are shown in Table 4.

Table 4.
Numbers of alleles and sequence types (ST) of 23 pathogenic Leptospira strains.
3.3. Phylogenetic Analysis
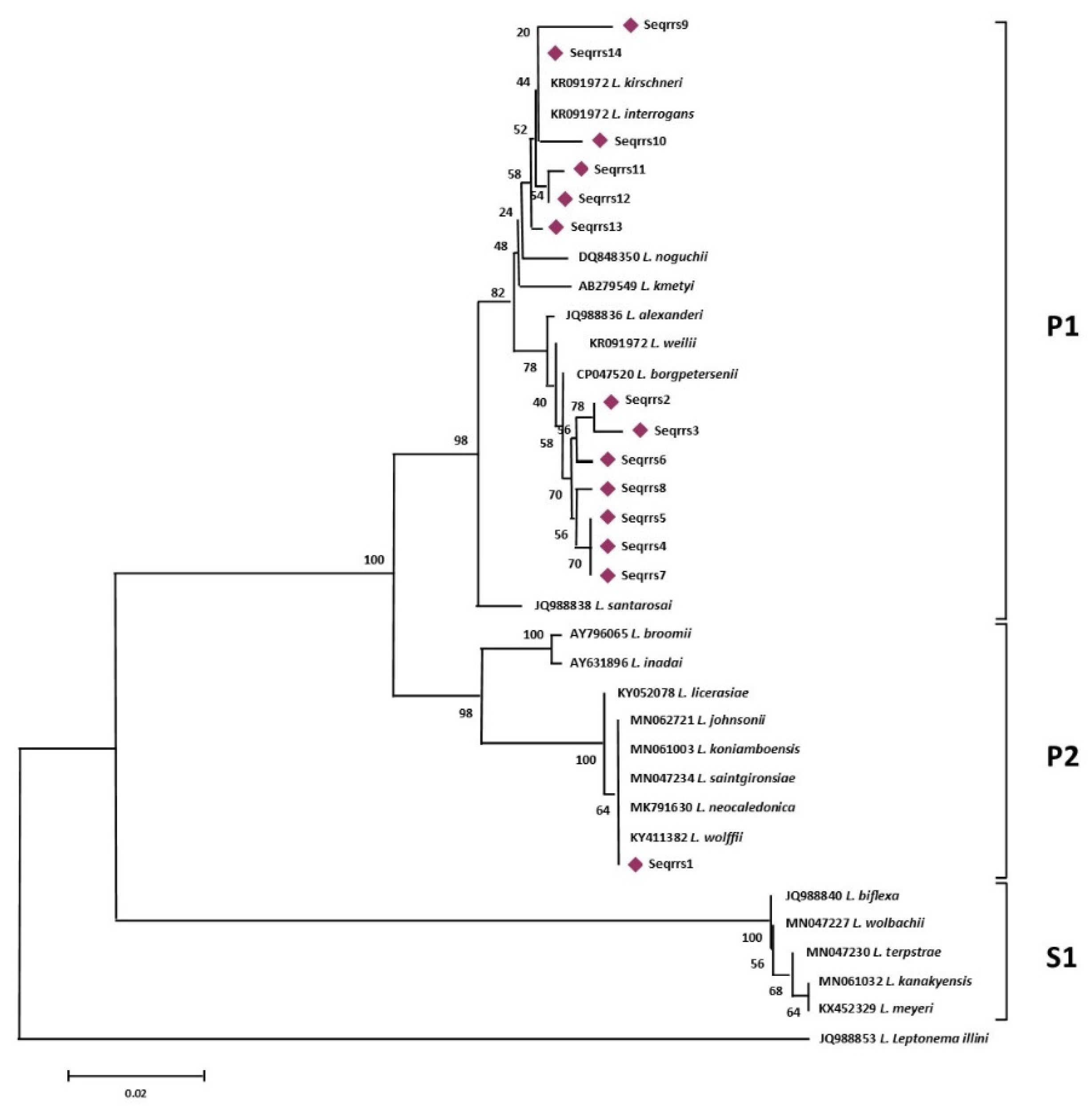
Phylogenetic analysis based on the alignment of the 14 rrs sequence types obtained in this study with the 22 Leptospira reference sequences (Figure 1), identified 3 main groups representative of the pathogenic, intermediate, and saprophytic Leptospira species.
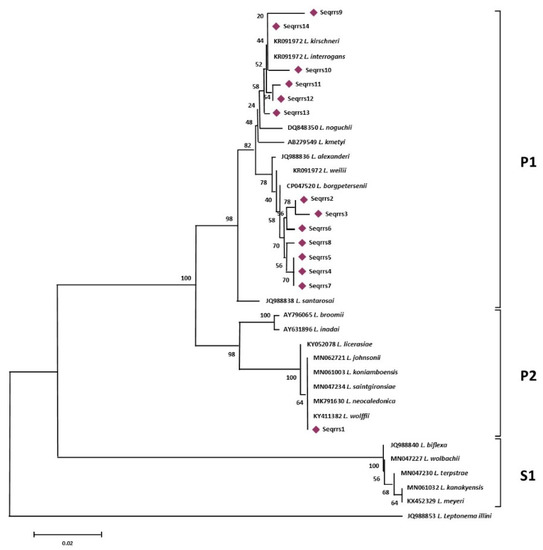
Figure 1.
rrs-based phylogenetic analyses of the sequence types generated in this study and of 22 sequences representative of the different species of the genus Leptospira. Evolutionary history was inferred using the Neighbor-Joining method. The optimal tree with the sum of branch length = 0.41105704 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches.
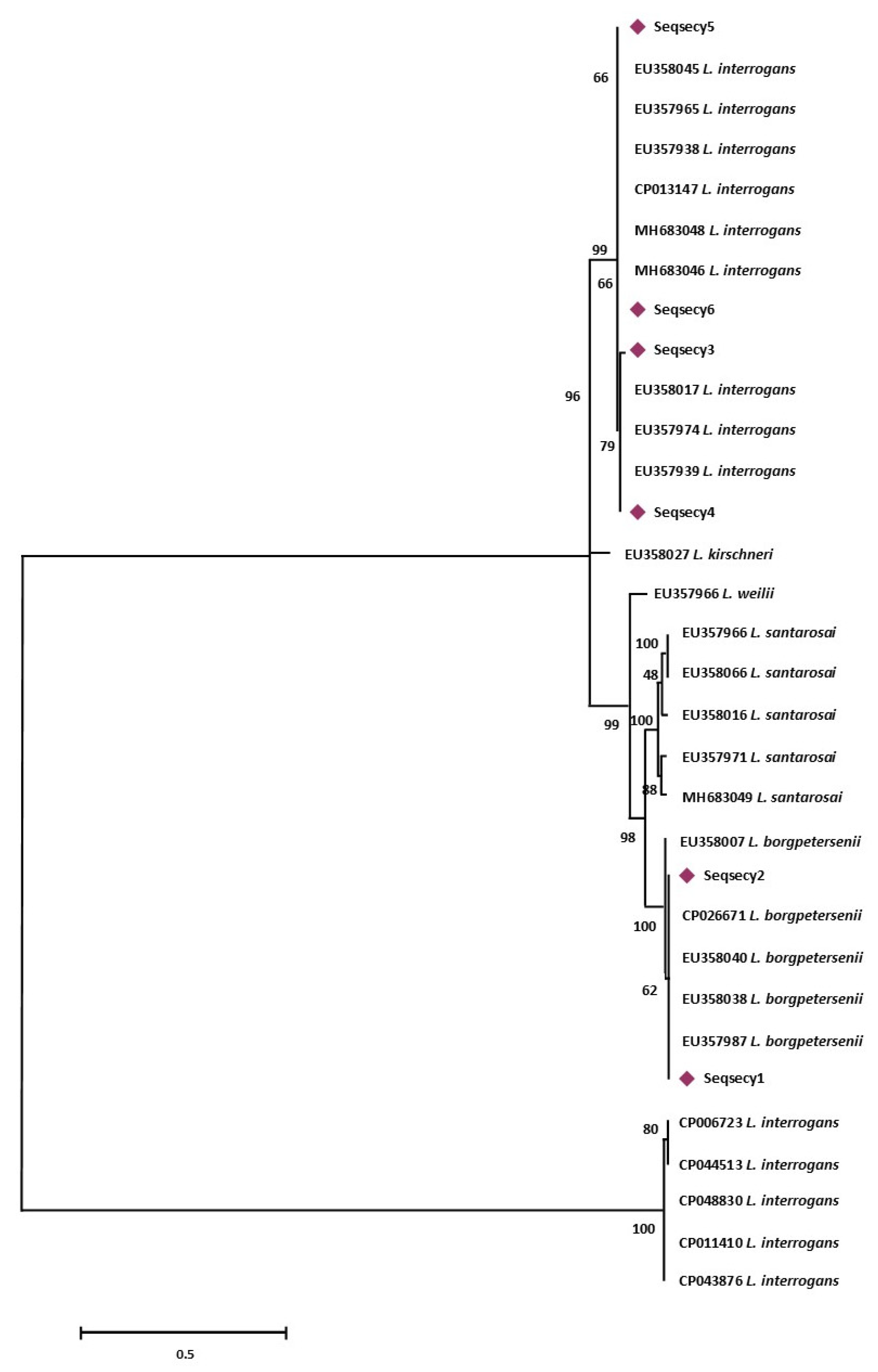
More specifically, sequence types named Seqrrs2, Seqrrs3, Seqrrs4, Seqrrs5, Seqrrs6, Seqrrs7, Seqrrs8, Seqrrs9 Seqrrs10, Seqrrs11, Seqrrs12, and Seqrrs13 grouped in a strongly supported clade with pathogenic Leptospira strains while Seqrrs1 was included in a separate clade including the intermediate Leptospira species. The phylogenetic trees obtained by aligning the six secY sequence types and the 6 STs resulted from MLST analyses with the Leptospira reference strains, indicated that all isolates from this study grouped with reference strains representative of L. interrogans and L. borgpetersenii, respectively. It indicated that all sequences here detected can be classified within the pathogenic Leptospira group. The sequence clusters obtained were statistically supported by bootstrap analyses (Figure 2 and Figure 3).
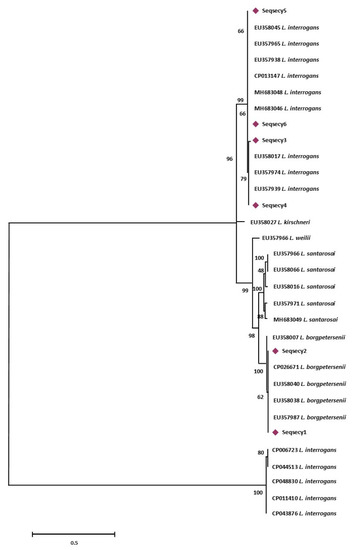
Figure 2.
Maximum Likelihood method based on the Tamura 3-parameter model. The analysis involved 32 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 486 positions in the final dataset. Statistical support for internal branches of tree was evaluated by bootstrapping with 1000 reiterations.
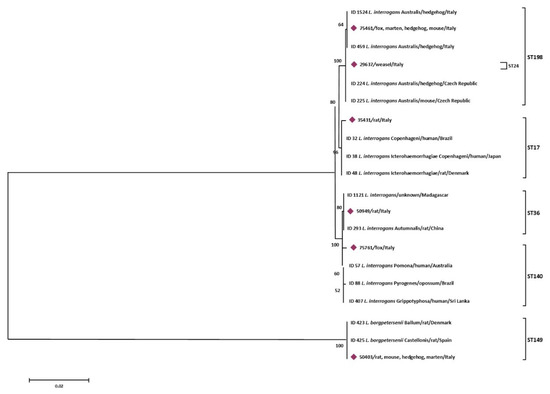
Figure 3.
Phylogenetic tree based on concatenated sequences of 7 housekeeping loci of the 6 STs obtained in this study was constructed by using the Maximum Likelihood method based on the Tamura 3-parameter model. The tree with the highest log likelihood (−6579,6990) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The analysis involved 20 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 3108 positions in the final dataset.
4. Discussion
The importance of rodents as reservoirs for a variety of Leptospira serovars has been widely studied in the world [37]. There is an increasing interest in monitoring of Leptospira spp. hosts, and studies on the prevalence of this pathogen in wild mammals are increasing over Europe. In Germany, 6% of animals tested positive for L. kirschneri and L. interrogans [38]. In France, studies conducted on reported serological positivity for L. interrogans, L. kirschneri, and L. borgpetersenii in 24 different mammalian species [39]. In Sardinia, studies on presence and typing of Leptospira species in wild mammals are still lacking.
This report examined circulating Leptospira strains in 15 different wild species, including rodents, using culture and DNA characterization tools. Our results indicate that all wild species examined are carriers of pathogenic Leptospira species in Sardinia. Pathogenic Leptospira were found with a frequency of 54% (95% CI: 38–70%) in hedgehogs, followed by mustelids with 40% (95% CI: 0–83%), end wild rodents with 21% (95% CI: 10–33%). The detection of L. interrogans serovar Australis and L. borgpetersenii serovar Ballum in hedgehogs was in agreement with other studies conducted in several European countries, including France [40], Italy [41], the Netherlands [42], and Scotland [43], which showed the presence of these Leptospira spp. from Erinaceomorphs. Moreover, pathogenic Leptospira were isolated from hedgehogs in France, as well as in China [44] recently. These findings show that hedgehogs could act as important source of pathogenic Leptospira spp. serogroup Australis and outline the importance of leptospirosis surveillance in this species. In this study, the presence of L. interrogans in the carnivore group was demonstrated for the first time on the island. Among the species analyzed was Vulpes vulpes ichnusae, a Sardinian species endemic to urban and peri-urban areas, including human environments. The hunting of this species is allowed on the island, and is regulated by the Regional Law number 23 of 1998. Foxes are known to be an important source of Leptospira in Europe, where the Grippotyphosa serogroup is known as the most frequently reported in Germany [45], and the Poi and Saxkoebing serogroups, and Sejroe are the most common in Poland [46]. The presence of pathogenic Leptospira in Sardinian wild foxes needs further investigation to clarify the role of wild carnivores as a reservoir of pathogenic Leptospira serovars on the island, as well as their epidemiological role in the zoonotic cycle. Still within the carnivore group, we also report the first molecular detection of L. borgpetersenii and L. interrogans from two species of Sardinian Mustela and one Martens. Together with the hedgehog and the fox, it is among the mammals most commonly hit by cars. Additionally, for these species the results obtained are in agreement with the studies conducted in France which show that the mustelid species have the highest risk of being infected by L. interrogans, L. borgpetersenii, and L. kirschneri [40]. This study also reveals the presence of L. interrogans in Sardinian rodents (Rattus norvegicus, Rattus rattus, and Apodemus sylvaticus). It has been known that wild rats (Rattus spp.) Are the most important sources of Leptospira infection, as they are abundant in urban and peri-domestic environments [39]. The brown rat is reported to be the primary host of L. interrogans related to the serogroup Icterohaemorrhagiae, which is responsible for the most severe forms of the disease in humans [47,48]. With the identification of 14 different rrs sequences, 6 for the secY gene, and 6 MLST profiles, our study reflects the wide diversity of Leptospira genotypes circulating in wildlife. Only 6 samples from biological matrix were added to the molecular analysis, the remaining 74 positive sample for Leptospira could not be amplified, most likely due to low DNA concentrations.
In the present study, the use of the rrs and secY genes represented a useful tool for detecting the Leptospira genomospecies in wild mammals and allows differentiating members of the pathogenic, intermediate and saprophytic Leptospira group. The 3 types obtained showed an obvious phylogenetic divergence from all recognized species belonging to the 3 clades of Leptospira and it was in accordance with previous studies [6,49]. Results obtained by using rrs and secY genes are the same as those obtained by MLST analyses except for those obtained from 1 Mustela nivalis. The partial ribosomal 16S gene sequence detected in this mammal hosts, was identical to that of a species already isolated from soil in Japan namely L. johnsonii and belonging to the intermediate clade [50]. However, the presence of this P2 intermediate species were not confirmed with secY and MLST analyses, indicating that the molecular identification of Leptospira strains needs the use of further genetic markers in order to confirm these results.
5. Conclusions
Our results show that several pathogenic strains of Leptospira are circulating in Sardinian fauna. Information on the possible role as sentinel or reservoirs of wild mammals is critical to understand the possible zoonotic potential of Leptospira. Therefore, the characterization of the genetic diversity of Leptospira strains is fundamental to designing epidemiological studies and control strategies for leptospirosis in the same area. Further studies are needed to better characterize isolates by analyzing more discriminative genes, and to identify the main reservoirs of Leptospira strains in Sardinia island.
Author Contributions
Conceptualization, I.P. and M.N.P.; methodology, I.P. and M.N.P.; validation, I.P. and V.C.; formal analysis, I.P.; investigation, I.P., B.P., M.N., A.P., and L.R.; resources, all authors; data curation, I.P. and M.N.P.; Writing—Original draft preparation, I.P. and V.C.; writing—Review and editing, all authors; visualization, I.P.; project administration, M.N.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Health of Italy, scientific project number IZS SA 02/11.
Institutional Review Board Statement
Ethics review and approval for this study were waived because the animals analyzed were found dead.
Acknowledgments
We wish to acknowledge all practitioners involved in this study for the help during the necropsy and processing of samples, and the staff of the IZS detached sections of Oristano, Nuoro, and Tortolì for sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wangdi, K.; Kasturiaratchi, K.; Nery, S.V.; Lau, C.L.; Gray, D.J.; Clements, A.C.A. Diversity of infectious aetiologies of acute undifferentiated febrile illnesses in south and Southeast Asia: A systematic review. BMC Infect. Dis. 2019, 19, 577. [Google Scholar] [CrossRef]
- Naidoo, K.; Moseley, M.; McCarthy, K.; Chingonzoh, R.; Lawrence, C.; Setshedi, G.M.; Frean, J.; Rossouw, J. Fatal Rodentborne Leptospirosis in Prison Inmates, South Africa, 2015. Emerg. Infect. Dis. 2020, 26, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Soo, Z.M.P.; Khan, N.A.; Siddiqui, R. Leptospirosis: Increasing importance in developing countries. Acta Trop. 2020, 201, 105183. [Google Scholar] [CrossRef] [PubMed]
- Llanos-Soto, S.; Najle, M.I.; Salgado, M.; González-Acuña, D. Evidence of Pathogenic Leptospira Infection in a Free-Ranging Andean Fox (Lycalopex culpaeus) from Central Chile. J. Wildl. Dis. 2019, 55, 958–960. [Google Scholar] [CrossRef]
- Thibeaux, R.; Girault, D.; Bierque, E.; Soupé-Gilbert, M.-E.; Rettinger, A.; Douyère, A.; Meyer, M.; Iraola, G.; Picardeau, M.; Goarant, C. Biodiversity of Environmental Leptospira: Improving Identification and Revisiting the Diagnosis. Front. Microbiol. 2018, 9, 816. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Khalid, M.K.N.M.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLOS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [PubMed]
- Arent, Z.J.; Gilmore, C.; Ayanz, J.M.S.-M.; Neyra, L.Q.; García-Peña, F.J. Molecular Epidemiology of Leptospira Serogroup Pomona Infections among Wild and Domestic Animals in Spain. EcoHealth 2017, 14, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.S.; Pinto, P.S.; Lilenbaum, W. A systematic review of Leptospirosis on wild animals in Latin America. Trop. Anim. Health Prod. 2018, 50, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, K.; Welcome, S.; Kenig, M.; Lim, B.; Rajeev, S. Epidemiology of Leptospira infection in livestock species in Saint Kitts. Trop. Anim. Health Prod. 2019, 51, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, J.; Zhang, T.; Qiu, H.; Li, Z.; Zhang, E.; Li, S.; Chang, Y.-F.; Guo, X.; Jiang, X.; et al. Genetic characteristics of pathogenic Leptospira in wild small animals and livestock in Jiangxi Province, China, 2002–2015. PLOS Negl. Trop. Dis. 2019, 13, e0007513. [Google Scholar] [CrossRef]
- Dezzutto, D.; Barbero, R.; Canale, G.; Acutis, P.L.; Biolatti, C.; Dogliero, A.; Mitzy, M.D.; Francone, P.; Colzani, A.; Bergagna, S.; et al. Detection of Leptospira spp. in Water Turtle (Trachemys scripta) Living in Ponds of Urban Parks. Vet. Sci. 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Rodamilans, G.M.; Fonseca, M.S.; Paz, L.N.; Fernandez, C.C.; Biondi, I.; Lira-Da-Silva, R.M.; Meyer, R.; Pinna, M.H.; Portela, R.D. Leptospira interrogans in wild Boa constrictor snakes from Northeast Brazil peri‑urban rainforest fragments. Acta Trop. 2020, 209, 105572. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fatla, A.; Zając, V.; Cisak, E.; Sroka, J.; Sawczyn, A.; Dutkiewicz, J. Leptospirosis as a tick-borne disease? Detection of Leptospira spp. in Ixodes ricinus ticks in eastern Poland. Ann. Agric. Environ. Med. 2012, 19, 656–659. [Google Scholar]
- Mateus, J.; Gómez, N.; Herrera-Sepúlveda, M.T.; Hidalgo, M.; Pérez-Torres, J.; Cuervo, C. Bats are a potential reservoir of pathogenic Leptospira species in Colombia. J. Infect. Dev. Ctries. 2019, 13, 278–283. [Google Scholar] [CrossRef]
- Adler, B.; Moctezuma, A.D.L.P. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Cilia, G.; Bertelloni, F.; Mignone, W.; Spina, S.; Berio, E.; Razzuoli, E.; Vencia, W.; Franco, V.; Cecchi, F.; Bogi, S.; et al. Molecular detection of Leptospira spp. in wild boar (Sus scrofa) hunted in Liguria region (Italy). Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101410. [Google Scholar] [CrossRef] [PubMed]
- Bertasio, C.; Papetti, A.; Scaltriti, E.; Tagliabue, S.; D’Incau, M.; Boniotti, M.B. Serological Survey and Molecular Typing Reveal New Leptospira Serogroup Pomona Strains among Pigs of Northern Italy. Pathogens 2020, 9, 332. [Google Scholar] [CrossRef]
- Coppola, F.; Cilia, G.; Bertelloni, F.; Casini, L.; D’Addio, E.; Fratini, F.; Cerri, D.; Felicioli, A. Crested Porcupine (Hystrix cristata L.): A New Potential Host for Pathogenic Leptospira Among Semi-Fossorial Mammals. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101472. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Di Bella, C.; Agnello, S.; Curro, V.; Vicari, D.; Vitale, F. Leptospira interrogans survey by PCR in wild rodents coming from different urban areas of Palermo, Italy. Rev. Cuba. Med. Trop. 2013, 59, 59–60. [Google Scholar]
- Pezzella, M.; Lillini, E.; Sturchio, E.; Ierardi, L.A.; Grassi, M.; Traditi, F.; Cristaldi, M. Leptospirosis survey in wild rodents living in urban areas of Rome. Ann. di Ig. Med. Prev. Comunita 2005, 16, 721–726. [Google Scholar]
- Bollo, E.; Pregel, P.; Gennero, S.; Pizzoni, E.; Rosati, S.; Nebbia, P.; Biolatti, B. Health status of a population of nutria (Myocastor coypus) living in a protected area in Italy. Res. Vet. Sci. 2003, 75, 21–25. [Google Scholar] [CrossRef]
- Vera, E.; Taddei, S.; Cavirani, S.; Schiavi, J.; Angelone, M.; Cabassi, C.S.; Schiano, E.; Quintavalla, F. Leptospira Seroprevalence in Bardigiano Horses in Northern Italy. Animals 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Zamagni, S.; Bertasio, C.; Boniotti, M.B.; Troìa, R.; Battilani, M.; Dondi, F. Identification of Serogroups Australis and Icterohaemorrhagiae in Two Dogs with a Severe Form of Acute Leptospirosis in Italy. Pathogens 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Agnello, S.; Chetta, M.; Amato, B.; Vitale, G.; Bella, C.D.; Vicari, D.; Presti, V.D. Human leptospirosis cases in Palermo Italy. The role of rodents and climate. J. Infect. Public Health 2018, 11, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Bertelloni, F.; Piredda, I.; Ponti, M.N.; Turchi, B.; Cantile, C.; Parisi, F.; Pinzauti, P.; Armani, A.; Palmas, B.; et al. Presence of pathogenic Leptospira spp. in the reproductive system and fetuses of wild boars (Sus scrofa) in Italy. PLoS Negl. Trop. Dis. 2020, 14, e0008982. [Google Scholar] [CrossRef] [PubMed]
- Piredda, I.; Palmas, B.; Noworol, M.; Tola, S.; Longheu, C.; Bertasio, C.; Scaltriti, E.; Denurra, D.; Cherchi, M.; Picardeau, M.; et al. Isolation of Leptospira interrogans from a Bottlenose Dolphin (Tursiops truncatus) in the Mediterranean Sea. J. Wildl. Dis. 2020, 56, 727. [Google Scholar] [CrossRef]
- Nagorsen, D.W. An Identification Manual to the Small Mammals of British Columbia; Ministry of Sustainable Resource Management Ministry of Water, Land and Air Protection Royal British Columbia Museum: Victoria, BC, Canada, 2002. [Google Scholar]
- Gleiser, C.A. The Laboratory Diagnosis of Leptospirosis. Am. J. Trop. Med. Hyg. 1955, 4, 158–159. [Google Scholar] [CrossRef]
- Stoddard, R.A.; Gee, J.E.; Wilkins, P.P.; McCaustland, K.; Hoffmaster, A.R. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009, 64, 247–255. [Google Scholar] [CrossRef]
- Ahmed, N.; Devi, S.M.; Valverde, M.D.L.A.; Vijayachari, P.; Machang’U, R.S.; Ellis, W.A.; Hartskeerl, R.A. Multilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira species. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 28. [Google Scholar] [CrossRef][Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.G.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A Single Multilocus Sequence Typing (MLST) Scheme for Seven Pathogenic Leptospira Species. PLOS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- de Faria, M.T.; Calderwood, M.S.; Athanazio, D.A.; McBride, A.J.; Hartskeerl, R.A.; Pereira, M.M.; Ko, A.I.; Reis, M.G. Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Trop. 2008, 108, 1–5. [Google Scholar] [CrossRef]
- Obiegala, A.; Woll, D.; Karnath, C.; Silaghi, C.; Schex, S.; Eßbauer, S.; Pfeffer, M. Prevalence and Genotype Allocation of Pathogenic Leptospira Species in Small Mammals from Various Habitat Types in Germany. PLoS Negl. Trop. Dis. 2016, 10, e0004501. [Google Scholar] [CrossRef]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira infection in rats: A literature review of global prevalence and distribution. PLoS Negl. Trop. Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef]
- Ayral, F.; Djelouadji, Z.; Raton, V.; Zilber, A.-L.; Gasqui, P.; Faure, E.; Baurier, F.; Vourc’H, G.; Kodjo, A.; Combes, B. Hedgehogs and Mustelid Species: Major Carriers of Pathogenic Leptospira, a Survey in 28 Animal Species in France (20122015). PLoS ONE 2016, 11, e0162549. [Google Scholar] [CrossRef]
- Babudieri, B.; Fariña, R. The Leptospirae of the Italian Hedge-Hog. Pathobiology 1964, 27, 103–116. [Google Scholar] [CrossRef]
- Hartskeerl, P.A.; Terpstra, W. Leptospirosis in wild animals. Vet. Q. 1996, 18, 149–150. [Google Scholar] [CrossRef]
- Broom, J.; Coghlan, J.; Kmety, E. Leptospira bratislava isolated from a hedgehog in scotland. Lancet 1960, 275, 1326–1327. [Google Scholar] [CrossRef]
- Ma, X.-J.; Gong, X.-Q.; Xiao, X.; Liu, J.-W.; Han, H.-J.; Qin, X.-R.; Lei, S.-C.; Gu, X.-L.; Yu, H.; Yu, X.-J. Detection of Leptospira interrogans in Hedgehogs from Central China. Vector-Borne Zoonotic Dis. 2020, 20, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Mayer-Scholl, A.; Imholt, C.; Spierling, N.G.; Heuser, E.; Schmidt, S.; Reil, D.; Rosenfeld, U.M.; Jacob, J.; Nöckler, K.; et al. Leptospira Genomospecies and Sequence Type Prevalence in Small Mammal Populations in Germany. Vector-Borne Zoonotic Dis. 2018, 18, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Żmudzki, J.; Arent, Z.; Jabłoński, A.; Nowak, A.; Zębek, S.; Stolarek, A.; Bocian, Ł.; Brzana, A.; Pejsak, Z. Seroprevalence of 12 serovars of pathogenic Leptospira in red foxes (Vulpes vulpes) in Poland. Acta Vet. Scand. 2018, 60, 34. [Google Scholar] [CrossRef] [PubMed]
- Haake, D.A.; Levett, P.N. Leptospirosis in Humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Guglielmini, J.; Bourhy, P.; Schiettekatte, O.; Zinini, F.; Brisse, S.; Picardeau, M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007374. [Google Scholar] [CrossRef]
- Masuzawa, T.; Saito, M.; Nakao, R.; Nikaido, Y.; Matsumoto, M.; Ogawa, M.; Yokoyama, M.; Hidaka, Y.; Tomita, J.; Sakakibara, K.; et al. Molecular and phenotypic characterization of Leptospira johnsonii sp. nov., Leptospira ellinghausenii sp. nov. and Leptospira ryugenii sp. nov. isolated from soil and water in Japan. Microbiol. Immunol. 2019, 63, 89–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).