Proteomic Analysis Identifies Potential Markers for Chicken Primary Follicle Development
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection
2.2. Isolation of Follicles
2.3. Protein Extraction, Digestion, and Peptide Labeling
2.4. Fractionation of TMT Reagent Labeled Peptides and LC–MS/MS Analyzes
2.5. Peptide Identification
2.6. Bioinformatics Analysis
2.7. Validation of Proteomics Data by Using Real-Time Quantitative PCR
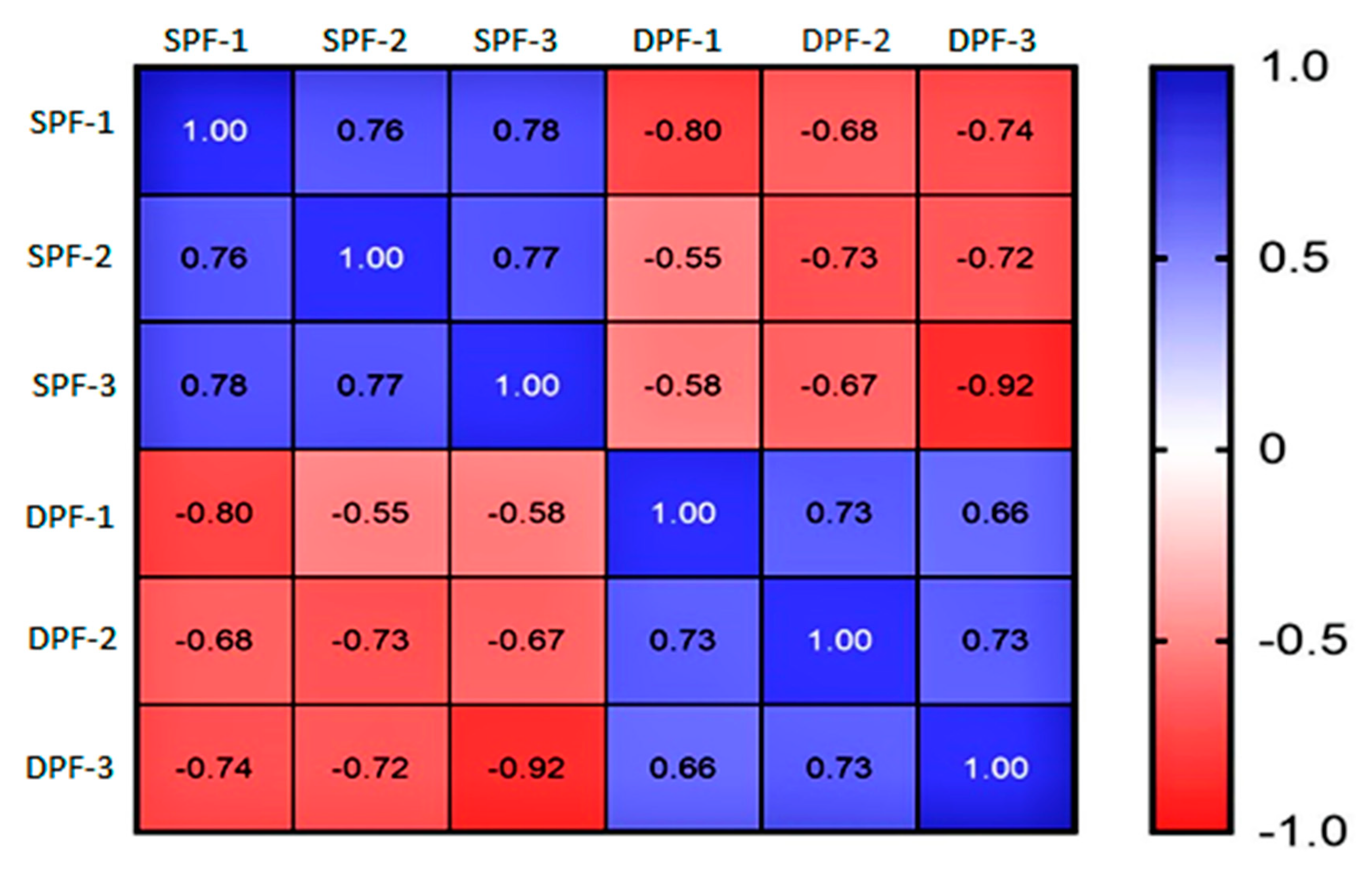
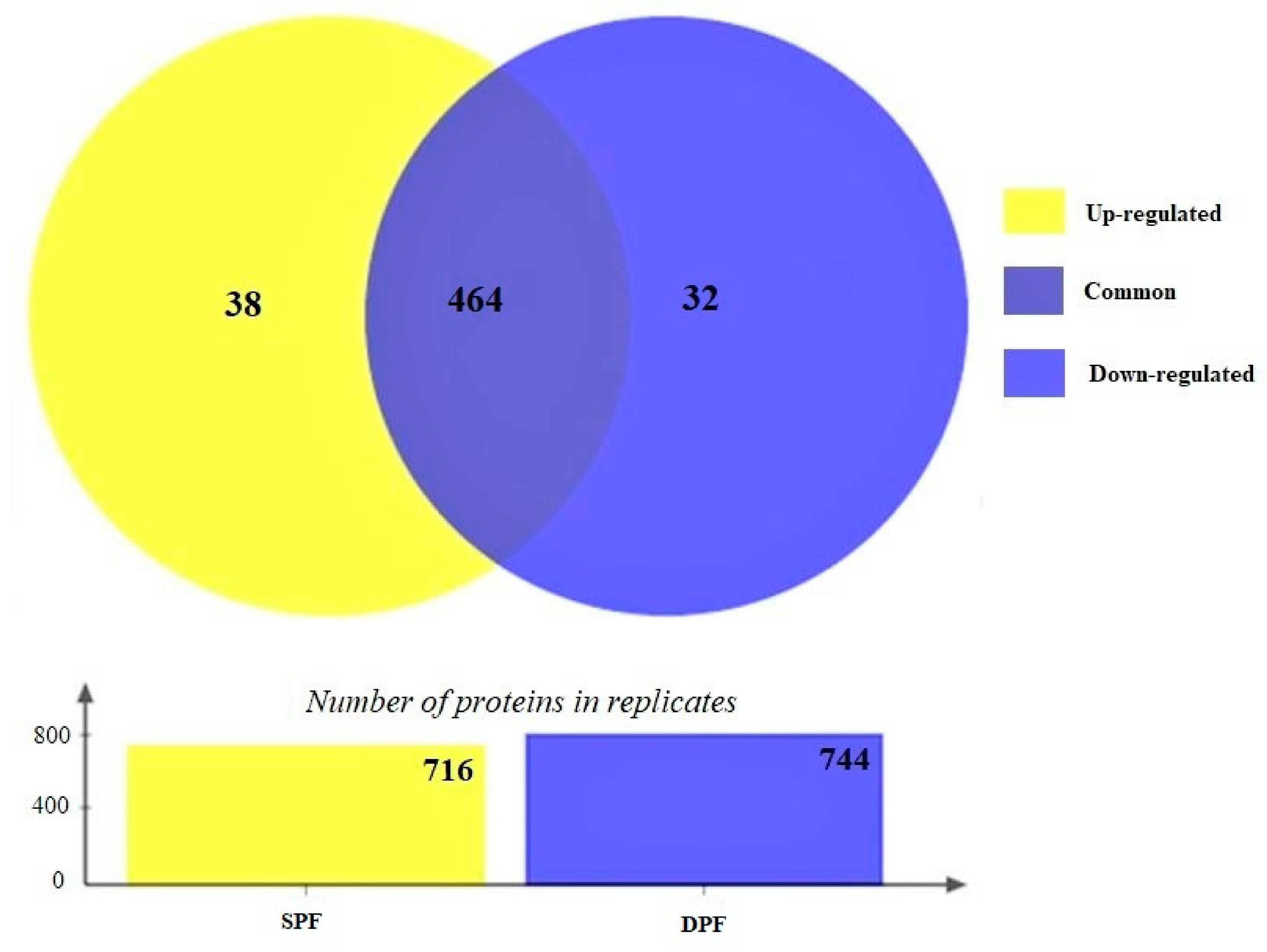
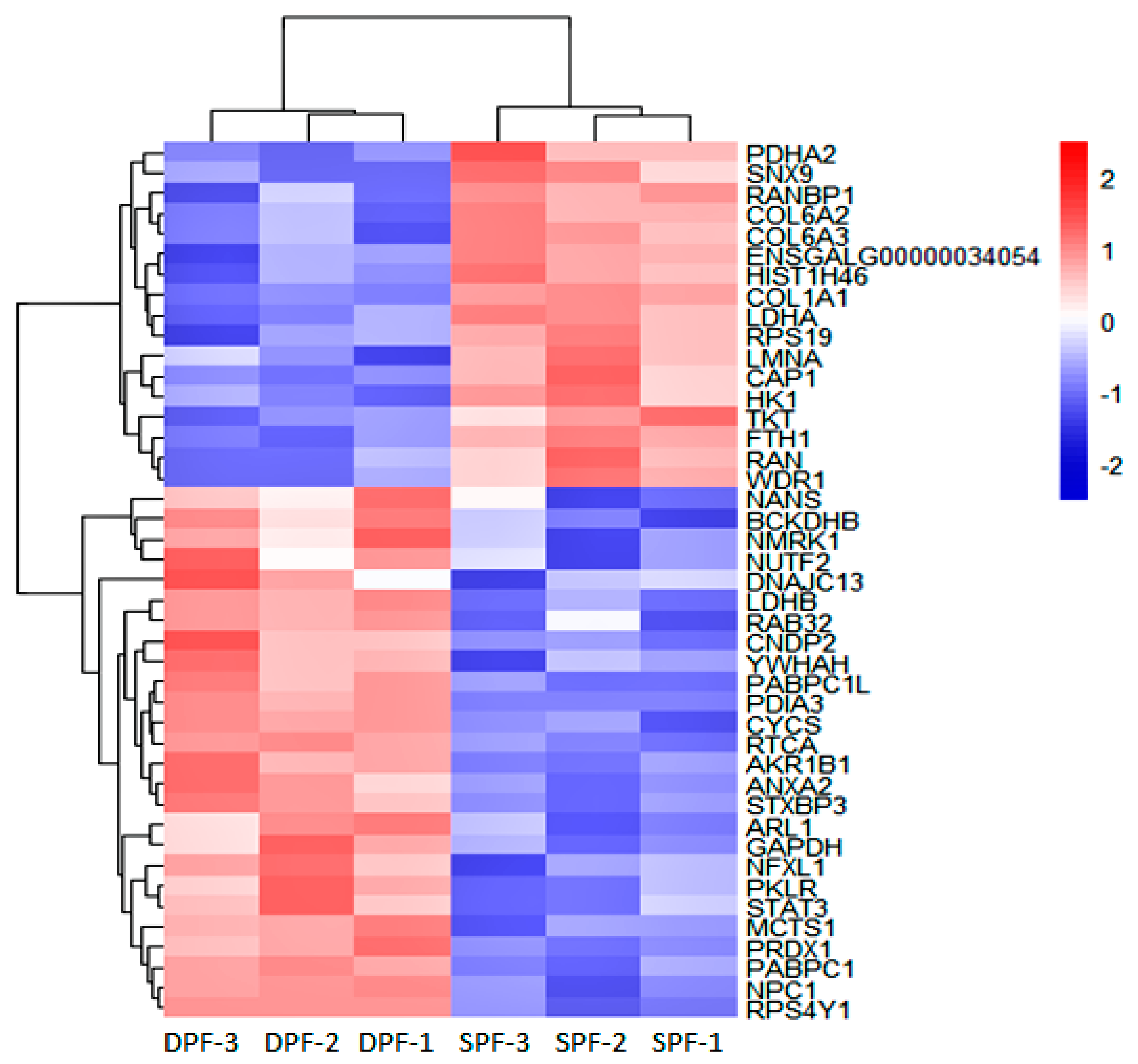
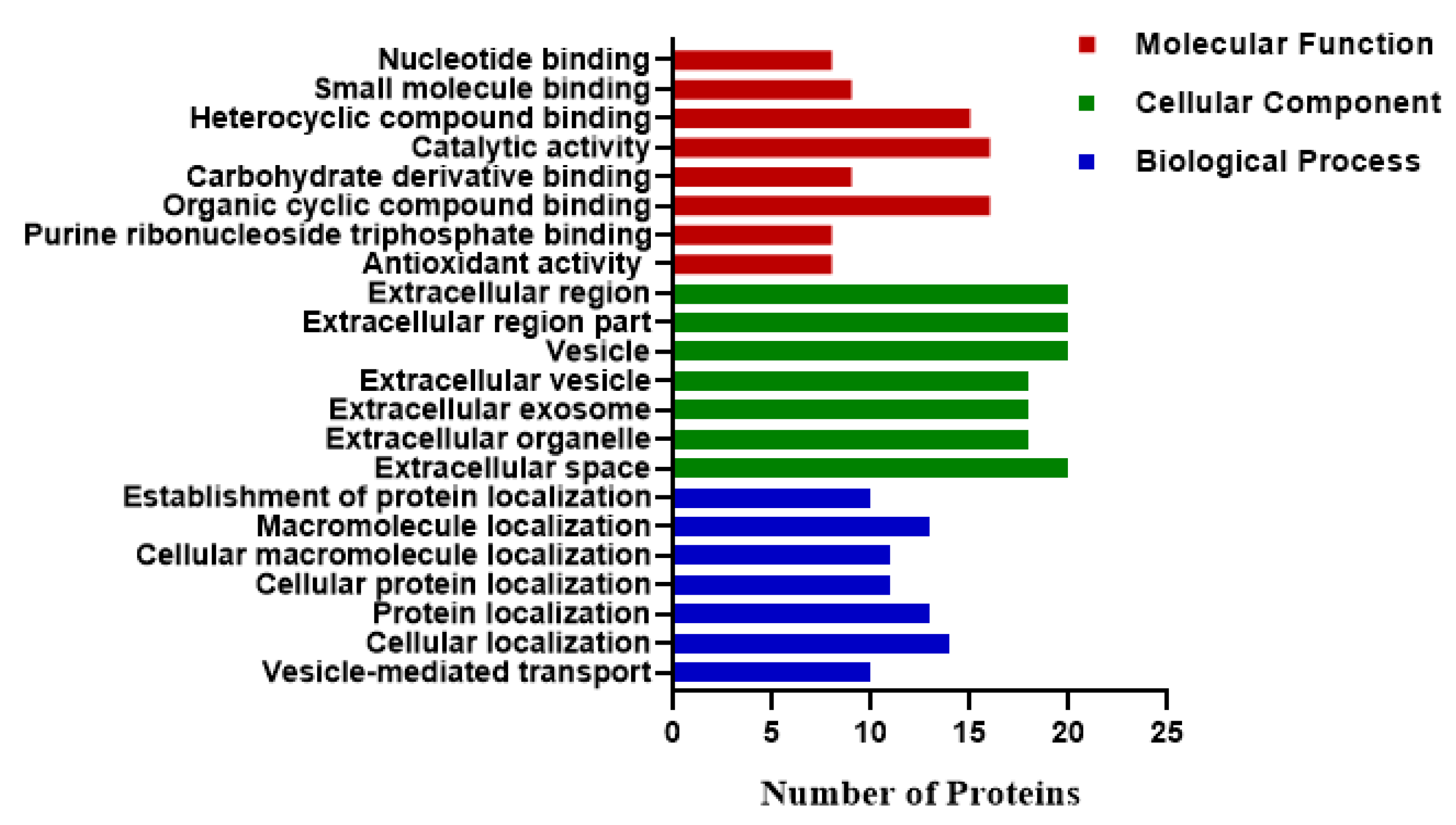
3. Results
4. Discussion
4.1. Role of Glycolysis during Primary Follicle Development
4.2. Role of ANXA2, PDIA3, and CAPZB during the Primary Follicle Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaheer, K. An updated review on chicken eggs: Production, consumption, management aspects and nutritional benefits to human health. Food Nutr. Sci. 2015, 6, 1208. [Google Scholar] [CrossRef]
- Diaz, F.J.; Anthony, K.; Halfhill, A.N. Early avian follicular development is characterized by changes in transcripts involved in steroidogenesis, paracrine signaling and transcription. Mol. Reprod. Dev. 2011, 78, 212–223. [Google Scholar] [CrossRef]
- Rodler, D.; Sinowatz, F. Expression of intermediate filaments in the Balbiani body and ovarian follicular wall of the Japanese quail (Coturnix japonica). Cells Tissues Organs 2013, 197, 298–311. [Google Scholar] [CrossRef]
- Lyu, Z.; Qin, N.; Tyasi, T.L.; Zhu, H.; Liu, D.; Yuan, S.; Xu, R. The Hippo/MST Pathway Member SAV1 Plays a Suppressive Role in Development of the Prehierarchical Follicles in Hen Ovary. PLoS ONE 2016, 11, e0160896. [Google Scholar] [CrossRef]
- Qin, N.; Fan, X.C.; Xu, X.X.; Tyasi, T.L.; Li, S.J.; Zhang, Y.Y.; Wei, M.L.; Xu, R.F. Cooperative Effects of FOXL2 with the Members of TGF-β Superfamily on FSH Receptor mRNA Expression and Granulosa Cell Proliferation from Hen Prehierarchical Follicles. PLoS ONE 2015, 10, e0141062. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Ritter, L.J.; Myllymaa, S.; Kaivo-Oja, N.; Dragovic, R.A.; Hickey, T.E.; Ritvos, O.; Mottershead, D.G. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J. Cell Sci. 2006, 119, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Shea, L.D. The Role of the extracellular matrix in ovarian follicle development. Reprod. Sci. 2007, 14, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Fan, X.C.; Zhang, Y.Y.; Xu, X.X.; Tyasi, T.L.; Jing, Y.; Mu, F.; Wei, M.L.; Xu, R.F. New insights into implication of the SLIT/ROBO pathway in the prehierarchical follicle development of hen ovary. Poult. Sci. 2015, 94, 2235–2246. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.-H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Liaoliao, H.; Yuehui, Z.; Huang, J.; Luo, T.; Zhong, Z.; Zheng, Y.; Zheng, L. Hippo Signaling Pathway Reveals a Spatio-Temporal Correlation with the Size of Primordial Follicle Pool in Mice. Cell. Physiol. Biochem. 2015, 35, 957–968. [Google Scholar] [CrossRef]
- Johnson, A.L.; Lee, J. Granulosa cell responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poult. Sci. 2016, 95, 108–114. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, X.; Mao, Y.; Kang, L.; Ma, X.; Jiang, Y. Characterization of annexin A2 in chicken follicle development: Evidence for its involvement in angiogenesis. Anim. Reprod. Sci. 2015, 161, 104–111. [Google Scholar] [CrossRef]
- Rosewell, K.; Al-Alem, L.; Li, F.; Kelty, B.; Curry, T.E. Identification of Hepsin and Protein Disulfide Isomerase A3 as Targets of Gelatinolytic Action in Rat Ovarian Granulosa Cells During the Periovulatory Period1. Biol. Reprod. 2011, 85, 858–866. [Google Scholar] [CrossRef]
- Ellerman, D.A.; Myles, D.G.; Primakoff, P. A Role for Sperm Surface Protein Disulfide Isomerase Activity in Gamete Fusion: Evidence for the Participation of ERp57. Dev. Cell 2006, 10, 831–837. [Google Scholar] [CrossRef]
- Primm, T.P.; Gilbert, H.F. Hormone Binding by Protein Disulfide Isomerase, a High Capacity Hormone Reservoir of the Endoplasmic Reticulum. J. Biol. Chem. 2001, 276, 281–286. [Google Scholar] [CrossRef]
- Etches, R.J.; Petitte, J.N. Reptilian and avian follicular hierarchies: Models for the study of ovarian development. J. Exp. Zoöl. 1990, 256, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sang, H. Prospects for transgenesis in the chick. Mech. Dev. 2004, 121, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Altelaar, A.F.M.; Munoz, J.; Heck, A.J.R. Next-generation proteomics: Towards an integrative view of proteome dynamics. Nat. Rev. Genet. 2012, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Mann, M. Proteomics to study genes and genomes. Nat. Cell Biol. 2000, 405, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Michaux, N.; Camboni, A.; Martinez-Madrid, B.; van Langendonckt, A.; Nottola, S.A.; Donnez, J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum. Reprod. 2005, 21, 413–420. [Google Scholar] [CrossRef]
- Du, M.; Han, H.; Jiang, B.; Zhao, C.; Qian, C.; Shen, H.; Xu, Y.; Li, Z. An Efficient Isolation Method for Domestic Hen (Gallus domesticus) Ovarian Primary Follicles. J. Reprod. Dev. 2006, 52, 569–576. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010, 5439. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Heiden, M.G.V. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef]
- Kolobova, E.; Tuganova, A.; Boulatnikov, I.; Popov, K.M. Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem. J. 2001, 358, 69–77. [Google Scholar] [CrossRef]
- Bao, H.; Jiang, M.; Zhu, M.; Sheng, F.; Ruan, J.; Ruan, C. Overexpression of Annexin II affects the proliferation, apoptosis, invasion and production of proangiogenic factors in multiple myeloma. Int. J. Hematol. 2009, 90, 177–185. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, F.; Yu, M.; Zhao, P.; Ji, W.; Zhang, H.; Han, J.; Niu, R. Up-regulation of Anxa2 gene promotes proliferation and invasion of breast cancer MCF-7 cells. Cell Prolif. 2012, 45, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.A.; Gibbins, J.M. Extracellular Disulfide Exchange and the Regulation of Cellular Function. Antioxid. Redox Signal. 2006, 8, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Maattanen, P.; Kozlov, G.; Gehring, K.; Thomas, D. ERp57 and PDI: Multifunctional protein disulfide isomerases with similar domain architectures but differing substrate–partner. Biochem. Cell Biol. 2006, 84, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Hrabia, A.; Wolak, D.; Kwaśniewska, M.; Kieronska, A.; Socha, J.K.; Sechman, A. Expression of gelatinases (MMP-2 and MMP-9) and tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary in relation to follicle development and atresia. Theriogenology 2019, 125, 268–276. [Google Scholar] [CrossRef]
- Huo, X.; Liu, Y.; Wang, X.; Ouyang, P.; Niu, Z.; Shi, Y.; Qiu, B. Co-expression of human protein disulfide isomerase (hPDI) enhances secretion of bovine follicle-stimulating hormone (bFSH) in Pichia pastoris. Protein Expr. Purif. 2007, 54, 234–239. [Google Scholar] [CrossRef]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 Inhibition Increases Mitochondrial Metabolism through SIRT1 Activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, S.H. Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 2004, 16, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ogienko, A.A.; Karagodin, D.A.; Lashina, V.V.; Baiborodin, S.I.; Omelina, E.S.; Baricheva, E.M. Capping protein beta is required for actin cytoskeleton organisation and cell migration during Drosophila oogenesis. Cell Biol. Int. 2013, 37, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mukaihara, K.; Suehara, Y.; Kohsaka, S.; Kubota, D.; Toda-Ishii, M.; Akaike, K.; Fujimura, T.; Kobayashi, E.; Yao, T.; Ladanyi, M.; et al. Expression of F-actin-capping protein subunit beta, CAPZB, is associated with cell growth and motility in epithelioid sarcoma. BMC Cancer 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | GeneBank Accession Number | Primer Sequence (5′-0 to 3′-0) | Product Length (bp) |
|---|---|---|---|
| Gapdh | NM_204305.1 | PF:5′-TGCCCAGAACATCATCCCA-3′ PR:5′-GCCAGCACCCGCATCAAAG-3′ | 295 |
| Pdia3 | NM_204110.3 | PF:5′-GGCGCAACCGAGTTATGATG-3′ PR:5′-CTCACCCACGCTGTTGTCTA-3′ | 131 |
| Capzb | NM_001173529.1 | PF:5′-GCGACTCTTCTCCGCACATA-3′ PR:5′-AAGTCTGCACAGACCTCAGC-3′ | 135 |
| Anxa2 | XM_025153803.1 | PF:5′-GAGGCAGTGATCTTGGGCTT-3′ PR:5′-CATCAGTTCCCAGGCCCTTC-3′ | 88 |
| β-actin | NM_205518.1 | PF:5′-GAGCTGAGAGTAGCCCCTGA-3′ PR:5′-CGCACAATTTCTCTCTCGGC-3′ | 353 |
| Hsp40 | XM_015278955.2 | PF:5′-AGGCTCTGCTTCCTCCAAGA-3′ PR:5′-ACGGTAGGTTTGCTCGCTTG-3′ | 95 |
| Faf2 | XM_414548.6 | PF:5′-GTCGGGTTACTGACCCAGTG-3′ PR:5′-AGTGCCTGGCTGTAAGTTCC-3′ | 109 |
| Col6a1 | NM_205107.1 | PF:5′-CCTCGTGGCGCAAGTTAAAG-3′ PR:5′-TCTCCTTGAGATGGGAGCCA-3′ | 278 |
| Rps19 | XM_025146195.1 | PF:5′-CAAGCTGAAGGTTCCGGACT-3′ PR:5′-ATCTTCGTCATGGAGCCCAC-3′ | 156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadood, A.A.; Wang, J.; Pu, L.; Shahzad, Q.; Waqas, M.; Liu, X.; Xie, L.; Yu, L.; Chen, D.; Akhtar, R.W.; et al. Proteomic Analysis Identifies Potential Markers for Chicken Primary Follicle Development. Animals 2021, 11, 1108. https://doi.org/10.3390/ani11041108
Wadood AA, Wang J, Pu L, Shahzad Q, Waqas M, Liu X, Xie L, Yu L, Chen D, Akhtar RW, et al. Proteomic Analysis Identifies Potential Markers for Chicken Primary Follicle Development. Animals. 2021; 11(4):1108. https://doi.org/10.3390/ani11041108
Chicago/Turabian StyleWadood, Armughan Ahmed, Jingyuan Wang, Liping Pu, Qaisar Shahzad, Muhammad Waqas, Xingting Liu, Long Xie, Lintian Yu, Dongyang Chen, Rana Waseem Akhtar, and et al. 2021. "Proteomic Analysis Identifies Potential Markers for Chicken Primary Follicle Development" Animals 11, no. 4: 1108. https://doi.org/10.3390/ani11041108
APA StyleWadood, A. A., Wang, J., Pu, L., Shahzad, Q., Waqas, M., Liu, X., Xie, L., Yu, L., Chen, D., Akhtar, R. W., & Lu, Y. (2021). Proteomic Analysis Identifies Potential Markers for Chicken Primary Follicle Development. Animals, 11(4), 1108. https://doi.org/10.3390/ani11041108







