Performance Evaluation of Two Slow-Medium Growing Chicken Strains Maintained under Organic Production System during Different Seasons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Weather Period
2.4. Data Collection
2.5. Statistical Analyses
3. Results
3.1. Weights
3.2. Average Daily Gain (ADG)
3.3. Feed Intake (FI)
3.4. Feed Conversion Ratio (FCR)
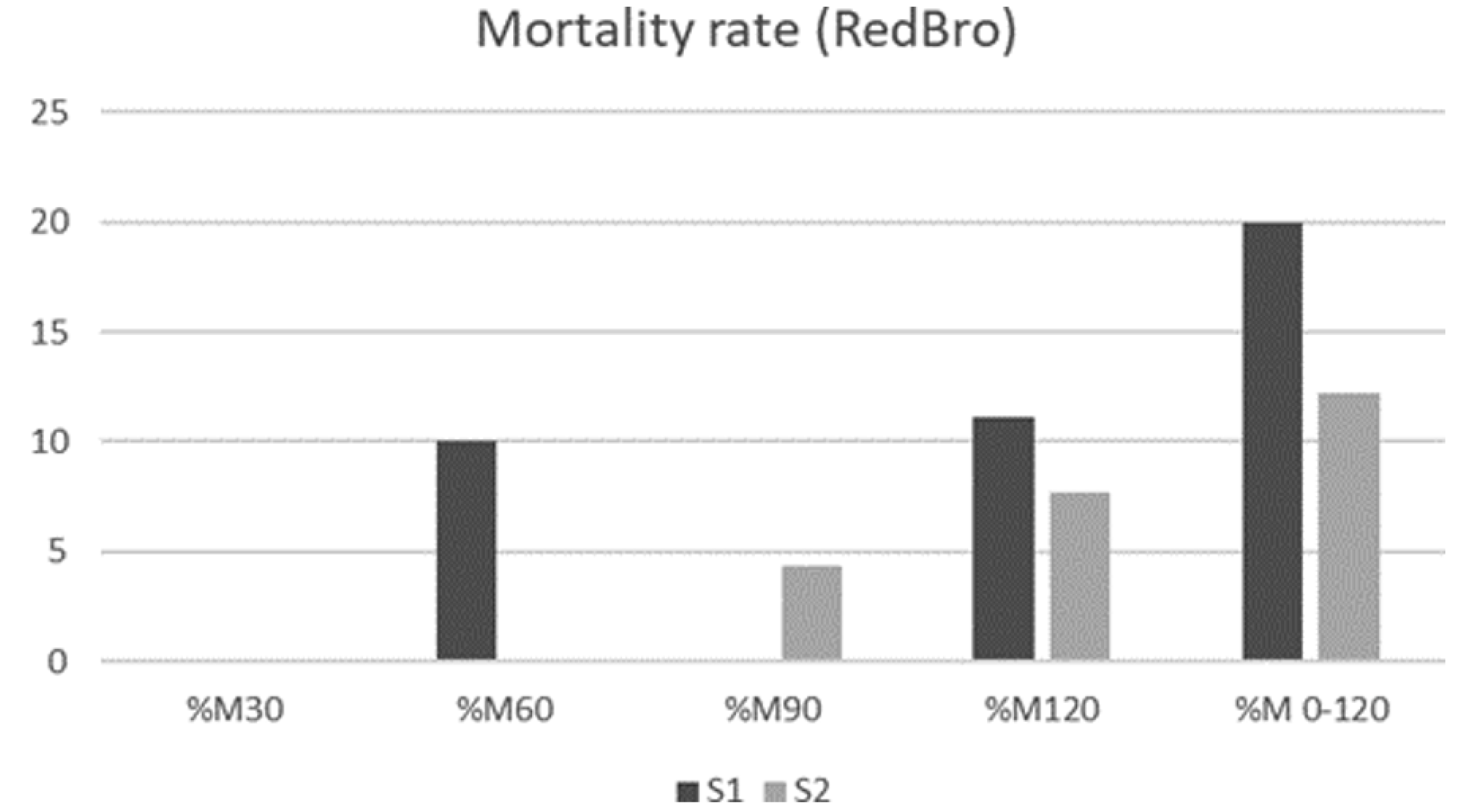
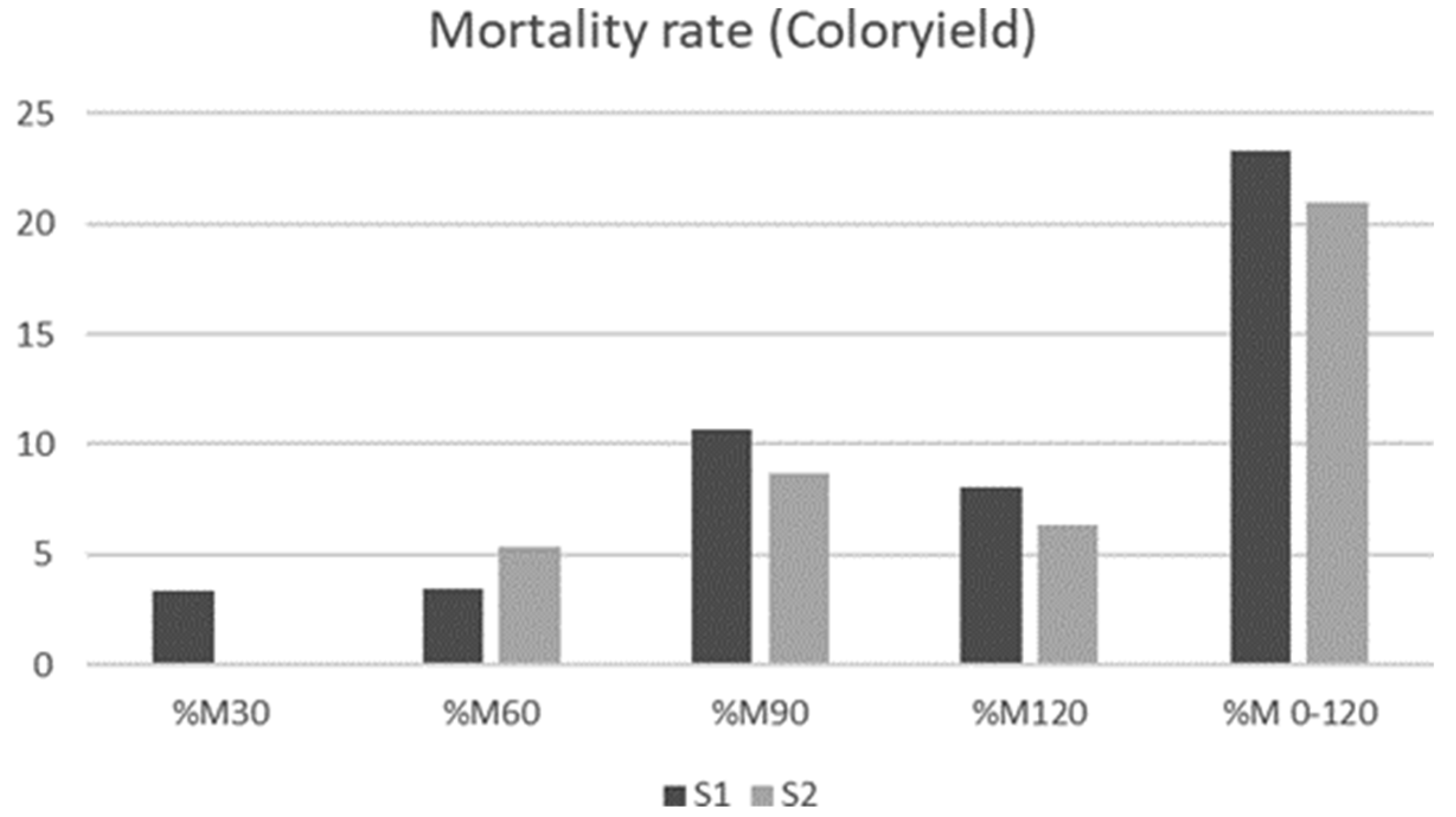
3.5. Mortality Rates
3.6. Live Weight Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fanatico, A.C.; Pillai, P.B.; Cavitt, L.C.; Owens, C.M.; Emmert, J.L. Evaluation of Slower-Growing Broiler Genotypes Grown with and without Outdoor Access: Growth Performance and Carcass Yield. Poult. Sci. 2005, 84, 1321–1327. [Google Scholar] [CrossRef]
- Fanatico, A.C.; Pillai, P.B.; Emmert, J.L.; Owens, C.M. Meat Quality of Slow- and Fast-Growing Chicken Genotypes Fed Low-Nutrient or Standard Diets and Raised Indoors or with Outdoor Access. Poult. Sci. 2007, 86, 2245–2255. [Google Scholar] [CrossRef]
- Sundrum, A.; Padel, S.; Arsenos, G.; Kuzniar, A.; Henriksen, B.I.F.; Walkenhorst, M.; Vaarst, M. Current and Proposed EU Legislation on Organic Livestock Production, with a Focus on Animal Health, Welfare and Food Safety: A Review. In Proceedings of the 5th SAFO Workshop, Odense, Denmark, 1 June 2006. [Google Scholar]
- Fanatico, A.C.; Pillai, P.B.; Hester, P.Y.; Falcone, C.; Mench, J.A.; Owens, C.M.; Emmert, J.L. Performance, Livability, and Carcass Yield of Slow- and Fast-Growing Chicken Genotypes Fed Low-Nutrient or Standard Diets and Raised Indoors or with Outdoor Access. Poult. Sci. 2008, 87, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.D.; Mugnai, C.; Amato, M.G.; Piottoli, L.; Cartoni, A.; Castellini, C. Effect of Slaughtering Age in Different Commercial Chicken Genotypes Reared According to the Organic System: 1. Welfare, Carcass and Meat Traits. Ital. J. Anim. Sci. 2014, 13, 3308. [Google Scholar] [CrossRef] [Green Version]
- Katogianni, I.; Zoiopoulos, P.; Adamidis, K.; Fegeros, K. Comparison of Two Broiler Genotypes Grown under the European Union Organic Legislation Vergleich Zweier Broilergenotypen für die Biomast nach EU-Verordnung; Verlag Eugen Ulmer: Stuttgart, Germany, 2008. [Google Scholar]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of Organic Production System on Broiler Carcass and Meat Quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C. Fatty Acid Composition of Meat and Estimated Indices of Lipid Metabolism in Different Poultry Genotypes Reared under Organic System. Poult. Sci. 2012, 91, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Sokołowicz, Z.; Krawczyk, J.; Świątkiewicz, S. Quality of Poultry Meat from Native Chicken Breeds—A Review. Anim. Sci 2016, 16, 347–368. [Google Scholar] [CrossRef] [Green Version]
- Bhadauria, P.; Kataria, J.M.; Majumdar, S.; Bhanja, S.K.; Kolluri, G. Impact of Hot Climate on Poultry Production System—A Review Thermoregulatory Mechanism of Poultry. Poult. Sci. 2014, 2, 56–63. [Google Scholar]
- Peana, I.; Francesconi, A.H.D.; Dimauro, C.; Cannas, A.; Sitzia, M. Effect of Winter and Spring Meteorological Conditions on Milk Production of Grazing Dairy Sheep in the Mediterranean Environment. Small Rumin. Res. 2017, 153, 194–208. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry; National Academies Press: Washington, DC, USA, 1994.
- Tao, X.; Xin, H. Temperature-Humidity-Velocity Index for Market-Size Broilers. In Proceedings of the ASAE Annual International Meeting, Las Vegas, NV, USA, 27–30 July 2003; pp. 27–30. [Google Scholar]
- Akşit, M.; Altan, Ö.; Büyüköztürk Karul, A.; Balkaya, M.; Özdemir, D. Effects of Cold Temperature and Vitamin E Supplementation on Oxidative Stress, Troponin-T Level, and Other Ascites-Related Traits in Broilers. Arch. Geflügelk 2008, 72, 221–230. [Google Scholar]
- Qureshi, S.; Khan, H.M.; Mir, M.S.; Raja, T.; Khan, A. Effect of Cold Stress and Various Suitable Remedies on Performance of Broiler Chicken. J. World Poult. Res. 2018, 8, 66–73. [Google Scholar]
- Bosco, A.D.; Mugnai, C.; Castellini, C. Performance and Meat Quality of Pure Ancona and Cornish × Ancona Chickens Organically Reared. Eur. Poult. Sci. 2011, 75, 7–12. [Google Scholar]
- Yalcin, S.; Settar, P.; Ozkan, S.; Cahaner, A. Comparative Evaluation of Three Commercial Broiler Stocks in Hot versus Temperate Climates. Poult. Sci. 1997, 76, 921–929. [Google Scholar] [CrossRef]
- Akşit, M.; Yalçin, S.; Ozkan, S.; Metin, K.; Ozdemir, D. Effects of Temperature during Rearing and Crating on Stress Parameters and Meat Quality of Broilers. Poult. Sci. 2006, 85, 1867–1874. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of Constant and Cyclic Heat Stress on Muscle Metabolism and Meat Quality of Broiler Breast Fillet and Thigh Meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Abdullah, A.Y.; Muwalla, M.M.; Maharmeh, H.O.; Matarneh, S.K.; Ishmais, M.A.A. Effects of Strain on Performance, and Age at Slaughter and Duration of Post-Chilling Aging on Meat Quality Traits of Broiler. Asian-Australas. J. Anim. Sci. 2010, 23, 1645–1656. [Google Scholar] [CrossRef]
- Blahová, J.; Dobšíková, R.; Straková, E.; Suchý, P. Effect of Low Environmental Temperature on Performance and Blood System in Broiler Chickens (Gallus domesticus). Acta Vet. Brno 2007, 76, S17–S23. [Google Scholar] [CrossRef] [Green Version]
- Van der Sluis, W. Floor Temperature Affects Broiler Performance. World Poult. 2004, 20, 17. [Google Scholar]
- Smith, D.; Lyon, C.; Lyon, B. The Effect of Age, Dietary Carbohydrate Source, and Feed Withdrawal on Broiler Breast Fillet Color. Poult. Sci. 2002, 81, 1584–1588. [Google Scholar] [CrossRef]
- Novák, P.; Zeman, L.; Ko, K.A.; Novák, L. Modelling of Body Mass Increase and Feed Conversion Ratio in Chickens ROSS 208. Acta Vet. Brno 2004, 73, 17–22. [Google Scholar] [CrossRef]
- Wen, C.; Yan, W.; Zheng, J.; Ji, C.; Zhang, D.; Sun, C.; Yang, N. Feed Efficiency Measures and Their Relationships with Production and Meat Quality Traits in Slower Growing Broilers. Poult. Sci. 2018, 97, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, M.; Zheng, S.; Xie, P.; Ma, A. Effects of High Temperature on Multiple Parameters of Broilers In Vitro and In Vivo. Poult. Sci. 2008, 87, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Toudic, C. Evaluating Uniformity in Broilers—Factors Affecting Variation. Available online: https://www.semanticscholar.org/paper/EVALUATING-UNIFORMITY-IN-BROILERS-%E2%80%93-FACTORS-Toudic/5ea700a89e43bf2fb6ae1a0cebaa949a459fa62d (accessed on 25 June 2020).
- Dalanezi, J.A.; Mendes, A.A.; Garcia, E.A.; Garcia, R.G.; Moreira, J.; Paz, I.C.L.A. Effect of Broiler Breeder Age on Performance and Carcass Yield of Broiler Chickens. Arq. Bras. Med. Vet. Zootec. 2005, 57, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Molenaar, R.; Reijrink, I.A.M.; Meijerhof, R.; van den Brand, H. Relationship between Hatchling Length and Weight on Later Productive Performance in Broilers. World’s Poult. Sci. J. 2008, 64, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Kosba, M.A.; Zeweil, H.S.; Ahmed, M.H.; Shabara, S.M.; Debes, A.A. Selection for Uniformity in Alexandria Local Chicken. 1—Response to Selection. Egypt. Poult. Sci. J. 2009, 29, 1157–1171. [Google Scholar]
- Griffin, A.M.; Renema, R.A.; Robinson, F.E.; Zuidhof, M.J. The Influence of Rearing Light Period and the Use of Broiler or Broiler Breeder Diets on Forty-Two-Day Body Weight, Fleshing, and Flock Uniformity in Broiler Stocks. J. Appl. Poult. Res. 2005, 14, 204–216. [Google Scholar] [CrossRef]
- Antruejo, A.E.; Savoy, J.P.; Perrotta, C.H.; Canet, Z.E.; Dottavio, A.M.; di Masso, R.J. Densidad de Alojamiento y Caracteres Productivos En Un Cruzamiento Experimental de Tres Vías de Pollo Campero. Cienc. Vet. 2018, 20, 67–80. [Google Scholar] [CrossRef]
| Starter (1–30 d) | Grower-Finisher (30–120 d) | |
|---|---|---|
| Ingredients (g/100 g) | ||
| Soybean meal | 35.19 | - |
| Corn | 30.00 | - |
| Wheat | 12.87 | 30.00 |
| Barley | 9.84 | 30.00 |
| Spring pea | 8.00 | 30.00 |
| Dicalcium phosphate | 1.93 | - |
| Calcium carbonate | 0.82 | - |
| Organic Premix 1 | 0.50 | 2.50 |
| Acidifier | 0.30 | - |
| Salt | 0.28 | - |
| Sodium bicarbonate | 0.16 | - |
| Enzymatic complex | 0.10 | - |
| Sunflower | - | 7.50 |
| Chemical composition (g/kg) | ||
| Metabolizable Energy (kcal/kg) | 2462 | 2179 |
| Moisture | 9.24 | 10.16 |
| Crude Protein | 21.45 | 15.94 |
| Crude Fiber | 3.68 | 5.74 |
| Fat | 5.69 | 5.04 |
| Ash | 7.09 | 4.89 |
| S1 | S2 | |
|---|---|---|
| MeanT (°C) | 9.32 ± 0.27 a | 16.86 ± 0.27 b |
| MaxT (°C) | 15.57 ± 0.33 a | 25.06 ± 0.31 b |
| MinT (°C) | 3.61 ± 0.22 a | 8.96 ± 0.25 b |
| Thermal Amplitude (°C) | 11.96 ± 0.24 a | 16.10 ± 0.22 b |
| Mean RH (%) | 75.14 ± 0.34 a | 60.59 ± 0.23 b |
| Max RH (%) | 94.38 ± 0.75 | 87.91 ± 0.79 |
| Min RH (%) | 48.92 ± 0.33 a | 33.04 ± 0.34 b |
| Wind speed (m/s) | 2.05 ± 0.03 a | 1.55 ± 0.05 b |
| Max Wind speed (m/s) | 7.09 ± 0.08 a | 6.23 ± 0.13 b |
| Solar Radiation (MJ/m2) | 15.56 ± 0.32 a | 18.97 ± 0.39 b |
| Rainfall (mm) | 1.64 ± 0.11 a | 0.87 ± 0.18 b |
| THI | 13.77 | 22.65 |
| THVI | 13.21 | 21.72 |
| VBT | −7.07 | −3.75 |
| Homeostasis | < 1 | < 1 |
| S1 (n = 80) | S2 (n = 80) | p-Values | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CY (n = 40) | RB (n = 40) | Total S1 | CY (n = 40) | RB (n = 40) | Total S2 | Strains | Weather Period | Interaction SxW | |||||||||||||
| LW0 | 0.074 | ± | 0.026 | 0.056 | ± | 0.015 | 0.065 | ± | 0.023 | 0.051 | ± | 0.003 | 0.059 | ± | 0.009 | 0.057 | ± | 0.009 | |||
| LW15 | 0.238 | ± | 0.076 | 0.237 | ± | 0.064 | 0.237 | ± | 0.070 | 0.188 | ± | 0.068 | 0.203 | ± | 0.035 | 0.200 | ± | 0.042 | 0.600 | 0.028 | 0.881 |
| LW30 | 0.421 | ± | 0.147 | 0.484 | ± | 0.136 | 0.452 | ± | 0.144 | 0.407 | ± | 0.075 | 0.435 | ± | 0.069 | 0.430 | ± | 0.070 | 0.107 | 0.576 | 0.311 |
| LW45 | 0.654 | ± | 0.168 | 0.728 | ± | 0.241 | 0.691 | ± | 0.209 | 0.680 | ± | 0.070 | 0.696 | ± | 0.120 | 0.693 | ± | 0.112 | 0.296 | 0.886 | 0.399 |
| LW60 | 1.090 | ± | 0.295 | 1.253 | ± | 0.322 | 1.171 | ± | 0.316 | 1.040 | ± | 0.159 | 0.939 | ± | 0.217 | 0.957 | ± | 0.210 | 0.633 | 0.026 | 0.066 |
| LW75 | 1.624 | ± | 0.421 | 1.824 | ± | 0.374 | 1.724 | ± | 0.407 | 1.499 | ± | 0.191 | 1.357 | ± | 0.263 | 1.383 | ± | 0.255 | 0.747 | 0.003 | 0.080 |
| LW90 | 2.235 | ± | 0.455 | 2.307 | ± | 0.522 | 2.271 | ± | 0.486 | 2.035 | ± | 0.238 | 1.803 | ± | 0.336 | 1.845 | ± | 0.330 | 0.504 | 0.005 | 0.208 |
| LW105 | 2.834 | ± | 0.506 | 2.905 | ± | 0.510 | 2.869 | ± | 0.504 | 2.638 | ± | 0.278 | 2.374 | ± | 0.395 | 2.422 | ± | 0.386 | 0.401 | 0.004 | 0.280 |
| LW120 | 3.579 | ± | 0.667 | 3.582 | ± | 0.488 | 3.580 | ± | 0.578 | 3.108 | ± | 0.365 | 3.217 | ± | 0.466 | 3.197 | ± | 0.446 | 0.756 | 0.004 | 0.594 |
| ADG 1–30 | 0.011 | ± | 0.004 | 0.014 | ± | 0.004 | 0.013 | ± | 0.004 | 0.011 | ± | 0.002 | 0.012 | ± | 0.002 | 0.012 | ± | 0.002 | 0.107 | 0.576 | 0.311 |
| ADG 30–60 | 0.027 | ± | 0.007 | 0.020 | ± | 0.007 | 0.024 | ± | 0.008 | 0.026 | ± | 0.007 | 0.016 | ± | 0.006 | 0.023 | ± | 0.007 | 0.803 | 0.021 | 0.099 |
| ADG 60–90 | 0.034 | ± | 0.007 | 0.038 | ± | 0.010 | 0.036 | ± | 0.008 | 0.030 | ± | 0.003 | 0.033 | ± | 0.010 | 0.031 | ± | 0.009 | 0.129 | 0.023 | 0.888 |
| ADG 90–120 | 0.039 | ± | 0.018 | 0.042 | ± | 0.009 | 0.041 | ± | 0.014 | 0.031 | ± | 0,005 | 0.034 | ± | 0.015 | 0.033 | ± | 0.014 | 0.300 | 0.458 | 0.060 |
| ADG 1–120 | 0.029 | ± | 0.005 | 0.029 | ± | 0.004 | 0.029 | ± | 0.004 | 0.025 | ± | 0.003 | 0.026 | ± | 0.003 | 0.026 | ± | 0.003 | 0.756 | 0.004 | 0.595 |
| S1 (n = 80) | S2 (n = 80) | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CY (n = 40) | RB (n = 40) | Total | CY (n = 40) | RB (n = 40) | Total | Strain | Weather period | Interaction | |
| FI 1–30 | 1.055 ± 0.110 | 1.073 ± 0.170 | 1.064 ± 0.143 | 1.111 ± 0.258 | 1.033 ± 0.205 | 1.087 ± 0.222 | 0.551 | 0.842 | 0.365 |
| FI 30–60 | 2.523 ± 0.893 | 3.070 ± 1.220 | 2.783 ± 1.120 | 2.337 ± 0.334 | 2.567 ± 0.630 | 2.448 ± 0.631 | 0.080 | 0.021 | 0.080 |
| FI 60–90 | 4.208 ± 0.256 | 5.519 ± 0.912 | 4.831 ± 0.244 | 5.523 ± 1.036 | 5.716 ± 0.244 | 5.593 ± 0.992 | 0.017 | 0.017 | 0.073 |
| FI 90–120 | 6.461 ± 0.560 | 7.531 ± 0.985 | 6.969 ± 0.345 | 7.095 ± 0.980 | 6.816 ± 0.870 | 6.993 ± 0.860 | 0.127 | 0.874 | 0.011 |
| FI 1–120 | 14.249 ± 6.029 | 17.060 ± 6.687 | 15.584 ± 6.296 | 16.770 ± 4.161 | 16.600 ± 3.087 | 16.710 ± 4.069 | 0.054 | 0.129 | 0.030 |
| FCR 1–30 | 3.191 ± 2.316 | 2.488 ± 1.476 | 2.850 ± 1.996 | 5.110 ± 0.303 | 2.403 ± 0.565 | 4.126 ± 0.565 | 0.002 | 0.078 | 0.055 |
| FCR 30–60 | 5.708 ± 1.494 | 5.607 ± 1.384 | 5.660 ± 1.443 | 4.465 ± 1.019 | 3.689 ± 2.114 | 4.183 ± 2.013 | 0.622 | 0.08 | 0.704 |
| FCR 60–90 | 5.351 ± 0.989 | 6.513 ± 5.957 | 5.903 ± 4.269 | 5.337 ± 0.413 | 4.707 ± 2.087 | 5.108 ± 1.904 | 0.863 | 0.557 | 0.563 |
| FCR 90–120 | 4.213 ± 2.146 | 6.601 ± 0.989 | 5.348 ± 1.659 | 5.357 ± 1.454 | 6.564 ± 1.770 | 5.796 ± 1.804 | 0.001 | 0.272 | 0.242 |
| FCR 1–120 | 4.164 ± 0.631 | 5.171 ± 0.833 | 4.642 ± 0.740 | 4.880 ± 0.213 | 4.554 ± 1.302 | 4.767 ± 1.248 | 0.240 | 0.850 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento-García, A.; Revilla, I.; Abecia, J.-A.; Palacios, C. Performance Evaluation of Two Slow-Medium Growing Chicken Strains Maintained under Organic Production System during Different Seasons. Animals 2021, 11, 1090. https://doi.org/10.3390/ani11041090
Sarmiento-García A, Revilla I, Abecia J-A, Palacios C. Performance Evaluation of Two Slow-Medium Growing Chicken Strains Maintained under Organic Production System during Different Seasons. Animals. 2021; 11(4):1090. https://doi.org/10.3390/ani11041090
Chicago/Turabian StyleSarmiento-García, Ainhoa, Isabel Revilla, José-Alfonso Abecia, and Carlos Palacios. 2021. "Performance Evaluation of Two Slow-Medium Growing Chicken Strains Maintained under Organic Production System during Different Seasons" Animals 11, no. 4: 1090. https://doi.org/10.3390/ani11041090
APA StyleSarmiento-García, A., Revilla, I., Abecia, J.-A., & Palacios, C. (2021). Performance Evaluation of Two Slow-Medium Growing Chicken Strains Maintained under Organic Production System during Different Seasons. Animals, 11(4), 1090. https://doi.org/10.3390/ani11041090








