Physiological Parameters Monitored on Bottlenose Dolphin Neonates (Tursiops truncatus, Montagu 1821) over the First 30 Days of Life
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Mothers
- Stage 1: Labor signs with vaginal discharge, milk discharge, decreased appetite, contractions, decreased basal temperature, increased intermammary distance;
- Stage 2: Starts with the appearance of the flukes (podalic parturition represents 98% of the cases) and finishes with the birth of the calf;
- Stage 3: Complete expulsion of the placenta.
2.3. Neonates
2.4. Statistical Analysis
3. Results
3.1. Parturition
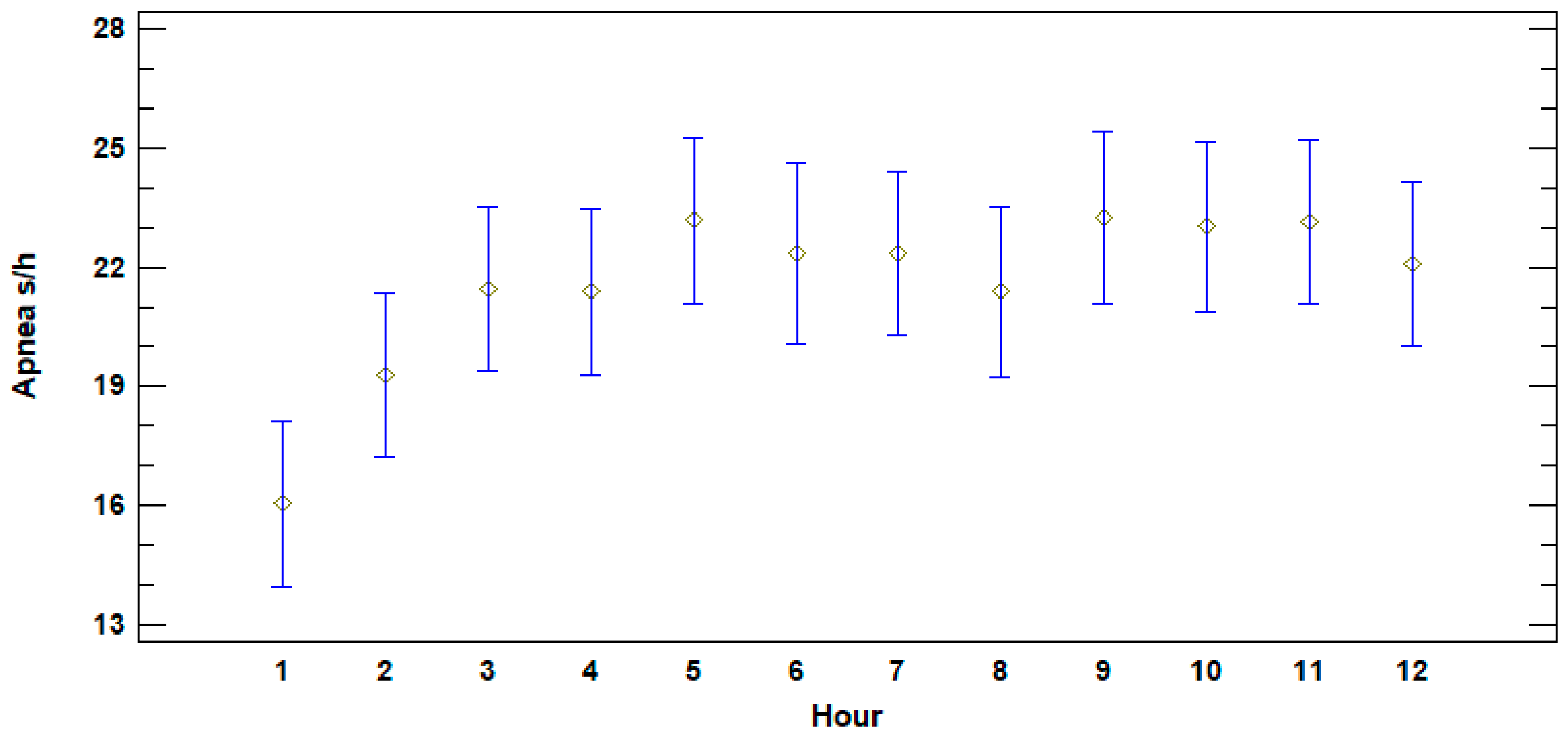
3.2. Apnea Duration
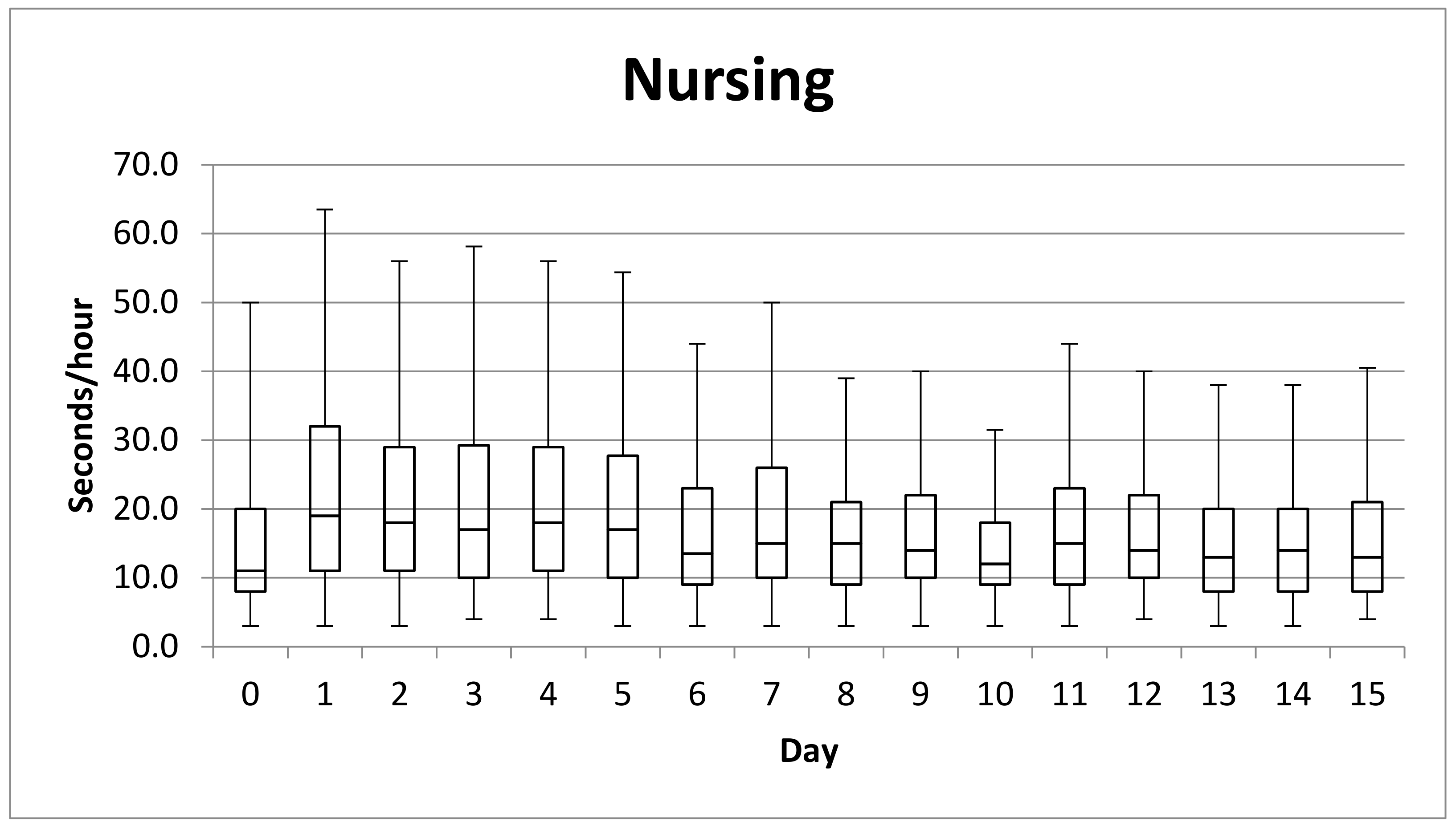
3.3. Nursing Activity
- -
- C1 showed two effective suckling events 17 h after birth, although it died two hours later.
- -
- C2 did not nurse and died on day 3.
- -
- Calf 11 was born from primiparous mother. Despite several attempts from the calf to nurse, the unexperienced mother did not accept any approach and even displayed aggressive behavior towards the calf. Due to lack of effective nursing events, the neonate was restrained for the first time at 14 h of life for a medical check-up and forced feeding. Colostrum was collected from the mother (38 mL) and administered by gastroesophageal tubing (9 mm diameter and 75 cm length, 50 cm mark) to Calf 11. During the following days, several attempts were conducted to allow the mother to take control of feeding the calf. However, the female was uncomfortable and still acting aggressively towards the calf, not allowing nursing to occur. Daily captures (from eight tube feeding sessions/day to two tube feeding sessions/day) were performed on Calf 11 for the first 24 days of life in order to ensure proper caloric intake, administering both maternal milk and formula.
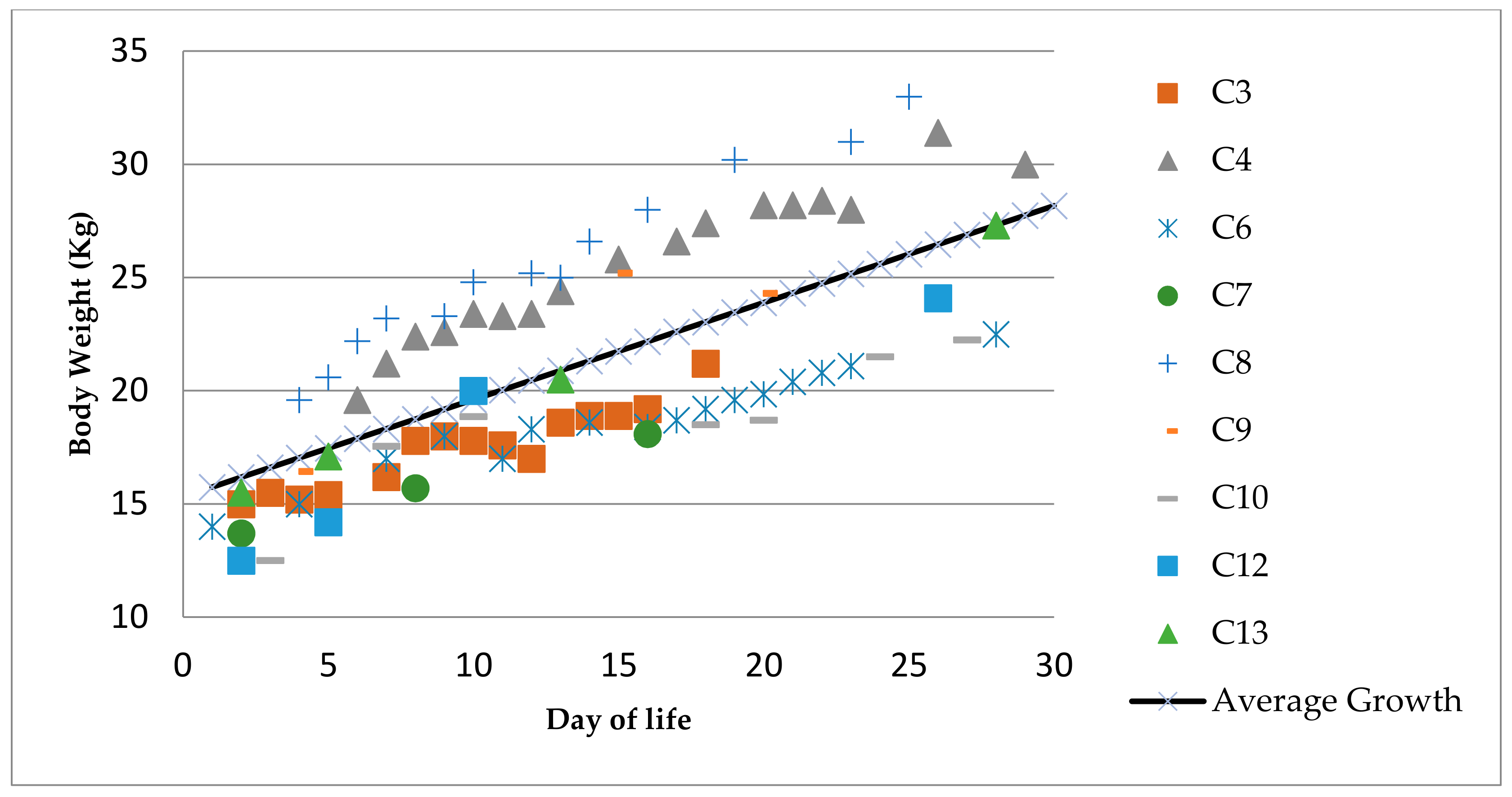
3.4. Growth and Body Weight
3.5. Blood Parameters
4. Discussion
5. Conclusions
- If the calf does not present an increase in apnea duration of at least 2.25 s between the first and the third hour of life.
- If the placenta is not expelled within 12 h after the birth of the calf.
- If the neonate does not successfully latch on within 20 h postpartum.
- If the percentage of synchronic surfacing by the dyad does not reach an average of 92.12 ± 6.76%.
- If any clinical signs of disease are observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, M.E. Zoo and Wild Animal Medicine, 2nd ed.; W.B Saunders Company: Philapdelphia, PA, USA, 1986; pp. 19–31. [Google Scholar]
- Waples, K.A.; Gales, N.J. Evaluating and minimising social stress in the care of captive bottlenose dolphins (Tursiops aduncus). Zoo Biol. 2002, 21, 5–26. [Google Scholar] [CrossRef]
- Sweeney, J.C.; Stone, R.; Campbell, M.; McBain, J.; St. Leger, J.; Xitco, M.; Jensen, E.; Ridgway, S. Comparative Survivability of Tursiops Neonates from three U.S. Institutions for the Decades 1990–1999 and 2000–2009. Aquat. Mamm. 2010, 36, 248–261. [Google Scholar] [CrossRef]
- Sweeney, J. Reproduction. In Zoo and Wild Animal Medicine; Fowler, M.E., Ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1986; pp. 789–791. [Google Scholar]
- Owen, J.S. A retrospective study of captive breeding programs involving Tursiops truncatus in South Florida. In Proceedings of the 21st Annual Conference of the International Association of Aquatic Animal Medicine, Vancouver, BC, Canada, 12–16 May 1990. [Google Scholar]
- Schroeder, J.P. Breeding Bottlenose Dolphins in Captivity. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 435–446. [Google Scholar]
- Mann, J.; Smuts, B.B. Behavioural development in wild Bottlenose dolphin newborns (Tursiops sp.). Behaviour 1999, 136, 529–566. [Google Scholar] [CrossRef]
- Mann, J.; Connor, R.C.; Barre, L.M.; Heithaus, M.R. Female reproductive success in Bottlenose dolphin (Tursiops sp.): Life history, habitat, provisioning and group-size effects. Behav. Ecol. 2000, 1, 210–219. [Google Scholar] [CrossRef]
- Makara, M.; Shimada, A.; Kawamura, N.; Murase, T.; Morita, T. Aspiration Pneumonia as a cause of neonatal death in three captive Bottlenose dolphins (Tursiops truncatus). J. Vet. Med. Sci. 2007, 69, 325–327. [Google Scholar] [CrossRef][Green Version]
- Venn-Watson, S.K.; Jensen, E.D.; Ridgway, S.H. Evaluation of population health among bottlenose dolphins (Tursiops truncatus) at the United States Navy Marine Mammal Program. J. Am. Vet. Med. Assoc. 2011, 238, 356–360. [Google Scholar] [CrossRef]
- Tursiops truncates. European Association for Zoos and Aquaria—EAZA EEP. The Netherlands. Available online: https://eaam.org/wp-content/uploads/2018/02/Tursiops-Truncatus-EEP-January-2013.pdf (accessed on 12 December 2019).
- Van Elk, C.E.; Hartmann, M.G.; Leus, K.; Fienieg, E.; de Man, D. Long-Term Management Plan for the Bottlenose Dolphin (Tursiops truncatus) European Endangered Species Programme (EEP); Dolfinarium Harderwijk: Harderwijk, The Netherlands, 2017; p. 59. [Google Scholar]
- Lacave, G. A survey of management practices for dolphin pregnancy with two examples of birth complications. Aquat. Mamm. 1991, 17, 37–41. [Google Scholar]
- Cozzi, B.; Huggenberger, S.; Oelschläger, H.H.A. The Anatomy of Dolphins: Insights into Body Structure and Function, 1st ed.; Academic Press: London, UK, 2017; pp. 389–391. [Google Scholar]
- Gage, L.J.; Walsh, T.M. Hand-rearing and artificial milk formula. In CRC Handbook of Marine Mammal Medicine; Gulland, F., Dierauf, L., Whitman, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 739–755. [Google Scholar]
- Jaakkola, K.; Willis, K. How long do dolphins live? Survival rates and life expectancies for bottlenose dolphins in zoological facilities vs. wild populations. Mar. Mammal. Sci. 2019, 36, 248–261. [Google Scholar] [CrossRef]
- Wells, R.S.; Scott, M.D. Estimating Bottlenose Dolphin Population Parameters from Individual Identification and Capture-Release Techniques; Report of the International Whaling Commission, Special Issue 12; International Whaling Commission: Cambridge, UK, 1990; pp. 407–415. [Google Scholar]
- Stolen, M.K.; Barlow, J.A. Model Life Table for Bottlenose dolphins (Tursiops truncatus) from the Indian River Lagoon System, Florida, USA. Mar. Mammal. Sci. 2003, 19, 630–649. [Google Scholar] [CrossRef]
- Neil, D.T.; Holmes, B.J. Survival of Bottlenose Dolphin (Tursiops sp.) Calves at a Wild Dolphin Provisioning Program, Tangalooma, Australia. Anthrozoos 2008, 21, 57–69. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Jensen, E.D.; Smith, C.R.; Xitco, M.; Ridgway, S.H. Evaluation of annual survival and mortality rates and longevity of bottlenose dolphins (Tursiops truncatus) at the United States Navy Marine Mammal Program from 2004 through 2013. J. Am. Vet. Med. Assoc. 2015, 246, 893–898. [Google Scholar] [CrossRef]
- Mann, J.; Smuts, B.B. Natal attraction: Allomaternal care and mother-infant separations in wild Bottlenose dolphins. Anim. Behav. 1998, 55, 1097–1113. [Google Scholar] [CrossRef]
- Robeck, T.R.; Atkinsons, S.K.C.; Brook, F. Reproduction. In CRC Handbook of Marine Mammal Medicine, 2nd ed.; Dierauf, L., Gulland, F.M.D., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 193–236. [Google Scholar] [CrossRef]
- Lacave, G.; Eggermont, M.; Verslycke, T.; Brook, F.; Salbany, F.; Roque, L.; Kinoshita, R. Prediction from ultrasonographic measurements of the expected delivery date in two species of Bottlenose dolphins Tursiops truncatus and Tursiops aduncus. Vet. Rec. 2004, 154, 228–233. [Google Scholar] [CrossRef]
- Biancani, B.; Da Dalt, L.; Lacave, G.; Romagnoli, S.; Gabai, G. Measuring fecal progestogens as a tool to monitor reproductive activity in captive female bottlenose dolphins (Tursiops truncatus). Theriogenology 2009, 72, 1282–1292. [Google Scholar] [CrossRef]
- Fowler, M.E.; Miller, R.E. Zoo and Wild Animal Medicine. Current Therapy, 6th ed.; Saunders Elsevier: St. Louis, MO, USA, 2008; pp. 66–67. [Google Scholar]
- Bossart, G.D.; Reidarson, T.; Dierauf, L.A.; Duffield, D. Clinical Pathology. In CRC Handbook of Marine Mammal Medicine, 2nd ed.; Dierauf, L., Gulland, F.M.D., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 383–436. [Google Scholar]
- Hall, A.J.; Wells, R.S.; Sweeney, J.C.; Townsend, F.I.; Balmer, B.C.; Hohn, A.A.; Rhinehart, H.L. Annual, seasonal and individual variation in hematology and clinical blood chemistry profiles in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida. Comp. Biochem. Physiol. A 2007, 148, 266–277. [Google Scholar] [CrossRef]
- Saviano, P.; Fiorucci, L.; Grande, F.; Macrelli, R.; Troisi, A.; Polisca, A.; Orlandi, R. Pregnancy and Fetal Development: Cephalic Presentation and Other Descriptive Ultrasonographic Findings from Clinically Healthy Bottlenose Dolphins (Tursiops truncatus) under Human Care. Animals 2020, 10, 908. [Google Scholar] [CrossRef]
- Terasawa, F.; Yokoyama, Y.; Kitaura, M. Rectal temperatures before and after parturition in bottlenose dolphins. Zoo Biol. 1999, 18, 153–156. [Google Scholar] [CrossRef]
- Hartmann, M.G. Dolphin neonate behaviour and management: A review. In Proceedings of the 41st Annual Symposium of the European Association for Aquatic Mammals, Nuremberg, Germany, 15–18 March 2013. [Google Scholar]
- Kinoshita, R.; Rayner, C.; Brook, F. A managed reproduction program for Tursiops truncatus aduncus. In Report from the Bottlenose Dolphin Breeding Workshop; Duffield, D.A., Robeck, T.R., Eds.; American Zoological Association Marine Mammal Taxon Advisory Group: Silver Spring, MD, USA, 1999; pp. 16–26. [Google Scholar]
- Blanchet, M.; Nance, T.; Ast, C.; Wahlberg, M.; Acquarone, M. First case of a monitored pregnancy of a harbour porpoise (Phocena phocena) under human care. Aquat. Mamm. 2008, 34, 9–20. [Google Scholar] [CrossRef]
- Tavolga, M.C.; Essapian, F.S. The behavior of the bottle-nosed dolphin (Tursiops truncatus): Mating, pregnancy, parturition and mother-infant behaviour. Zoologica 1957, 42, 11–31. [Google Scholar]
- Joseph, B.; Duffield, D.A.; Robeck, T.R. Summary data on reproduction of bottlenose dolphins in controlled environment. In Bottlenose Dolphin Reproduction Workshop; Duffield, D.A., Robeck, T.R., Eds.; American Zoological Association Marine Mammal Taxon Advisory Group: Silver Spring, MD, USA, 1999; pp. 42–56. [Google Scholar]
- Robeck, T.R.; O’Brien, J.K.; Atkinson, S. Reproduction. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F., Dierauf, L., Whitman, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 169–207. [Google Scholar]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; p. 182. [Google Scholar]
- Townsend, F.I. Hand-rearing techniques for neonate cetaceans. In Zoo and Wild Animal Medicine; Fowler, M.E., Ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1999; pp. 493–497. [Google Scholar]
- Baumgartner, K.; Lacave, G.; Sweeney, J.C.; Will, H. A Suggested Birth Protocol for Bottlenose Dolphins (Tursiops truncatus)—Updated 2015, Zoo Nuremberg. Aquat. Mamm. 2018, 44, 100–109. [Google Scholar] [CrossRef]
- Flower, J.E.; Langan, J.N.; Nevitt, B.N.; Chinnadurai, S.K.; Stacey, R.; Ivančić, M.; Adkesson, M.J. Neonatal Critical Care and Hand-Rearing of a Bottlenose Dolphin (Tursiops truncatus) Calf. Aquat. Mamm. 2018, 44, 482–490. [Google Scholar] [CrossRef]
- Krames, B.; Krames, J. Pre-natale care and post-natal observation of four Tursiops truncatus first—Time mothers. Mar. Mamm. 1996, 2, 10–23. [Google Scholar]
- Peddemors, V.M. Respiratory development in a captive-born Bottlenose dolphin, Tursiops truncatus, calf. S. Afr. J. Zool. 1990, 25, 178–184. [Google Scholar] [CrossRef][Green Version]
- Kleiva, Z. Analysis of Health Surveys of the Black Sea Bottlenose Dolphins (Tursiops truncatus ponticus). Ph.D. Thesis, Lithuanian University of Health Science Veterinary Academy, Kaunas, Lithuania, 2013. [Google Scholar]
- Cockcroft, V.G.; Ross, G.J.B. Observations on the early development of a captive Bottlenose dolphin calf. In The Bottlenose Dolphin; Leatherwood, S., Reeves, R.R., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 461–478. [Google Scholar]
- Peddemors, V.M.; Fothergill, M.; Cockcroft, V.G. Feeding and growth in a captive-born Bottlenose dolphin, Tursiops truncatus. S. Afr. J. Zool. 1992, 27, 74–80. [Google Scholar] [CrossRef]
- West, K.L.; Oftedal, O.T.; Carpenter, J.R.; Krames, B.J.; Campbell, M.; Sweeney, J.C. Effect of lactation stage and concurrent pregnancy on milk composition in the bottlenose dolphin. J. Zool. 2007, 273, 148–160. [Google Scholar] [CrossRef]
- McKenna, L.P.; Campbell, M.E. Care and handling of neonate bottlenose dolphins (Tursiops truncatus): A clinical and behavioral approach. Soundings 2012, 37, 27–31. [Google Scholar]
- von Streit, C.; Ganslosser, U.; von Fersen, L. Behavioral development of two captive mother-calf dyads of bottlenose dolphins (Tursiops truncatus) in the calves’ first year. Int. J. Comp. Psychol. 2013, 26, 176–196. [Google Scholar]
- Ridgway, S.; Kamolnick, T.; Reddy, M.; Curry, C. Orphan-induced lactation in Tursiops and analysis of collected milk. Mar. Mammal. Sci. 1995, 11, 172–182. [Google Scholar] [CrossRef]
- Wells, R.S. Learning from nature: Bottlenose dolphin care and husbandry. Zoo Biol. 2009, 28, 635–651. [Google Scholar] [CrossRef]
- Goldstein, J.D.; Reese, E.; Reif, J.S.; Varela, R.A.; McCulloch, S.D.; Defran, R.H.; Fair, P.A.; Bossart, G.D.; Hansen, L. Hematologic, biochemical, and cytologic findings from apparently healthy atlantic bottlenose dolphins (Tursiops truncatus) inhabiting the Indian River Lagoon, Florida, USA. J. Wild. Dis. 2006, 42, 447–454. [Google Scholar] [CrossRef]
- Gulland, F.; Dierauf, L.; Whitman, K. (Eds.) CRC Handbook of Marine Mammal Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1003–1004. [Google Scholar]
- Venn-Watson, S.; Jensen, E.D.; Ridgway, S.H. Effects of age and sex on clinicopathologic reference ranges in a healthy managed Atlantic bottlenose dolphin population. J. Am. Vet. Med. Assoc. 2007, 231, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Geraci, J.R. Cortisol, aldosterone, and leucocytes in the stress response sf bottlenose dolphins, Tursiops truncatus. Can. J. Fish. Aquat. Sci. 1986, 43, 1010–1016. [Google Scholar] [CrossRef]
- St. Aubin, D.J.; Ridgway, S.H.; Wells, R.S.; Rhinehart, H. Dolphin thyroid and adrenal hormones: Circulating levels in wild and semidomesticated Tursiops truncatus, and influence of sex, age, and season. Mar. Mamm. Sci. 1996, 12, 1–13. [Google Scholar] [CrossRef]
- Fair, P.A.; Schaefer, A.M.; Romano, T.A.; Bossart, G.D.; Lamb, S.V.; Reif, J.S. Stress response of wild bottlenose dolphins (Tursiops truncatus) during capture–release health assessment studies. Gen. Comp. Endocr. 2014, 206, 203–212. [Google Scholar] [CrossRef]
- Biancani, B.; Dalt, L.D.; Gallina, G.; Capolongo, F.; Gabai, G. Fecal cortisol radioimmunoassay to monitor adrenal gland activity in the bottlenose dolphin (Tursiops truncatus) under human care. Mar. Mamm. Sci. 2017, 33, 1014–1034. [Google Scholar] [CrossRef]
- Crocker, D.E. Endocrinology. In CRC Handbook of Marine Mammal Medicine; Gulland, F., Dierauf, L., Whitman, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 137–151. [Google Scholar]
- Atkinson, S.; Dierauf, L.A. Stress and Marine Mammals. In CRC Handbook of Marine Mammal Medicine; Gulland, F., Dierauf, L., Whitman, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 153–168. [Google Scholar]
- Ortiz, R.M.; Worthy, A.J. Effects of capture on adrenal steroid and vasopressin concentrations in free-ranging bottlenose dolphins (Tursiops truncatus). Comp. Biochem. Phys. A 2000, 125, 317–324. [Google Scholar] [CrossRef]
- Hansen, L.J.; Wells, R.S. Bottlenose Dolphin Health Assessment: Field Report on Sampling Near Beaufort, North Carolina, during July, 1995; NMFS-SEFSC-382; NOAA: Charleston, SC, USA, 1996; p. 28.
- Mellor, D.J. Operational Details of the Five Domains Model and Its Key Applications to the Assessment and Management of Animal Welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef]
| Facility | Shape | Water Surface Area (m2) | Maximum Depth (m) | Water Volume (m3) |
|---|---|---|---|---|
| Mediterraneo Marine Park | Circular | 78 | 3.5 | 432 |
| Oltremare | Rectangular with rounded edges | 240 | 3 | 850 |
| Acquario di Genova | Rectangular with rounded edges | 198 | 4 | 1000 |
| Mother | Country and Year of Birth | Previous Parturition | Calf | Facility |
|---|---|---|---|---|
| M1 | WB (USA) 1979 | YES | C1 | OLT |
| M2 | WB (CUBA) 1997 | YES | C2 | MMP |
| M2 | WB (CUBA) 1997 | NO | C5 | MMP |
| M2 | WB (CUBA) 1997 | YES | C13 | MMP |
| M3 | CB (ITALY) 1997 | YES | C3 | OLT |
| M4 | CB (ITALY) 1994 | YES | C4 | OLT |
| M4 | CB (ITALY) 1994 | YES | C8 | OLT |
| M5 | WB (CUBA) 1998 | NO | C6 | MMP |
| M6 | WB (CUBA) 1999 | NO | C7 | MMP |
| M6 | WB (CUBA) 1999 | YES | C10 | MMP |
| M6 | WB (CUBA) 1997 | YES | C12 | MMP |
| M7 | CB (ITALY) 2001 | NO | C9 | ADG |
| M8 | CB (ITALY) 1995 | NO | C11 | ADG |
| Mother ID | Calf ID | Calf Sex | Date of Birth (Day/Month/Year) | Time of the Birth | Length of Stage 2 (Minutes) | Length of Stage 3 (Minutes after Birth) | First Effective Nursing (Minutes after Birth) |
|---|---|---|---|---|---|---|---|
| M1 | C1 | Female | 19/01/2015 | 16:43 | 43 | 285 | n/a |
| M2 | C2 | Male | 13/08/2016 | 01:37 | 37 | 220 | n/a |
| M3 | C3 | Female | 31/05/2010 | 04:20 | 105 | 300 | 510 |
| M4 | C4 | Male | 02/07/2010 | 08:30 | 150 | 265 | 240 |
| M2 | C5 | Male | 20/07/2010 | 03:21 | 60 | 290 | 390 |
| M5 | C6 | Male | 08/08/2010 | 19:05 | 100 | 360 | 990 |
| M6 | C7 | Male | 20/12/2010 | 14:51 | 60 | 360 | 1080 |
| M4 | C8 | Male | 09/08/2014 | 20:59 | 225 | 570 | 180 |
| M7 | C9 | Female | 01/09/2014 | 03:48 | 45 | 240 | 780 |
| M6 | C10 | Female | 31/10/2014 | 19:00 | 20 | 345 | 900 |
| M8 | C11 | Male | 20/08/2015 | 02:13 | 110 | 410 | n/a |
| M6 | C12 | Male | 09/12/2018 | 22:03 | 50 | 240 | 780 |
| M2 | C13 | Male | 01/10/2019 | 22:52 | 29 | 332 | 667 |
| Day 1–4 | ||||||
|---|---|---|---|---|---|---|
| N | Samples | Min | Max | Av | SD | |
| Body weight (Kg) | 11 | 14 | 12.5 | 20.6 | 15.23 | 2.36 |
| Length (cm) | 11 | 13 | 95.5 | 118 | 107.98 | 4.91 |
| Girth (cm) | 9 | 11 | 48.3 | 72.5 | 61.80 | 6.41 |
| Day 5–10 | ||||||
| N | Samples | Min | Max | Av | SD | |
| Body weight (Kg) | 8 | 23 | 14.2 | 24.8 | 19.43 | 3.00 |
| Length (cm) | 6 | 19 | 106 | 117.5 | 112.59 | 3.55 |
| Girth (cm) | 7 | 20 | 61 | 74 | 68.30 | 4.04 |
| Day 11–20 | ||||||
| N | Samples | Min | Max | Av | SD | |
| Body weight (Kg) | 8 | 33 | 17 | 30.2 | 21.83 | 3.90 |
| Length (cm) | 8 | 25 | 108 | 125 | 116.65 | 4.32 |
| Girth (cm) | 8 | 28 | 64 | 80.5 | 71.91 | 4.13 |
| Day 21–30 | ||||||
| N | Samples | Min | Max | Av | SD | |
| Body weight (Kg) | 6 | 15 | 20.4 | 33 | 26.00 | 4.38 |
| Length (cm) | 5 | 10 | 112 | 131 | 125.50 | 5.33 |
| Girth (cm) | 5 | 10 | 69 | 85.5 | 78.55 | 4.57 |
| Parameter | 0–Tx | Tx–7 | 8–14 | 15–30 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12, Sample = 12 | n = 6, Sample = 18 | n = 7, Sample = 19 | n = 7, Sample = 30 | |||||||||
| n | 25% | 75% | n | 25% | 75% | n | 25% | 75% | n | 25% | 75% | |
| RBC (106/uL) | 11 | 3.85 | 4.04 | 18 | 3.86 | 4.40 | 17 | 3.60 | 4.23 | 29 | 3.41 | 3.93 |
| HGB (g/dL) | 11 | 14.65 | 15.40 | 18 | 15.03 | 16.73 | 17 | 13.90 | 15.10 | 29 | 12.80 | 14.40 |
| HCT (%) | 11 | 43.80 | 46.5 | 18 | 43.13 | 46.30 | 17 | 41.00 | 43.20 | 29 | 37.40 | 41.70 |
| MCV (fl) | 11 | 112 | 115.05 | 18 | 105.53 | 109.83 | 17 | 102.10 | 113.00 | 29 | 107.80 | 111.30 |
| MCH (pg) | 11 | 36.60 | 39.30 | 18 | 35.10 | 39.68 | 17 | 35.70 | 39.10 | 29 | 36.00 | 38.60 |
| MCHC (g/dL) | 11 | 33.10 | 34.00 | 18 | 33.75 | 36.00 | 17 | 33.80 | 35.20 | 29 | 33.20 | 34.90 |
| WBC (103/uL) | 11 | 4.12 | 7.40 | 18 | 3.44 | 7.10 | 17 | 5.09 | 8.40 | 29 | 5.20 | 8.00 |
| Neutrophils (band) (%) | 12 | 0.00 | 0.00 | 18 | 0.00 | 0.00 | 16 | 0.00 | 0.00 | 29 | 0.00 | 0.00 |
| Neutrophils (mature) (%) | 12 | 56.25 | 77.25 | 18 | 44.25 | 68.13 | 17 | 39.20 | 79.00 | 29 | 60.00 | 75.00 |
| Lymphocytes (%) | 12 | 18.50 | 28.25 | 18 | 16.73 | 24.93 | 17 | 13.00 | 43.50 | 29 | 16.60 | 23.00 |
| Monocytes (%) | 12 | 2.00 | 5.75 | 18 | 5.25 | 34.53 | 17 | 3.00 | 8.40 | 29 | 3.00 | 9.00 |
| Eosinophils (%) | 12 | 0.00 | 2.00 | 18 | 1.18 | 2.93 | 17 | 1.40 | 3.00 | 29 | 2.00 | 6.60 |
| Reticulocytes (%) | 0 | N.D. | N.D. | 2 | 1.27 | 1.36 | 1 | 3.00 | 3.00 | 8 | 5.46 | 6.49 |
| Platelets (103/uL) | 11 | 119.50 | 169.00 | 17 | 157.00 | 218.00 | 17 | 187.00 | 242.00 | 29 | 327.00 | 419.00 |
| Glucose (mg/dL) | 9 | 91.50 | 130.45 | 16 | 92.00 | 121.25 | 16 | 116.50 | 150.88 | 22 | 128.00 | 148.75 |
| BUN (mg/dL) | 9 | 42.00 | 63.50 | 16 | 47.00 | 69.50 | 17 | 49.00 | 58.00 | 25 | 43.00 | 49.00 |
| Creatinine (mg/dL) | 9 | 0.50 | 0.70 | 14 | 0.50 | 0.71 | 16 | 0.55 | 0.70 | 25 | 0.68 | 0.80 |
| Cholesterol (mg/dL) | 5 | 174.00 | 210.00 | 10 | 217.50 | 248.00 | 11 | 219.81 | 278.10 | 16 | 208.25 | 262.26 |
| Triglycerides (mg/dL) | 5 | 221.30 | 328.00 | 10 | 151.00 | 238.75 | 11 | 116.50 | 237.50 | 19 | 137.00 | 200.50 |
| Bilirubin (mg/dL) | 8 | 0.32 | 0.68 | 12 | 0.28 | 0.43 | 11 | 0.05 | 0.20 | 19 | 0.10 | 0.28 |
| ALP (U/L) | 8 | 1825.50 | 2270.75 | 13 | 1699.00 | 2266.00 | 11 | 2343.50 | 4503.50 | 21 | 3250.00 | 5864.50 |
| GGT (U/L) | 6 | 22.25 | 52.75 | 10 | 45.00 | 58.50 | 11 | 43.50 | 49.50 | 20 | 32.00 | 37.25 |
| GOT-AST (U/L) | 7 | 172.50 | 207.50 | 12 | 164.00 | 225.25 | 16 | 144.50 | 193.75 | 24 | 127.75 | 179.50 |
| GPT-ALT (U/L) | 9 | 35.00 | 40.00 | 15 | 26.05 | 46.00 | 16 | 14.00 | 28.13 | 25 | 13.00 | 25.00 |
| α-Amylase (U/L) | 5 | 2.00 | 3.00 | 7 | 1.00 | 2.35 | 4 | 1.00 | 2.20 | 16 | 1.00 | 2.00 |
| Lipase (U/L) | 3 | 10.00 | 15.50 | 6 | 13.25 | 20.00 | 3 | 8.00 | 10.00 | 14 | 16.00 | 21.00 |
| CK (U/L) | 6 | 623.00 | 1182.75 | 10 | 595.50 | 1083.25 | 10 | 458.75 | 551.25 | 19 | 520.50 | 838.50 |
| LDH (U/L) | 6 | 753.00 | 1026.25 | 10 | 999.05 | 2245.25 | 10 | 697.50 | 898.23 | 20 | 533.75 | 1857.25 |
| Tot. protein (g/L) | 9 | 53.00 | 62.00 | 15 | 58.70 | 61.25 | 17 | 52.00 | 57.00 | 24 | 51.83 | 54.70 |
| Albumin (g/L) | 8 | 38.00 | 41.25 | 12 | 39.23 | 45.40 | 14 | 37.53 | 41.30 | 20 | 33.75 | 39.48 |
| Globulin (g/L) | 8 | 12.53 | 19.75 | 12 | 17.08 | 21 | 14 | 15.33 | 17.65 | 20 | 12.68 | 21.00 |
| Alb/Glob | 9 | 1.82 | 3.75 | 14 | 1.90 | 2.32 | 16 | 2.16 | 2.67 | 23 | 1.66 | 3.25 |
| Sodium (mmol/L) | 9 | 158.00 | 162.00 | 15 | 153.70 | 160.50 | 15 | 153.50 | 157.00 | 24 | 151.60 | 154.00 |
| Potassium (mmol/L) | 9 | 4.60 | 4.90 | 15 | 3.45 | 4.45 | 15 | 3.70 | 4.20 | 25 | 3.70 | 4.10 |
| Chloride (mmol/L) | 7 | 112.50 | 117.00 | 10 | 101.30 | 110.00 | 14 | 106.00 | 111.00 | 24 | 108.98 | 112.00 |
| Phosphorus (mmol/L) | 7 | 1.28 | 2.10 | 15 | 1.50 | 2.23 | 14 | 2.08 | 2.45 | 23 | 1.87 | 2.26 |
| Calcium (mmol/L) | 9 | 2.30 | 2.52 | 14 | 2.06 | 2.54 | 15 | 2.12 | 2.70 | 22 | 2.23 | 2.45 |
| Magnesium (mmol/L) | 4 | 1.14 | 1.40 | 8 | 0.65 | 0.82 | 12 | 0.60 | 0.81 | 17 | 0.70 | 1.00 |
| Fibrinogen (mg/dL) | 4 | 125.00 | 230.00 | 10 | 211.50 | 323.00 | 8 | 272.75 | 354.00 | 22 | 254.00 | 637.00 |
| Cortisol (µg/dL) | 5 | 1.40 | 1.90 | 6 | 0.63 | 2.00 | 9 | 0.40 | 1.00 | 18 | 0.30 | 1.00 |
| Iron (µg/dL) | 9 | 251.23 | 413.40 | 13 | 249.00 | 502.00 | 15 | 228.70 | 343.00 | 27 | 183.00 | 300.00 |
| ESR (mm/h) | 3 | 0.50 | 6.50 | 4 | 0.00 | 3.00 | 5 | 1.00 | 4.00 | 13 | 1.00 | 4.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biancani, B.; Sánchez-Contreras, G.J.; Furlati, S.; Benaglia, F.; Arija, C.M.; Gili, C. Physiological Parameters Monitored on Bottlenose Dolphin Neonates (Tursiops truncatus, Montagu 1821) over the First 30 Days of Life. Animals 2021, 11, 1066. https://doi.org/10.3390/ani11041066
Biancani B, Sánchez-Contreras GJ, Furlati S, Benaglia F, Arija CM, Gili C. Physiological Parameters Monitored on Bottlenose Dolphin Neonates (Tursiops truncatus, Montagu 1821) over the First 30 Days of Life. Animals. 2021; 11(4):1066. https://doi.org/10.3390/ani11041066
Chicago/Turabian StyleBiancani, Barbara, Guillermo J. Sánchez-Contreras, Stefano Furlati, Francesco Benaglia, Carmen M. Arija, and Claudia Gili. 2021. "Physiological Parameters Monitored on Bottlenose Dolphin Neonates (Tursiops truncatus, Montagu 1821) over the First 30 Days of Life" Animals 11, no. 4: 1066. https://doi.org/10.3390/ani11041066
APA StyleBiancani, B., Sánchez-Contreras, G. J., Furlati, S., Benaglia, F., Arija, C. M., & Gili, C. (2021). Physiological Parameters Monitored on Bottlenose Dolphin Neonates (Tursiops truncatus, Montagu 1821) over the First 30 Days of Life. Animals, 11(4), 1066. https://doi.org/10.3390/ani11041066







