Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
2.2. Growth Parameters and Digestibility Trial
2.3. Sample Collection and Analytical Procedures
2.4. Serum Biochemical Analysis
2.5. Chemical Copmostion of Meat
2.6. Antioxidant Potential of Broiler Meat
2.6.1. Total Phenolic Content (TPC)
2.6.2. 2,2-Diphenyl-1-picrihydrzyl (DPPH) Assay
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.7. TBARS Assay
2.8. Real-Time PCR to Assess Nutrient Transporter Encoding Genes
2.9. Microbiological Assay
2.10. Statistical Analysis
3. Results
3.1. Growth Performance and Nutrient Digestibility
3.2. Serum Biochemical Parameters
3.3. Chemical Composition of Meat:
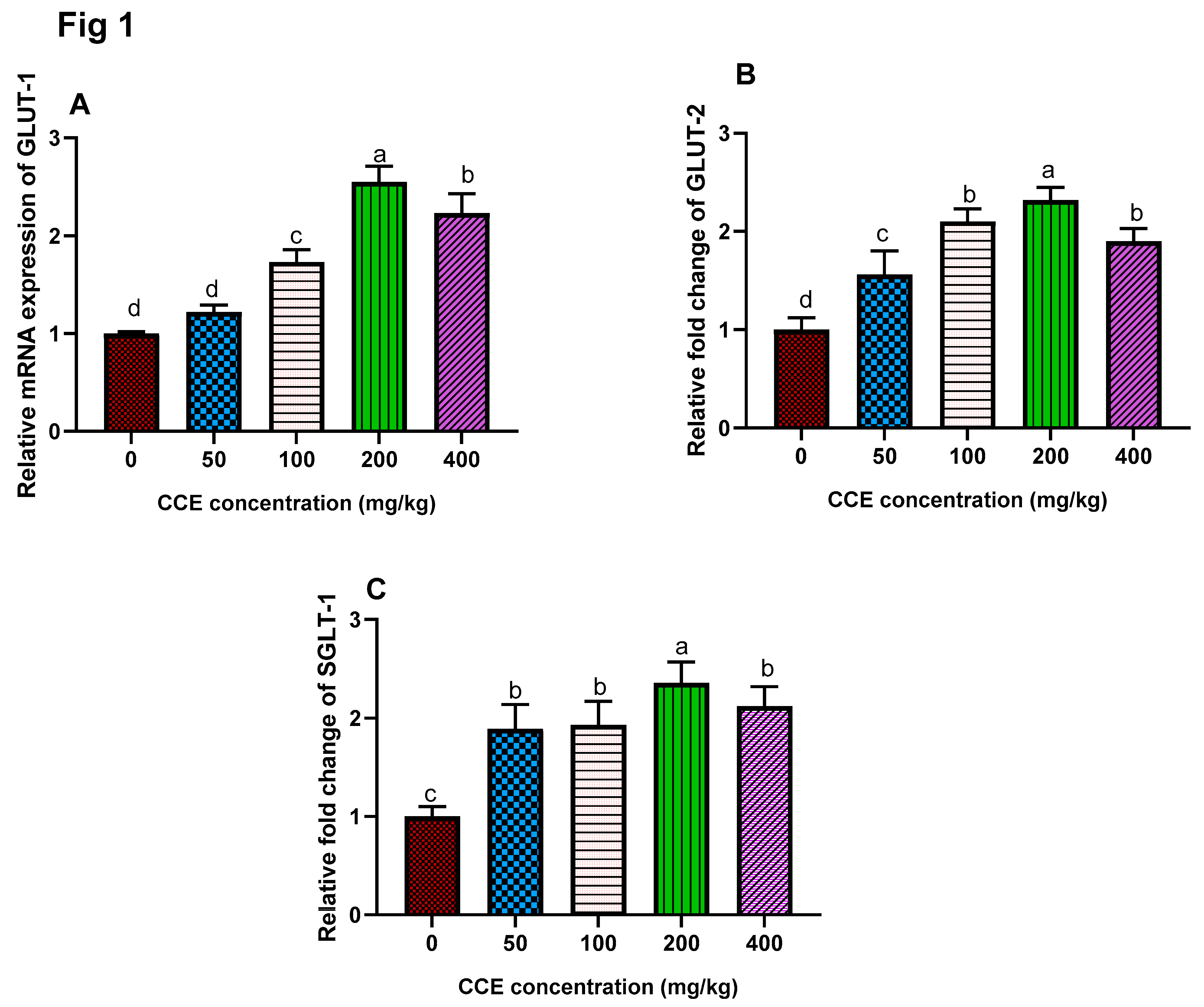
3.4. Gene Expression of Glucose Transporter
3.5. Gut Microbiota
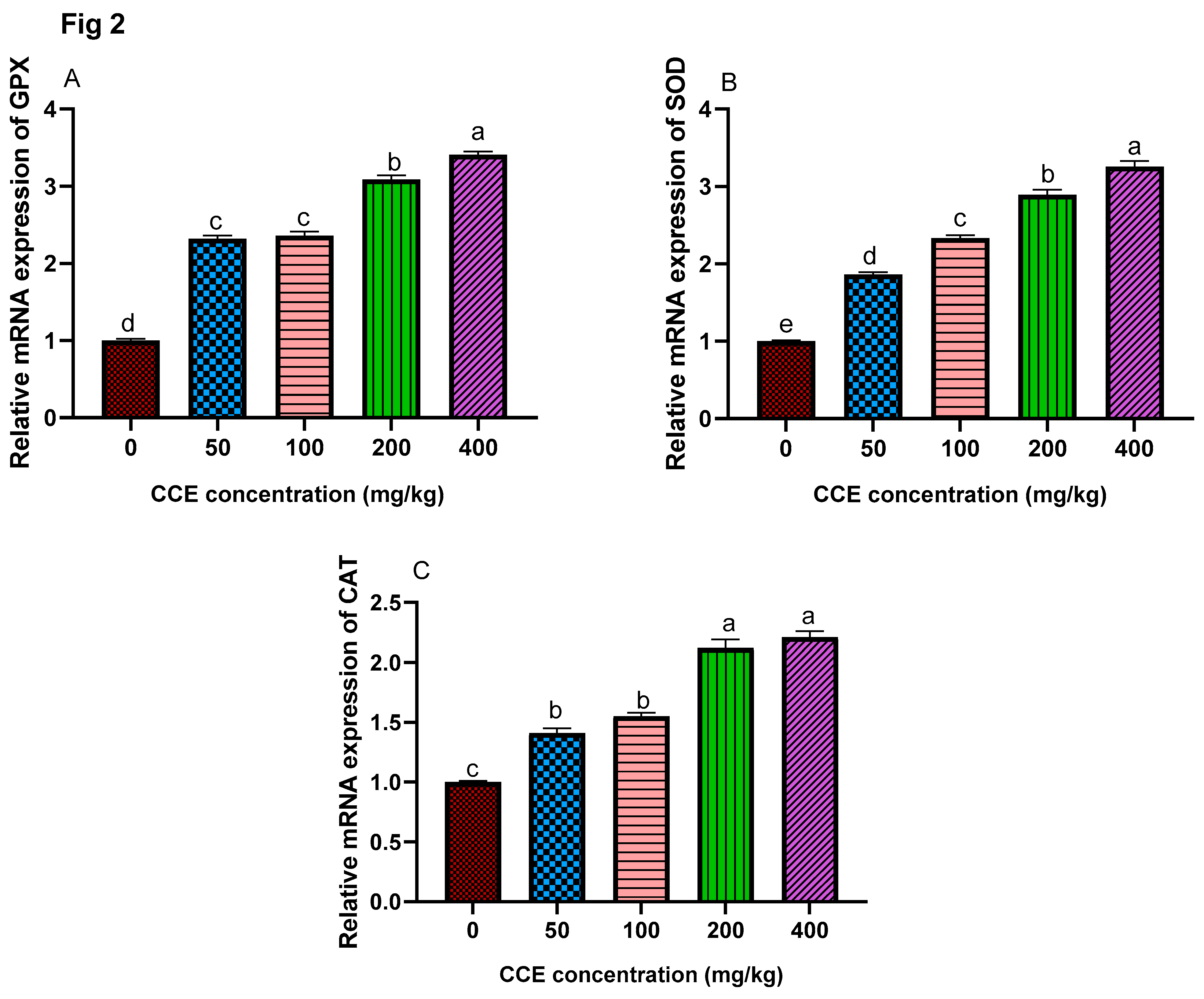
3.6. Antioxidant-Related Genes
3.7. Antioxidant Potential of Breast Meat
3.7.1. Total Phenolic Content (TPC) in Breast Meat
3.7.2. The Free (DPPH) Radical Scavenging Activity
3.7.3. FRAP Reducing Activity
3.8. Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurekci, C.; Al Jassim, R.; Hassan, E.; Bishop-Hurley, S.L.; Padmanabha, J.; McSweeney, C.S. Effects of feeding plant-derived agents on the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2014, 93, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Prakash, U.N.; Srinivasan, K. Beneficial influence of dietary spices on the ultrastructure and fluidity of the intestinal brush border in rats. Br. J. Nutr. 2010, 104, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.; Kettunen, H.; Bento, M.H.L.; Saarinen, M.; Lahtinen, S.; Ouwehand, A.; Schulze, H.; Rautonen, N. The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 2010, 51, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Hu, L.; Suo, Y.; Zhang, L.; Jin, F.; Feng, X.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Applegate, T.; Klose, V.; Steiner, T.; Ganner, A.; Schatzmayr, G. Probiotics and phytogenics for poultry: Myth or reality? J. Appl. Poult. Res. 2010, 19, 194–210. [Google Scholar] [CrossRef]
- Kumar, P.; Patra, A. Beneficial uses of black cumin (Nigella sativa L.) seeds as a feed additive in poultry nutrition. Worlds Poult. Sci. J. 2017, 73, 872–885. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Mehaisen, G.M.; Eshak, M.G.; Elkaiaty, A.M.; Atta, A.-R.M.; Mashaly, M.M.; Abass, A.O. Comprehensive growth performance, immune function, plasma biochemistry, gene expressions and cell death morphology responses to a daily corticosterone injection course in broiler chickens. PLoS ONE 2017, 12, e0172684. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Tural, S.; Koca, I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci. Hortic. 2008, 116, 362–366. [Google Scholar] [CrossRef]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) fruit as a potential source of natural health-promoting compounds: Physico-chemical characterisation of bioactive components. Plant Foods Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.; Arriaga, Â.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Sarker, M.; Sharmin, M.; Huque, K.; Yang, C. Effect of Medicinal Plants and Probiotics on Thiobarbituric acid value in broiler chicken meat. In Proceedings of the 58th International Congress of Meat Science and Technology, Montreal, QC, Canada, 12–17 August 2012. [Google Scholar]
- Quah, Y.; Lee, S.-J.; Lee, E.-B.; Birhanu, B.T.; Ali, M.; Abbas, M.A.; Boby, N.; Im, Z.-E.; Park, S.-C. Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities. Nutrients 2020, 12, 3317. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS characterization and identification of bioactive iridoids in Cornus mas fruit. J. Anal. Methods Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Krośniak, M.; Gąstoł, M.; Szałkowski, M.; Zagrodzki, P.; Derwisz, M. Cornelian cherry (Cornus mas L.) juices as a source of minerals in human diet. J. Toxicol. Env. Health A 2010, 73, 1155–1158. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef]
- Krisch, J. Effect of fruit juices and pomace extracts on the growth of Gram-positive and Gram-negative bacteria. Acta Biol. Szeged 2008, 52, 267–270. [Google Scholar]
- Ersoy, N.; Bagci, Y.; Gok, V. Antioxidant properties of 12 cornelian cherry fruit types (Cornus mas L.) selected from Turkey. Sci. Res. Essays 2011, 6, 98–102. [Google Scholar]
- Narimani-Rad, M.; Zendehdel, M.; Abbasi, M.M.; Abdollahi, B.; Lotfi, A. Cornelian cherry (Cornus mas L.) extract affects glycemic status in Wistar rats. Bull. Env. Pharm. Life Sci. 2013, 2, 48–50. [Google Scholar]
- Waksmundzka-Hajnos, M.; Sherma, J. High Performance Liquid Chromatography in Phytochemical Analysis; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Aziza, A.; Quezada, N.; Cherian, G. Antioxidative effect of dietary Camelina meal in fresh, stored, or cooked broiler chicken meat. Poult. Sci. 2010, 89, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, I.; Röhe, I.; Goodarzi Boroojeni, F.; Knorr, F.; Mader, A.; Hafeez, A.; Zentek, J. Feed supplemented with organic acids does not affect starch digestibility, nor intestinal absorptive or secretory function in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 29–35. [Google Scholar] [CrossRef]
- Aviagen, W. Ross 308: Broiler Nutrition Specification. Available online: https://tmea.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf (accessed on 30 September 2019).
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- McDonald, P. Animal Nutrition; Pearson Education: London, UK, 2002. [Google Scholar]
- Senevirathne, M.; Kim, S.-H.; Siriwardhana, N.; Ha, J.-H.; Lee, K.-W.; Jeon, Y.-J. Antioxidant potential of Ecklonia cava on reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci. Technol. Int. 2006, 12, 27–38. [Google Scholar] [CrossRef]
- Hwang, K.-E.; Choi, Y.-S.; Choi, S.-M.; Kim, H.-W.; Choi, J.-H.; Lee, M.-A.; Kim, C.-J. Antioxidant action of ganghwayakssuk (Artemisia princeps Pamp.) in combination with ascorbic acid to increase the shelf life in raw and deep fried chicken nuggets. Meat Sci. 2013, 95, 593–602. [Google Scholar] [CrossRef]
- Oyaızu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Starčević, K.; Krstulović, L.; Brozić, D.; Maurić, M.; Stojević, Z.; Mikulec, Ž.; Bajić, M.; Mašek, T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef]
- Johannah, N.; Joseph, A.; Maliakel, B.; Krishnakumar, I. Dietary addition of a standardized extract of turmeric (TurmaFEED TM) improves growth performance and carcass quality of broilers. J. Anim. Sci. Technol. 2018, 60, 8. [Google Scholar]
- El-Hack, A.; Mohamed, E.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Erener, G.; Ocak, N.; Altop, A.; Cankaya, S.; Aksoy, H.M.; Ozturk, E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian-Australas. J. Anim. Sci. 2011, 24, 1128–1135. [Google Scholar] [CrossRef]
- Herrero-Encinas, J.; Blanch, M.; Pastor, J.; Mereu, A.; Ipharraguerre, I.; Menoyo, D. Effects of a bioactive olive pomace extract from Olea europaea on growth performance, gut function, and intestinal microbiota in broiler chickens. Poult. Sci. 2020, 99, 2–10. [Google Scholar] [CrossRef]
- Wang, M.; Suo, X.; Gu, J.; Zhang, W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin extract in broiler chickens: Effect on chicken coccidiosis and antioxidant status. Poult. Sci. 2008, 87, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.; Warner, R.D.; Dunshea, F.R. A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens. Animals 2020, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; De Mejia, E.G. Phenolic compounds from fermented berry beverages modulated gene and protein expression to increase insulin secretion from pancreatic β-cells in vitro. J. Agric. Food Chem. 2016, 64, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Amasheh, S.; Aschenbach, J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds–A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3237–3266. [Google Scholar] [CrossRef]
- Ibrahim, D.; Sewid, A.H.; Arisha, A.H.; Abd El-Fattah, A.H.; Abdelaziz, A.M.; Al-Jabr, O.A.; Kishawy, A.T. Influence of Glycyrrhiza glabra Extract on Growth, Gene Expression of Gut Integrity, and Campylobacter jejuni Colonization in Broiler Chickens. Front. Vet. Sci. 2020, 7, 2063. [Google Scholar] [CrossRef]
- Sarker, M.S.K.; Kim, G.M.; Sharmin, F.; Yang, C.J. Effects of medicinal plants, Alisma canaliculatum, Laminaria japonica and Cornus officinalis, treated with probiotics on growth performance, meat composition and internal organ development of broiler chicken. Asian J. Med. Biol. Res. 2016, 2, 696–702. [Google Scholar] [CrossRef][Green Version]
- Son, H.-K.; Kang, S.-T.; Lee, J.-J. Effects of Peucedanum japonicum Thunb. on lipid metabolism and antioxidative activities in rats fed a high-fat/high-cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2014, 43, 641–649. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wei, C.; Chen, J.; Ye, X. Proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves regulate lipid metabolism and glucose consumption by activating AMPK pathway in HepG2 cells. J. Funct. Foods 2017, 29, 217–225. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Allison, R.W. Laboratory evaluation of plasma and serum proteins. In Thrall MA. Veterinary Hematology and Clinical Chemistry, 2nd ed.; Blackwell Publishing: London, UK, 2012; pp. 460–475. [Google Scholar]
- Gopi, M.; Dutta, N.; Pattanaik, A.K.; Jadhav, S.E.; Madhupriya, V.; Tyagi, P.K.; Mohan, J. Effect of polyphenol extract on performance, serum biochemistry, skin pigmentation and carcass characteristics in broiler chickens fed with different cereal sources under hot-humid conditions. Saudi J. Biol. Sci. 2020, 27, 2719–2726. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS ONE 2012, 7, e43197. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Arisha, A.H.; Abd El-Aziz, R.M.; Sherief, W.R.; Adil, S.H.; El Sayed, R.; Metwally, A.E. Impact of feeding anaerobically fermented feed supplemented with acidifiers on its quality and growth performance, intestinal villi and enteric pathogens of mulard ducks. Livest. Sci. 2020, 242, 104299. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharm. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Savaş, E.; Tavşanlı, H.; Çatalkaya, G.; Çapanoğlu, E.; Tamer, C.E. The antimicrobial and antioxidant properties of garagurt: Traditional Cornelian cherry (Cornus mas) marmalade. Qual. Assur. Saf. Crop. Foods 2020, 12, 12–23. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Polewski, M.A.; Esquivel-Alvarado, D.; Wedde, N.S.; Kruger, C.G.; Reed, J.D. Isolation and Characterization of Blueberry Polyphenolic Components and Their Effects on Gut Barrier Dysfunction. J. Agric. Food Chem. 2019, 68, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Hansen, T.V.; Krych, L.; Ahmad, H.F.B.; Nielsen, D.S.; Skovgaard, K.; Thamsborg, S.M. Dietary cinnamaldehyde enhances acquisition of specific antibodies following helminth infection in pigs. Vet. Immunol. Immunopathol. 2017, 189, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, I.; Röhe, I.; Krämer, C.; Goodarzi Boroojeni, F.; Knorr, F.; Mader, A.; Schulze, E.; Hafeez, A.; Neumann, K.; Löwe, R. The effects of particle size, milling method, and thermal treatment of feed on performance, apparent ileal digestibility, and pH of the digesta in laying hens. Poult. Sci. 2015, 94, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-talk between PPAR and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009, 2009. [Google Scholar] [CrossRef]
- Brubaker, P.L. The glucagon-like peptides: Pleiotropic regulators of nutrient homeostasis. Ann. N. Y. Acad. Sci. 2006, 1070, 10–26. [Google Scholar] [CrossRef]
- Guo, X.; Sun, W.; Luo, G.; Wu, L.; Xu, G.; Hou, D.; Hou, Y.; Guo, X.; Mu, X.; Qin, L. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS 1–PI 3K–AKT signaling pathway and GLUT 4 expression. FEBS Open Bio 2019, 9, 1008–1019. [Google Scholar] [CrossRef]
- Changxing, L.; Chenling, M.; Alagawany, M.; Jianhua, L.; Dongfang, D.; Gaichao, W.; Wenyin, Z.; Syed, S.; Arain, M.; Saeed, M. Health benefits and potential applications of anthocyanins in poultry feed industry. Worlds Poult. Sci. J. 2018, 74, 251–264. [Google Scholar] [CrossRef]
- Hu, X.; Guo, Y.; Huang, B.; Bun, S.; Zhang, L.; Li, J.; Liu, D.; Long, F.; Yang, X.; Jiao, P. The effect of glucagon-like peptide 2 injection on performance, small intestinal morphology, and nutrient transporter expression of stressed broiler chickens. Poult. Sci. 2010, 89, 1967–1974. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S.; Shahin, S.E.; Omar, A.E.; Mohammed, H.A.; Mahmoud, H.I.; Ibrahim, D. Effects of graded levels of microbial fermented or enzymatically treated dried brewer’s grains on growth, digestive and nutrient transporter genes expression and cost effectiveness in broiler chickens. BMC Vet. Res. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis—Analogies and differences of two medicinal plants traditionally used. Front. Pharm. 2018, 9, 894. [Google Scholar] [CrossRef]
- Crespo, I.; García-Mediavilla, M.V.; Almar, M.; González, P.; Tuñón, M.J.; Sánchez-Campos, S.; González-Gallego, J. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem. Toxicol. 2008, 46, 1555–1569. [Google Scholar] [CrossRef]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Erratum: Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pr. 2013, 7, 146. [Google Scholar] [CrossRef]
- Kotunia, A.; Wolinski, J.; Laubitz, D.; Jurkowska, M.; Rome, V.; Guilloteau, P.; Zabielski, R. Effect of sodium butyrate on the small intestine. J. Physiol. Pharm. 2004, 55, 59–68. [Google Scholar]
- Saleh, H.; Golian, A.; Kermanshahi, H.; Mirakzehi, M.T. Effects of dietary α-tocopherol acetate, pomegranate peel, and pomegranate peel extract on phenolic content, fatty acid composition, and meat quality of broiler chickens. J. Appl. Anim. Res. 2017, 45, 629–636. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, T.; Ahmad, H.; Zhang, J.; Zhang, L.; Zhong, X. Effects of different formulations of α-tocopherol acetate (vitamin E) on growth performance, meat quality and antioxidant capacity in broiler chickens. Br. Poult. Sci. 2015, 56, 687–695. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Golian, A.; Buyse, J.; Wang, Y.; De Smet, S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat quality of cyclically heat challenged finishing broilers fed Oreganum compactum and Curcuma xanthorrhiza essential oils. Poult. Sci. 2014, 93, 1930–1941. [Google Scholar] [CrossRef]
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L. Influence of temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules 2014, 19, 8177–8188. [Google Scholar] [CrossRef]
- Zhong, R.; Tan, C.; Han, X.; Tang, S.; Tan, Z.; Zeng, B. Effect of dietary tea catechins supplementation in goats on the quality of meat kept under refrigeration. Small Rumin. Res. 2009, 87, 122–125. [Google Scholar] [CrossRef]
- Sierżant, K.; Korzeniowska, M.; Król, B.; Orda, J.; Wojdyło, A. Oxidative stability of the meat of broilers fed diets supplemented with various levels of Blackcurrant extract (Ribes nigrum L.) during different time period. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Dave, R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr. J. Microbiol. Res. 2009, 3, 981–996. [Google Scholar]
- Ibrahim, D.; Kishawy, A.T.; Khater, S.I.; Hamed Arisha, A.; Mohammed, H.A.; Abdelaziz, A.S.; El-Rahman, A.; Ghada, I.; Elabbasy, M.T. Effect of dietary modulation of selenium form and level on performance, tissue retention, quality of frozen stored meat and gene expression of antioxidant status in Ross broiler chickens. Animals 2019, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Cerit, İ.; Şenkaya, S.; Tulukoğlu, B.; Kurtuluş, M.; Seçilmişoğlu, Ü.R.; Demirkol, O. Enrichment of functional properties of white chocolates with cornelian cherry, spinach and pollen powders. Gida J. Food 2016, 41, 311–316. [Google Scholar] [CrossRef]
- Faiz, F.; Khan, M.I.; Butt, M.S.; Nawaz, H. Enhancement of broiler meat oxidative stability through dietary supplementation of citrus processing waste. Pak. J. Agric. Sci. 2017, 54, 893–898. [Google Scholar]
- Jang, A.; Liu, X.-D.; Shin, M.-H.; Lee, B.-D.; Lee, S.-K.; Lee, J.-H.; Jo, C. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult. Sci. 2008, 87, 2382–2389. [Google Scholar] [CrossRef]
- Banerjee, R.; Verma, A.K.; Das, A.K.; Rajkumar, V.; Shewalkar, A.; Narkhede, H. Antioxidant effects of broccoli powder extract in goat meat nuggets. Meat Sci. 2012, 91, 179–184. [Google Scholar] [CrossRef]
| Ingredients, g/kg | Starter | Grower–Finisher |
|---|---|---|
| Yellow corn grain | 57.40 | 60.1 |
| Soybean meal, 47.5% | 34.66 | 29.00 |
| Corn gluten, 60% | 3.00 | 4.00 |
| Soybean oil | 1.10 | 3.00 |
| Calcium carbonate | 1.00 | 1.00 |
| Dicalcium phosphate | 1.80 | 1.90 |
| Common salt | 0.30 | 0.30 |
| Premix * | 0.30 | 0.30 |
| DL- Methionine, 98% | 0.18 | 0.14 |
| Lysine, HCl, 78% | 0.16 | 0.16 |
| Anti-mycotoxin | 0.10 | 0.10 |
| Analyzed Chemical Composition | ||
| ME, Kcal/Kg ** | 3004 | 3158 |
| CP % | 23.01 | 21.10 |
| EE % | 3.63 | 5.55 |
| CF % | 2.66 | 2.53 |
| Ca % | 0.97 | 0.98 |
| Available P % | 0.47 | 0.47 |
| Lysine % | 1.37 | 1.22 |
| Methionine % | 0.56 | 0.51 |
| Gene | Gene Full Name | Primer Sequence (5′–3′) | Reference No |
|---|---|---|---|
| Glucose transporters | |||
| GLUT1 | Glucose transporter 1 | F-TCCTCCTGATCAACCGCAAT R-TGTGCCCCGGAGCTTCT | NM_205209.1 |
| GLUT2 | Glucose transporter 2 | F-TGATCGTGGCACTGATGGTT R-CCACCAGGAAGAC↓GGAGATA | NM_207178.1 |
| SGLT-1 | Sodium-dependent glucose transporter | F-TGCCGGAGTATCTGAGGAAG R-CCCCATGGCCAACTGTATAA | XM_015275173.2 |
| Antioxidant related genes | |||
| GPX1 | Glutathione peroxidase | F- GCTGTTCGCCTTCCTGAGAG R- GTTCCAGGAGACGTCGTTGC | NM_001277853.1 |
| SOD1 | Superoxide dismutase | F- CACTGCATCATTGGCCGTACCA R- GCTTGCACACGGAAGAGCAAGT | NM_205064.1 |
| CAT | Catalase | F- TGGCGGTAGGAGTCTGGTCT R- GTCCCGTCCGTCAGCCATTT | NM_001031215.1 |
| House keeping | |||
| GAPDH | Glyceraldahyde-3-phosphate dehydrogenase | F-GGTGGTGCTAAGCGTGTTA R-CCCTCCACAATGCCAA | NM205518 |
| TBP | TATA-binding protein | F: GTCCACGGTGAATCTTGGTT R: GCGCAGTAGTACGTGGTTCTC | Acc:8484 |
| CCE (mg/kg Diet) | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | 0 | 50 | 100 | 200 | 400 | p-Value | SEM |
| Total growing period | |||||||
| BW (g/bird) | 2423 c | 2439 b,c | 2508 b | 2649 a | 2503 b | <0.001 | 14.10 |
| BWG (g/bird) | 2377 c | 2393 b,c | 2462 b | 2603 a | 2457 b | <0.001 | 14.12 |
| FI (g/bird) | 4331 b | 4219 c | 4224 c | 4503 a | 4400 b | <0.001 | 17.53 |
| FCR | 1.82 a | 1.76 a,b,c | 1.72 c | 1.73 b,c | 1.79 a,b | <0.001 | 0.01 |
| Digestibility % | |||||||
| Dry matter | 69.37 d | 71.87 b,c | 73.33 a,b | 74.80 a | 71.54 c | <0.05 | 0.50 |
| Crude protein | 62.45 c | 63.63 b,c | 65.92 a,b | 69.22 a | 63.69 b | <0.001 | 0.40 |
| Crude fiber | 27.30 | 27.37 | 27.95 | 29.47 | 29.18 | <0.20 | 0.60 |
| CCE (mg/kg Diet) | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | 0 | 50 | 100 | 200 | 400 | p-Value | SEM |
| ALT, U/L | 20.82 | 20.78 | 20.98 | 20.60 | 19.58 | 0.18 | 0.40 |
| AST, U/L | 55.16 | 55.40 | 54.00 | 54.64 | 53.58 | 0.06 | 0.48 |
| Uric acid, mg/dL | 5.70 | 5.96 | 5.74 | 6.04 | 5.91 | 0.55 | 0.06 |
| Creatinine, mg/dL | 0.96 | 1.02 | 0.98 | 1.1 | 0.94 | 0.69 | 0.01 |
| Total Cholesterol, mg/dL | 109.16 a | 109.30 a | 107.76 a | 109.34 a | 100.78 b | <0.001 | 3.62 |
| TGs, mg/dL | 61.18 | 60.78 | 61.24 | 60.32 | 60.28 | 0.34 | 0.38 |
| HDL-C, mg/dL | 43.34 | 44.58 | 44.48 | 44.78 | 43.90 | 0.17 | 0.36 |
| LDL-C, mg/dL | 53.58 a | 52.56 a | 51.03 a | 52.49 a | 44.82 b | 0.02 | 4.20 |
| VLDL-C, mg/dL | 12.23 | 12.16 | 12.24 | 12.06 | 12.00 | 0.34 | 0.02 |
| Total protein (g/dL) | 4.43 | 4.40 | 4.41 | 4.47 | 4.44 | 0.96 | 0.03 |
| Albumin (g/dL) | 2.28 | 2.25 | 2.32 | 2.25 | 2.25 | 0.97 | 0.03 |
| Globulin (g/dL) | 2.15 | 2.16 | 2.09 | 2.21 | 2.19 | 0.96 | 0.05 |
| CCE (mg/kg Diet) | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | 0 | 50 | 100 | 200 | 400 | p-Value | SEM |
| Breast Muscle Analysis % of Wet Basis | |||||||
| DM | 25.76 | 25.26 | 25.61 | 24.73 | 25.35 | 0.614 | 0.21 |
| CP | 23.61 | 23.04 | 24.19 | 23.24 | 23.92 | 0.322 | 0.19 |
| EE | 4.52 | 4.52 | 4.63 | 4.64 | 4.56 | 0.949 | 0.06 |
| Ash | 1.28 | 1.20 | 1.27 | 1.22 | 1.25 | 0.864 | 0.02 |
| Thigh Muscles Analysis% of Wet Basis | |||||||
| DM | 28.42 | 28.34 | 27.21 | 26.52 | 28.36 | 0.273 | 0.34 |
| CP | 22.06 | 20.66 | 20.38 | 21.95 | 21.74 | 0.051 | 0.24 |
| EE | 6.94 | 7.21 | 6.62 | 6.31 | 6.91 | 0.087 | 0.11 |
| Ash | 1.18 | 1.23 | 1.29 | 1.11 | 1.24 | 0.399 | 0.03 |
| CCE (mg/kg Diet) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | p-Value | SEM | |
| Bifidobacterium | 6.17 b | 6.57 b | 7.70 a | 7.90 a | 8.17 a | <0.01 | 0.20 |
| Lactobacillus | 6.50 d | 6.70 d | 7.73 c | 8.13 b | 8.87 a | <0.001 | 0.10 |
| Escherichia coli | 8.23 a | 7.9 ab | 7.23 b | 6.47 c | 6.27 c | <0.008 | 0.16 |
| CCE (mg/kg Diet) | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | 0 | 50 | 100 | 200 | 400 | p-Value | SEM |
| TPC at d 7 of storage | 68.71 e | 111.06 d | 124.06 c | 131.68 b | 143.72 a | 4.91 | <0.001 |
| TPC at d 90 of storage | 49.77 d | 96.71 c | 109.51 b | 121.95 a | 128.02 a | 3.27 | <0.001 |
| DPPH assay at d 7 of storage | 86.99 e | 118.20 d | 133.67 c | 148.58 b | 155.88 a | 4.45 | <0.001 |
| DPPH at d 90 of storage | 77.58 e | 106.93 d | 124.61 c | 134.58 b | 144.03 a | 3.89 | <0.001 |
| FRAP assay at d 7 of storage | 229.44 e | 435.81 d | 447.99 c | 514.62 b | 618.37 a | 8.09 | <0.001 |
| FRAP assay at d 90 of storage | 175.44 d | 292.34 c | 301.89 c | 369.88 b | 474.55 a | 7.51 | <0.001 |
| MDA content at d 7 of storage | 0.47 a | 0.26 b | 0.22 c | 0.15 d | 0.19 d | 0.12 | <0.03 |
| MDA content at d 90 of storage | 0.67 a | 0.46 a | 0.30 b | 0.28 b | 0.21 c | 0.03 | <0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.; Moustafa, A.; Metwally, A.S.; Nassan, M.A.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A.T.Y. Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals 2021, 11, 1038. https://doi.org/10.3390/ani11041038
Ibrahim D, Moustafa A, Metwally AS, Nassan MA, Abdallah K, Eldemery F, Tufarelli V, Laudadio V, Kishawy ATY. Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals. 2021; 11(4):1038. https://doi.org/10.3390/ani11041038
Chicago/Turabian StyleIbrahim, Doaa, Amira Moustafa, Aya Sh. Metwally, Mohamed A. Nassan, Karima Abdallah, Fatma Eldemery, Vincenzo Tufarelli, Vito Laudadio, and Asmaa T. Y. Kishawy. 2021. "Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat" Animals 11, no. 4: 1038. https://doi.org/10.3390/ani11041038
APA StyleIbrahim, D., Moustafa, A., Metwally, A. S., Nassan, M. A., Abdallah, K., Eldemery, F., Tufarelli, V., Laudadio, V., & Kishawy, A. T. Y. (2021). Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals, 11(4), 1038. https://doi.org/10.3390/ani11041038









