The Comparative Analysis of the Ruminal Bacterial Population in Reindeer (Rangifer tarandus L.) from the Russian Arctic Zone: Regional and Seasonal Effects
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
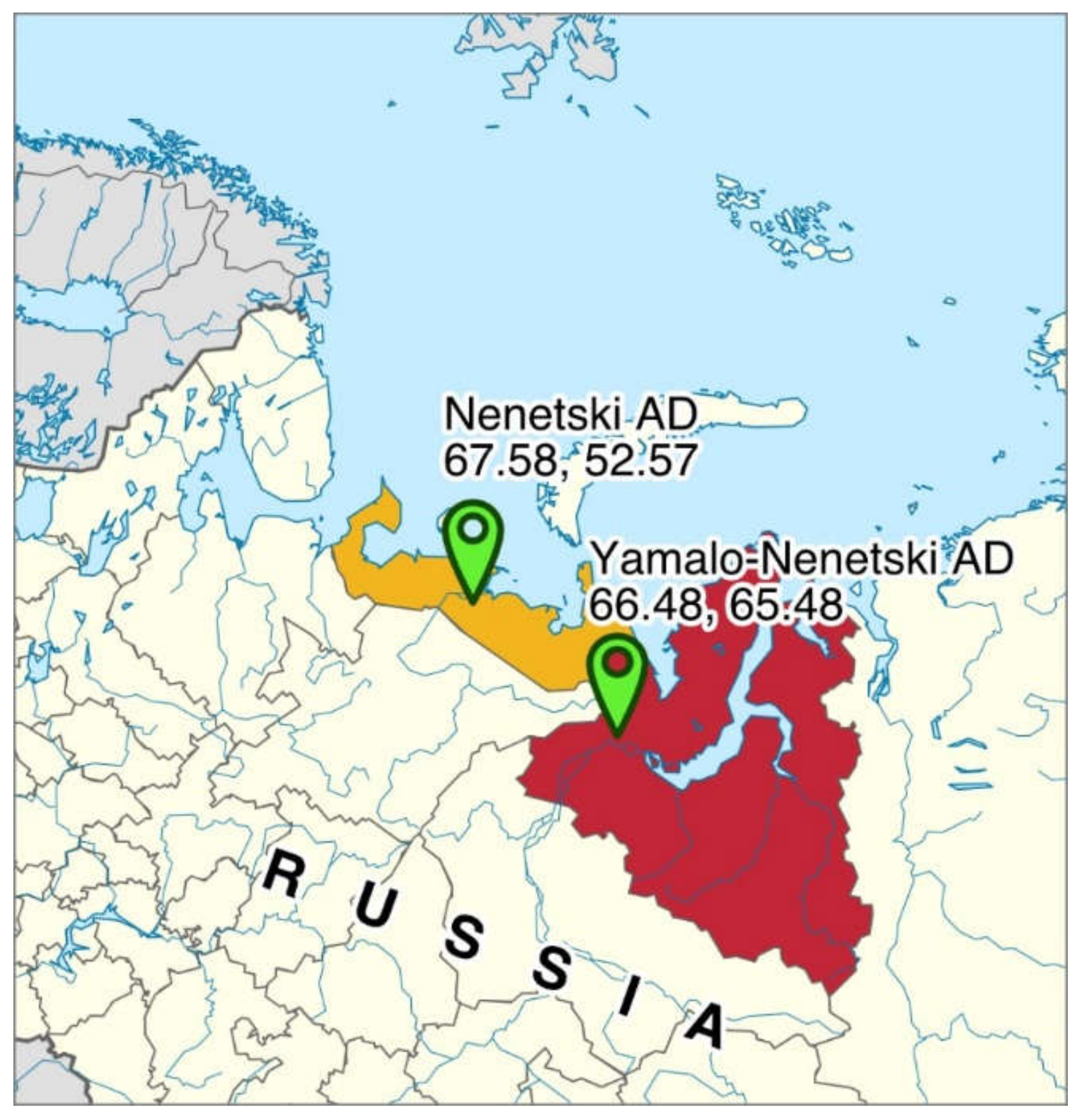
2.1. Sampling of Ruminal Content
2.2. DNA Isolation and Sequencing
2.3. Statistical Analysis
2.4. Accession Numbers
3. Results
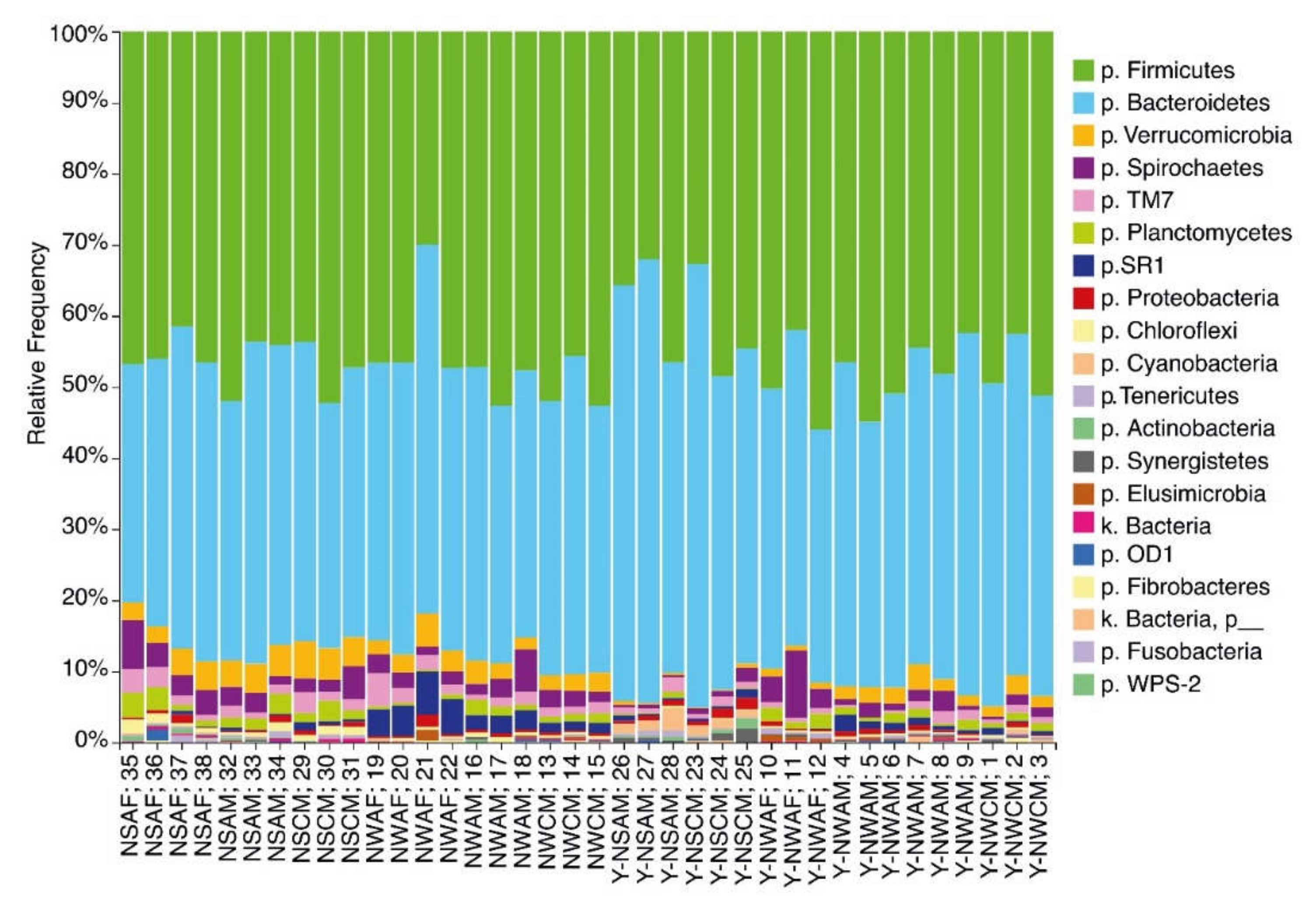
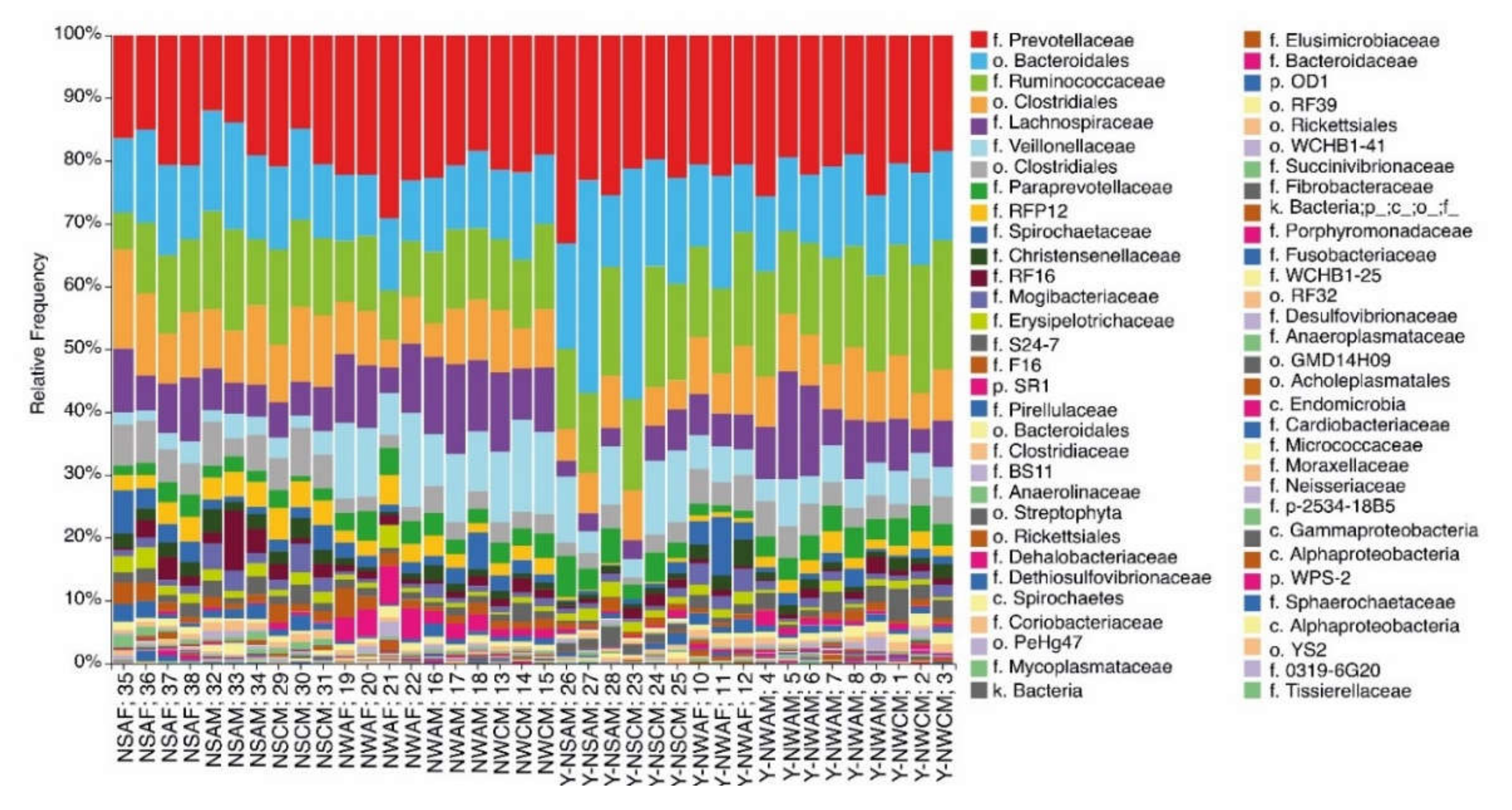
3.1. Analysis of the Ruminal Microbial Population Biodiversity in Reindeer
3.2. The Alpha Biodiversity of the Ruminal Microbial Population in Reindeer
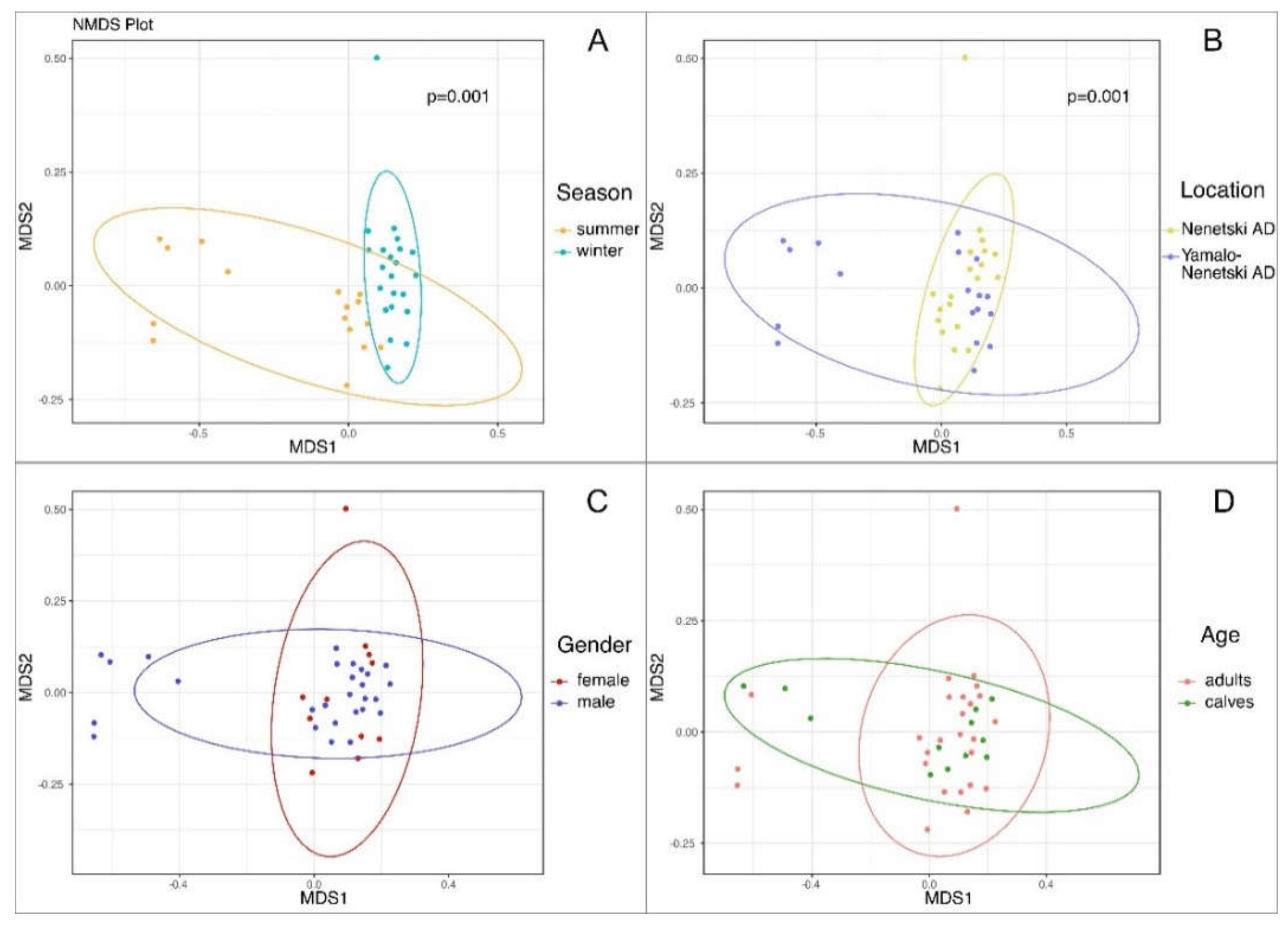
3.3. The Beta Biodiversity of the Ruminal Microbial Population in Reindeer
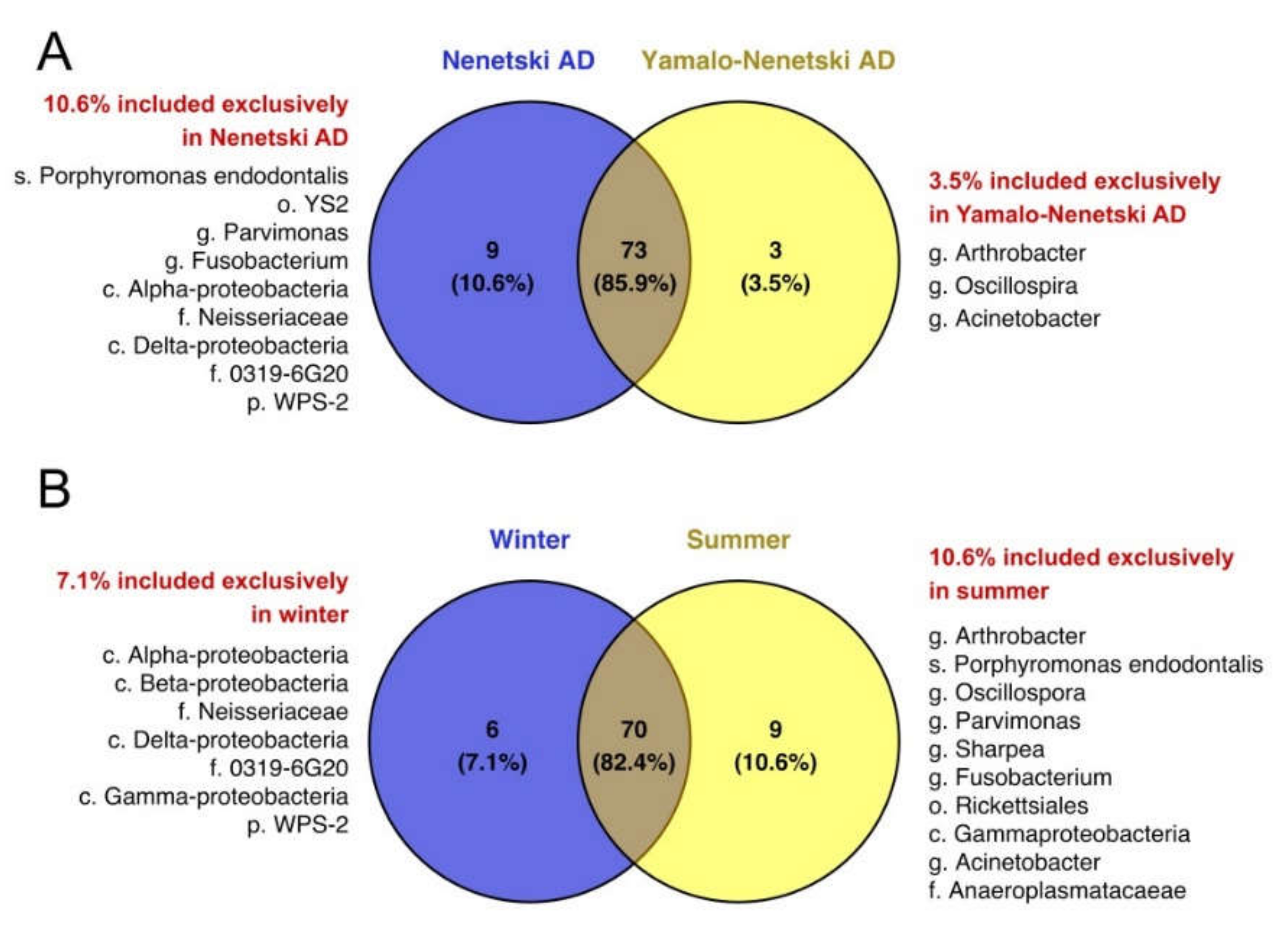
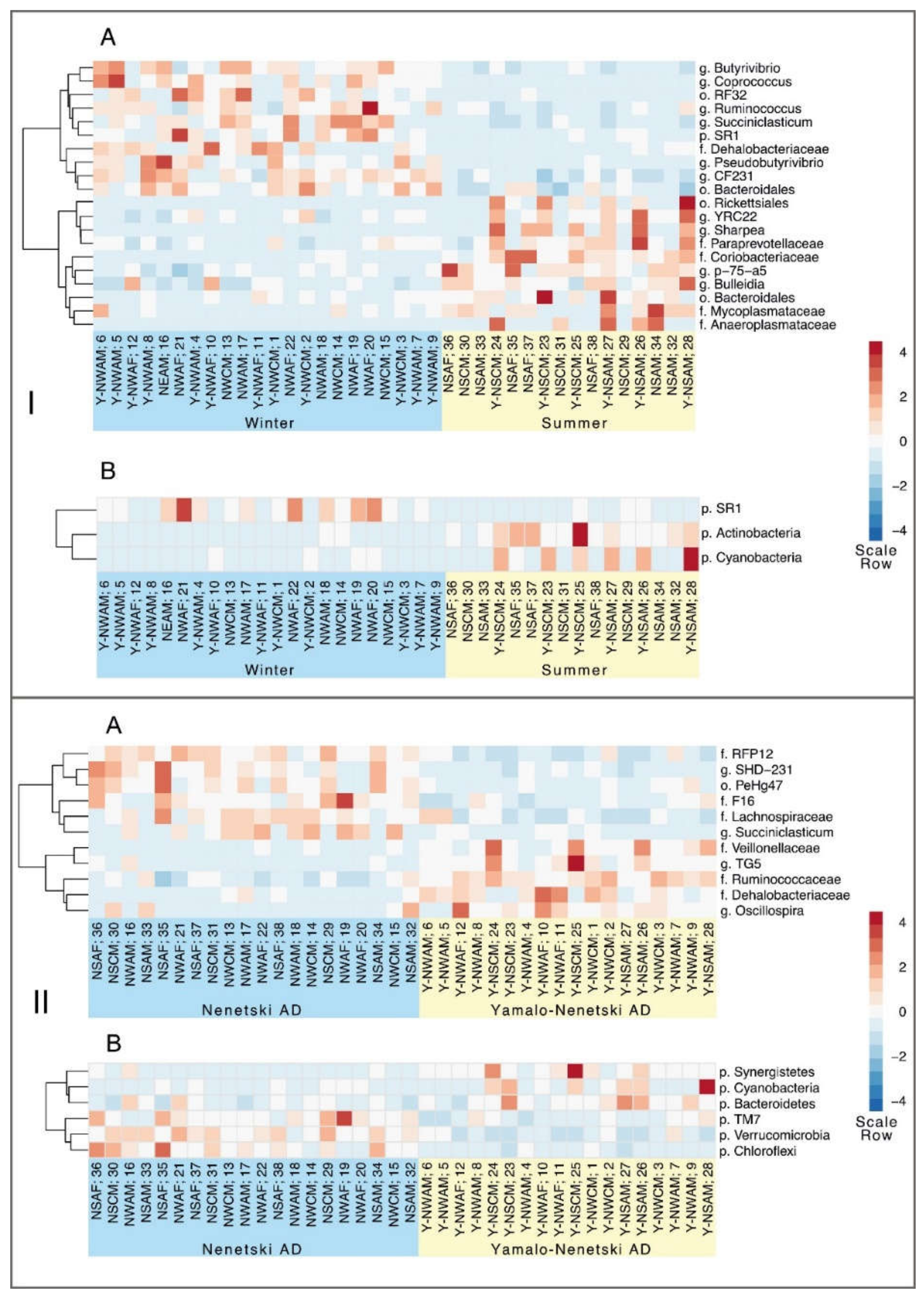
3.4. The Effects of Ecological and Physiological Factors on the Ruminal Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mitrofanova, O.V.; Dementieva, N.V.; Krutikova, A.A.; Tyshchenko, V.I.; Goncharov, V.V. Assessment of genetic polymor-phisms of several populations of reindeer (Rangifer tarandus). Genet. Breed. Anim. 2017, 1, 49–52. [Google Scholar]
- Mukhachev, A.D.; Layshev, K.A. The World of Reindeer; Research Institute of Agriculture of the Far North: Norilsk, Russia, 2007. [Google Scholar]
- Reindeer Rumen Microbiome (Rangifer tarandus) of the Arctic Regions of Russia; AlfaMig Ltd.: Saint-Petersburg, Russia, 2020; ISBN 978-5-6041818-4-3.
- Sundset, M.A.; Salgado-Flores, A.; Wright, A.-D.G.; Pope, P.B. The Reindeer Rumen Microbiome. Encycl. Metagenomics 2015, 722–732. [Google Scholar] [CrossRef]
- Mathiesen, S.D.; Mackie, R.I.; Aschfalk, A.; Ringø, E.; Sundset, M.A. Microbial ecology of the gastrointestinal tract in reindeer—Changes through season. In Microbial Ecology of the Growing Animal. Biology of the Growing Animals; Elsevier Press: Oxford, UK, 2005; pp. 73–100. [Google Scholar]
- Ilina, L.A.; Layshev, K.A.; Yildirim, E.A.; Filippova, V.A.; Dunyashev, T.P.; Dubrovin, A.V.; Nokonov, I.N.; Novikova, N.I.; Laptev, G.Y.; Yuzhakov, A.A.; et al. Comparative analysis of rumen bacterial community of young and adult Rangifer tarandus reindeers from arctic regions of Russia in the summer-autumn period. Agric. Biol. 2018, 53, 355–363. [Google Scholar] [CrossRef]
- Hofmann, R.R. The ruminant stomach. Stomach structure and feeding habits of east African game ruminants. Ecol. Afr. Monogr. Biol. Nairobi 1973, 2, 354. [Google Scholar]
- Hungate, R.E. The Rumen and its Microbes; Academic Press: Cambridge, MA, USA, 1966. [Google Scholar]
- Jami, E.; Mizrahi, I. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Xu, C.; Zhao, J.; Liu, H.; Fan, Z.; Yang, F.; Wright, A.-D.G.; Li, G. Bacteria and Methanogens Differ along the Gastrointestinal Tract of Chinese Roe Deer (Capreolus pygargus). PLoS ONE 2014, 9, e114513. [Google Scholar] [CrossRef]
- Henderson, G.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H.; Abecia, L.; Angarita, E.; Aravena, P.; Cox, F.; Global Rumen Census Collaborators; et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Delgado, M.L.; Singh, P.; Funk, J.A.; Moore, J.A.; Cannell, E.M.; Kanesfsky, J.; Manning, S.D.; Scribner, K.T. Intestinal Microbial Community Dynamics of White-Tailed Deer (Odocoileus virginianus) in an Agroecosystem. Microb. Ecol. 2017, 74, 496–506. [Google Scholar] [CrossRef]
- Groussin, M.; Mazel, F.; Sanders, J.G.; Smillie, C.S.; Lavergne, S.; Thuiller, W.; Alm, E.J. Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nat. Commun. 2017, 8, 14319. [Google Scholar] [CrossRef]
- Yang, B.; Le, J.; Wu, P.; Liu, J.; Guan, L.L.; Wang, J. Alfalfa Intervention Alters Rumen Microbial Community Development in Hu Lambs During Early Life. Front. Microbiol. 2018, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Sundset, M.A.; Barboza, P.S.; Green, T.K.; Folkow, L.P.; Blix, A.S.; Mathiesen, S.D. Microbial degradation of usnic acid in the reindeer rumen. Naturwissenschaften 2009, 97, 273–278. [Google Scholar] [CrossRef]
- Mathiesen, S.D.; Haga, O.E.; Kaino, T.; Tyler, N.J.C. Diet composition, rumen papillation and maintenance of carcass mass in female Norwegian reindeer (Rangifer tarandus tarandus) in winter. J. Zoöl. 2000, 251, 129–138. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Kelly, W.; Janssen, P.H.; Attwood, G. Rumen microbial (meta)genomics and its application to ruminant production. Animals 2013, 7, 184–201. [Google Scholar] [CrossRef]
- Podterob, A.P. Chemical composition of lichens and their medical applications. Pharm. Chem. J. 2008, 42, 582–588. [Google Scholar] [CrossRef]
- Roach, J.A.G.; Musser, S.M.; Morehouse, K.; Woo, J.Y.J. Determination of usnic acid in lichen toxic to elk by liquid chroma-tography with ultraviolet and tandem mass spectrometry detection. J. Agric. Food. Chem. 2006, 54, 2484–2490. [Google Scholar] [CrossRef]
- Orpin, C.G.; Mathiesen, S.D.; Greenwood, Y.; Blix, A.S. Seasonal changes in the ruminal microflora of the high-arctic Svalbard reindeer (Rangifer tarandusplatyrhynchus). Appl. Environ. Microb. 1985, 50, 144–151. [Google Scholar] [CrossRef]
- Sundset, M.A.; Edwards, J.E.; Cheng, Y.F.; Senosiain, R.S.; Fraile, M.N.; Northwood, K.S.; Præsteng, K.E.; Glad, T.; Mathiesen, S.D.; Wright, A.-D.G. Molecular Diversity of the Rumen Microbiome of Norwegian Reindeer on Natural Summer Pasture. Microb. Ecol. 2008, 57, 335–348. [Google Scholar] [CrossRef]
- Sundset, M.A.; Kohn, A.; Mathiesen, S.D.; Præsteng, K.E. Eubacterium rangiferina, a novel usnic acid-resistant bacterium from the reindeer rumen. Naturwissenschaften 2008, 95, 741–749. [Google Scholar] [CrossRef]
- Glad, T.; Barboza, P.; Mackie, R.I.; Wright, A.-D.G.; Brusetti, L.; Mathiesen, S.D.; Sundset, M.A. Dietary Supplementation of Usnic Acid, an Antimicrobial Compound in Lichens, Does Not Affect Rumen Bacterial Diversity or Density in Reindeer. Curr. Microbiol. 2014, 68, 724–728. [Google Scholar] [CrossRef]
- Church, D.C. Ruminant Animal: Digestive Physiology and Nutrition; Prentice Hall: New Jersey, NJ, USA, 1993. [Google Scholar]
- Grilli, D.J.; Fliegerová, K.; Kopečný, J.; Lama, S.P.; Egea, V.; Sohaefer, N.; Pereyra, C.; Ruiz, M.S.; Sosa, M.A.; Arenas, G.N.; et al. Analysis of the rumen bacterial diversity of goats during shift from forage to concentrate diet. Anaerobe 2016, 42, 17–26. [Google Scholar] [CrossRef]
- Zielińska, S.; Ekidawa, D.; Estempniewicz, L.; Ełoś, M.; Łoś, J.M. New Insights into the Microbiota of the Svalbard Reindeer Rangifer tarandus platyrhynchus. Front. Microbiol. 2016, 7, 170. [Google Scholar] [CrossRef]
- Sundset, M.A.; Præsteng, K.E.; Cann, I.K.O.; Mathiesen, S.D.; Mackie, R.I. Novel Rumen Bacterial Diversity in Two Geographically Separated Sub-Species of Reindeer. Microb. Ecol. 2007, 54, 424–438. [Google Scholar] [CrossRef]
- Smith, C.C.R.; Snowberg, L.K.; Caporaso, J.G.; Knight, R.; Bolnick, D. Dietary input of microbes and host genetic variation shape among-population differences in stickleback gut microbiota. ISME J. 2015, 9, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Durso, L.M.; Harhay, G.P.; Smith, T.P.L.; Bono, J.L.; DeSantis, T.Z.; Harhay, D.M.; Andersen, G.L.; Keen, J.E.; Laegreid, W.W.; Clawson, M.L. Animal-to-Animal Variation in Fecal Microbial Diversity among Beef Cattle. Appl. Environ. Microbiol. 2010, 76, 4858–4862. [Google Scholar] [CrossRef]
- Fonty, G.; Joblin, K.; Chavarot, M.; Roux, R.; Naylor, G.; Michallon, F. Establishment and Development of Ruminal Hydrogenotrophs in Methanogen-Free Lambs. Appl. Environ. Microbiol. 2007, 73, 6391–6403. [Google Scholar] [CrossRef]
- Rustomo, B.; Al Zahal, O.; Cant, J.P.; Fan, M.Z.; Duffield, T.F.; Odongo, N.E.; McBride, B.W. Acidogenic value of feeds. II. Effects of rumen acid load from feeds on dry matter intake, ruminal pH, fiber degradability and milk k production in the lactating dairy cow. Can. J. Anim. Sci. 2006, 86, 119–126. [Google Scholar]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; González, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P.T.M. Subacute ruminal acidosis in dairy cows. Rev. J. Vet. Med. 2003, 50, 406–414. [Google Scholar] [CrossRef]
- Rustomo, B.; Cant, J.P.; Fan, M.Z.; Duffield, T.F.; Odongo, N.E.; McBride, B.W. Acidogenic value of feeds. I. The relationship between the acidogenic value of feeds and in vitro ruminal pH changes. Can. J. Anim. Sci. 2006, 86, 109–117. [Google Scholar]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihara, M.; Kamagata, Y. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 2010, 16, 27–33. [Google Scholar] [CrossRef]
- Layshev, K.A.; Ilina, L.A.; Yildirim, E.A.; Filippova, V.A.; Dunyashev, T.P.; Dubrovin, A.V.; Sobolev, D.V.; Novikova, N.I.; Laptev, G.Y.; Yuzhakov, A.A.; et al. The rumen microbiota of reindeer (Rangifer tarandus) with clinical manifes-tations of necrobacteriosis. Agric. Biol. 2019, 54, 744–753. [Google Scholar] [CrossRef]
- Fauchald, P.; Park, T.; Tømmervik, H.; Myneni, R.B.; Hausner, V.H. Arctic greening from warming promotes declines in caribou populations. Sci. Adv. 2017, 3, e1601365. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, S.T.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Bonchev, D. Network Topology Analysis of Post-Mortem Brain Microarrays Identifies More Alzheimer’s Related Genes and MicroRNAs and Points to Novel Routes for Fighting with the Disease. PLoS ONE 2016, 11, e0144052. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; Da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Aagnes, T.H.; Sørmo, W.; Mathiesen, S.D. Ruminal microbial digestion in free living, in captive lichen-fed and in starved reindeer (Rangifer tarandustarandus) in winter. Appl. Environ. Microb. 1995, 61, 583–591. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Sensen, C.W.; McAllister, T.A.; Forster, R.J. Diversity of Rumen Bacteria in Canadian Cervids. PLoS ONE 2014, 9, e89682. [Google Scholar] [CrossRef]
- Hu, X.; Liu, G.; Shafer, A.B.A.; Wei, Y.; Zhou, J.; Lin, S.; Wu, H.; Zhou, M.; Hu, D.; Liu, S. Comparative Analysis of the Gut Microbial Communities in Forest and Alpine Musk Deer Using High-Throughput Sequencing. Front. Microbiol. 2017, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Flores, A.; Hagen, L.H.; Ishaq, S.L.; Zamanzadeh, M.; Wright, A.-D.G.; Pope, P.B.; Sundset, M.A. Rumen and Cecum Microbiomes in Reindeer (Rangifer tarandus tarandus) Are Changed in Response to a Lichen Diet and May Affect Enteric Methane Emissions. PLoS ONE 2016, 11, e0155213. [Google Scholar] [CrossRef] [PubMed]
- Pope, P.B.; MacKenzie, A.K.; Gregor, I.; Smith, W.; Sundset, M.A.; McHardy, A.C.; Morrison, M.; Eijsink, V.G. Metagenomics of the Svalbard Reindeer Rumen Microbiome Reveals Abundance of Polysaccharide Utilization Loci. PLoS ONE 2012, 7, e38571. [Google Scholar] [CrossRef]
- Schären, M.; Drong, C.; Kiri, K.; Riede, S.; Gardener, M.; Meyer, U.; Hummel, J.; Urich, T.; Breves, G.; Dänicke, S. Differential effects of monensin and a blend of essential oils on rumen microbiota composition of transition dairy cows. J. Dairy Sci. 2017, 100, 2765–2783. [Google Scholar] [CrossRef]
- Li, F.; Guan, L.L. Metatranscriptomic Profiling Reveals Linkages between the Active Rumen Microbiome and Feed Efficiency in Beef Cattle. Appl. Environ. Microbiol. 2017, 83, 00061-17. [Google Scholar] [CrossRef]
- Gharechahi, J.; Zahiri, H.S.; Noghabi, K.A.; Salekdeh, G.H. In-depth diversity analysis of the bacterial community resident in the camel rumen. Syst. Appl. Microbiol. 2015, 38, 67–76. [Google Scholar] [CrossRef]
- Newbold, C.J.; Wallace, R.J.; McIntosh, F.M. Mode of action of the yeast Saccharomyces cerevisiaeas a feed additive for ruminants. Br. J. Nutr. 1996, 76, 249–261. [Google Scholar] [CrossRef]
- Neves, A.L.A.; Li, F.; Ghoshal, B.; McAllister, T.; Guan, L.L. Enhancing the Resolution of Rumen Microbial Classification from Metatranscriptomic Data Using Kraken and Mothur. Front. Microbiol. 2017, 8, 2445. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Kachalkin, A.V.; Korchikov, E.S.; Dobrovolskaya, T.G. Lichen microbial communities. Microbiology 2017, 3, 265–283. [Google Scholar] [CrossRef]
- Bates, S.T.; Cropsey, G.W.G.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial Communities Associated with the Lichen Symbiosis. Appl. Environ. Microbiol. 2010, 77, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Sigurbjörnsdóttir, M.A.; Heiðmarsson, S.; Jónsdóttir, A.R.; Vilhelmsson, O. Novel bacteria associated with Arctic seashore lichens have potential roles in nutrient scavenging. Can. J. Microbiol. 2014, 60, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, K.M.B.; Krych, L.; Sørensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sørensen, S.J.; Hansen, A.K. Gut Microbiota Composition Is Correlated to Grid Floor Induced Stress and Behavior in the BALB/c Mouse. PLoS ONE 2012, 7, e46231. [Google Scholar] [CrossRef]
- Clavel, T.; Lepage, P.; Charrier, C. The Family Coriobacteriaceae; Springer: Berlin/Heidelberg, Germany, 2014; pp. 201–238. ISBN 9783642301377. [Google Scholar]
- Zhou, M.; Peng, Y.-J.; Chen, Y.; Klinger, C.M.; Oba, M.; Liu, J.-X.; Guan, L.L. Assessment of microbiome changes after rumen transfaunation: Implications on improving feed efficiency in beef cattle. Microbiome 2018, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lombardo Bedran, T.B.; Marcantonio, R.A.; Spin Neto, R.; Alves Mayer, M.P.; Grenier, D.; Spolidorio, L.C.; Spolidorio, D.P. Porphyromonas endodontalisin chronic periodontitis: A clinical and microbiological cross-sectional study. J. Oral Microbiol. 2012, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; López-García, A.; González-Recio, O.; Elcoso, G.; Fàbregas, F.; Chaucheyras-Durand, F.; Castex, M. Changes in the rumen and colon microbiota and effects of live yeast dietary supplementation during the transition from the dry period to lactation of dairy cows. J. Dairy Sci. 2019, 102, 6180–6198. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Shiratori, C.; Murakami, M.; Takami, H.; Toh, H.; Kato, Y.; Nakajima, F.; Takagi, M.; Akita, H.; Masaoka, T.; et al. Sharpea azabuensis gen. nov., sp. nov., a Gram-positive, strictly anaerobic bacterium isolated from the faces of thorough bred horses. Int. J. Syst. Evol. Microbiol. 2008, 58, 2682–2686. [Google Scholar] [CrossRef]
- Abbas, W.; Howard, J.T.; Paz, H.A.; Hales, K.E.; Wells, J.E.; Kuehn, L.A.; Erickson, G.E.; Spangler, M.L.; Fernando, S.C. Influence of host genetics in shaping the rumen bacterial community in beef cattle. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Ben Shabat, S.K.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Miller, M.E.B.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.M.; Qi, S.; Hackmann, T.J.; Staples, C.R.; Adesogan, A.T. Effects of the dose and viability of Saccharomyces cerevisiae. 1. Diversity of ruminal microbes as analyzed by Illumina MiSeq sequencing and quantitative PCR. J. Dairy Sci. 2017, 100, 325–342. [Google Scholar] [CrossRef]
- Martinez-Fernandez, G.; Denman, S.E.; Cheung, J.; McSweeney, C.S. Phloroglucinol Degradation in the Rumen Promotes the Capture of Excess Hydrogen Generated from Methanogenesis Inhibition. Front. Microbiol. 2017, 8, 1871. [Google Scholar] [CrossRef]
- Tarakanov, B.V. Methods for Studying Microflora of the Digestive Tract of Agricultural Animals and Poultry; Nauchny Mir: Moscow, Russia, 2006. [Google Scholar]
- Yamano, H.; Ichimura, Y.; Sawabe, Y.; Koike, S.; Suzuki, Y.; Kobayashi, Y. Seasonal differences in rumen bacterial flora of wild Hokkaido sika deer and partial characterization of an unknown bacterial group possibly involved in fiber digestion in winter. Anim. Sci. J. 2019, 90, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Deusch, S.; Camarinha-Silva, A.; Conrad, J.; Beifuss, U.; Rodehutscord, M.; Seifert, J. A Structural and Functional Elucidation of the Rumen Microbiome Influenced by Various Diets and Microenvironments. Front. Microbiol. 2017, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, K.; Zhang, C.; Feng, Y.; Zhang, X.; Wang, X.; Wu, G. Dynamics and stabilization of the rumen microbiome in yearling Tibetan sheep. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Gylswyk, N.O. Succiniclasticum ruminis gen. nov., sp. nov., a Ruminal Bacterium Converting Succinate to Propionate as the Sole Energy-Yielding Mechanism. Int. J. Syst. Bacteriol. 1995, 45, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospiraand related bacteria—From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef]
- Snelling, T.J.; Auffret, M.D.; Duthie, C.-A.; Stewart, R.D.; Watson, M.; Dewhurst, R.J.; Roehe, R.; Walker, A.W. Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Anim. Microbiome 2019, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilina, L.A.; Filippova, V.A.; Brazhnik, E.A.; Dubrovin, A.V.; Yildirim, E.A.; Dunyashev, T.P.; Laptev, G.Y.; Novikova, N.I.; Sobolev, D.V.; Yuzhakov, A.A.; et al. The Comparative Analysis of the Ruminal Bacterial Population in Reindeer (Rangifer tarandus L.) from the Russian Arctic Zone: Regional and Seasonal Effects. Animals 2021, 11, 911. https://doi.org/10.3390/ani11030911
Ilina LA, Filippova VA, Brazhnik EA, Dubrovin AV, Yildirim EA, Dunyashev TP, Laptev GY, Novikova NI, Sobolev DV, Yuzhakov AA, et al. The Comparative Analysis of the Ruminal Bacterial Population in Reindeer (Rangifer tarandus L.) from the Russian Arctic Zone: Regional and Seasonal Effects. Animals. 2021; 11(3):911. https://doi.org/10.3390/ani11030911
Chicago/Turabian StyleIlina, Larisa A., Valentina A. Filippova, Evgeni A. Brazhnik, Andrey V. Dubrovin, Elena A. Yildirim, Timur P. Dunyashev, Georgiy Y. Laptev, Natalia I. Novikova, Dmitriy V. Sobolev, Aleksandr A. Yuzhakov, and et al. 2021. "The Comparative Analysis of the Ruminal Bacterial Population in Reindeer (Rangifer tarandus L.) from the Russian Arctic Zone: Regional and Seasonal Effects" Animals 11, no. 3: 911. https://doi.org/10.3390/ani11030911
APA StyleIlina, L. A., Filippova, V. A., Brazhnik, E. A., Dubrovin, A. V., Yildirim, E. A., Dunyashev, T. P., Laptev, G. Y., Novikova, N. I., Sobolev, D. V., Yuzhakov, A. A., & Laishev, K. A. (2021). The Comparative Analysis of the Ruminal Bacterial Population in Reindeer (Rangifer tarandus L.) from the Russian Arctic Zone: Regional and Seasonal Effects. Animals, 11(3), 911. https://doi.org/10.3390/ani11030911







