Steatitis in Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Animals
2.3. Data Collection
2.4. Sample Collection
2.5. Sample Analyses
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wibbels, T.; Bevan, E. Lepidochelys kempii (Errata Version Published in 2019). Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T11533A155057916.en (accessed on 3 April 2020).
- National Marine Fisheries Service; U.S. Fish and Wildlife Service; SEMARNAT. Fish and Wildlife Service; SEMARNAT. Bi-National Recovery Plan for the Kemp’s Ridley Sea Turtle (Lepidochelys kempii), 2nd ed.; Last Revised 22 September 2011. Available online: https://www.fws.gov/kempsridley/Finals/kempsridley_revision2.pdf (accessed on 5 May 2020).
- Witherington, B.E. Sea Turtles in Context: Their Life History and Conservation. In Sea Turtle Health and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing, Inc.: Plantation, FL, USA, 2017; pp. 3–24. ISBN 978-160-427-099-0. [Google Scholar]
- Innis, C.J.; Ravich, J.B.; Tlusty, M.F.; Hoge, M.S.; Wunn, D.S.; Boerner-Neville, L.B.; Merigo, C.; Weber, E.S. Hematologic and Plasma Biochemical Findings in Cold-Stunned Kemp’s Ridley Turtles: 176 cases (2001–2005). J. Am. Vet. Med. Assoc. 2009, 235, 426–432. [Google Scholar] [CrossRef]
- Hunt, K.E.; Innis, C.; Rolland, R.M. Corticosterone and Thyroxine in Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii). J. Zoo Wildl. Med. 2012, 43, 479–493. [Google Scholar] [CrossRef]
- Witherington, B.E.; Ehrhart, L.M. Hypothermic Stunning and Mortality of Marine Turtles in the Indian River Lagoon System, Florida. Copeia 1989, 1989, 696–703. [Google Scholar] [CrossRef]
- Griffin, L.P.; Griffin, C.R.; Finn, J.T.; Prescott, R.L.; Faherty, M.; Still, B.M.; Danylchuk, A.J. Warming Seas Increase Cold-Stunning Events for Kemp’s Ridley Sea Turtles in the Northwest Atlantic. PLoS ONE 2019, 14, e0211503. [Google Scholar] [CrossRef] [PubMed]
- Innis, C.J.; Staggs, L.A. Cold-Stunning. In Sea Turtle Health and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing, Inc.: Plantation, FL, USA, 2017; pp. 675–686. ISBN 978-160-427-099-0. [Google Scholar]
- Innis, C.; Nyaoke, A.C.; Williams, C.R.; Dunnigan, B.; Merigo, C.; Woodward, D.L.; Weber, E.S.; Frasca, S. Pathologic and Parasitologic Findings of Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii) Stranded on Cape Cod, Massachusetts, 2001–2006. J. Wildl. Dis. 2009, 45, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Innis, C.J.; Braverman, H.; Cavin, J.M.; Ceresia, M.L.; Baden, L.R.; Kuhn, D.M.; Frasca, S.; McGowan, J.P.; Hirokawa, K.; Weber, E.S.; et al. Diagnosis and Management of Enterococcus spp Infections During Rehabilitation of Cold-Stunned Kemp’s Ridley Turtles (Lepidochelys kempii): 50 cases (2006–2012). J. Am. Vet. Med. Assoc. 2014, 245, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.L.; Tuxbury, K.A.; Cavin, J.M.; Stacy, B.A.; Frasca, S., Jr.; Stacy, N.I.; O’Sullivan Brisson, J.; Williams, S.R.; Mccarthy, R.J.; Innis, C.J. Osteomyelitis in Rehabilitating Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii): 25 cases (2008–2018). J. Am. Vet. Med. Assoc. 2021, in press. [Google Scholar]
- Mumford, S.L.; Johnson, L.; Herbst, L. Multifocal Steatitis Lesions in a Kemp’s Ridley Sea Turtle (Lepidochelys kempii). In Proceedings of the 1999 IAAAM Conference, Boston, MA, USA, 2–5 May 1999. [Google Scholar]
- Innis, C.J.; Ceresia, M.L.; Merigo, C.; Scott Weber, E., III; Papich, M.G. Single-Dose Pharmacokinetics of Ceftazidime and Fluconazole During Concurrent Clinical Use in Cold-Stunned Kemp’s Ridley Turtles (Lepidochelys kempii). J. Vet. Pharmacol. Ther. 2012, 35, 82–89. [Google Scholar] [CrossRef]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Nutrition and Skin Disease. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; Elsevier Mosby: St. Louis, MO, USA, 2013; pp. 685–694. ISBN 978-133-624-364-4. [Google Scholar]
- Oros, J.; Monagas, P.; Calabuig, P.; Luzardo, O.P.; Camacho, M. Pansteatitis Associated with High Levels of Polychlorinated Biphenyls in a Wild Loggerhead Sea Turtle Caretta caretta. Dis. Aquat. Org. 2013, 102, 237–242. [Google Scholar] [CrossRef]
- German, A.J.; Foster, A.P.; Holden, D.; Hotston Moore, A.; Day, M.J.; Hall, E.J. Sterile Nodular Panniculitis and Pansteatitis in Three Weimaraners. J. Small Anim. Pract. 2003, 44, 449–455. [Google Scholar] [CrossRef]
- Lane, E.P.; Huchzermeyer, F.W.; Govender, D.; Bengis, R.G.; Buss, P.E.; Hofmeyr, M.; Myburgh, J.G.; Steyl, J.C.; Pienaar, D.J.; Kotze, A. Pansteatitis of Unknown Etiology Associated with Large-Scale Nile Crocodile (Crocodylus niloticus) Mortality in Kruger National Park, South Africa: Pathologic Findings. J. Zoo Wildl. Med. 2013, 44, 899–910. [Google Scholar] [CrossRef]
- Larsen, R.E.; Buergelt, C.; Cardeilhac, P.T.; Jacobson, E.R. Steatitis and Fat Necrosis in Captive Alligators. J. Am. Vet. Med. Assoc. 1983, 183, 1202–1204. [Google Scholar]
- Osthoff, G.; Hugo, A.; Bouwman, H.; Buss, P.; Govender, D.; Joubert, C.C.; Swarts, J.C. Comparison of the Lipid Properties of Captive, Healthy Wild, and Pansteatitis-Affected Wild Nile Crocodiles (Crocodylus niloticus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Manawatthana, S.; Kasorndorkbua, C. Steatitis and Vitamin E Deficiency in Captive Olive Ridley Turtles (Lepidochelys olivacea). In Proceedings of the 2nd International Symposium on SEASTAR2000 and Asian Bio-logging Science, Bangkok, Thailand, 13–14 December 2005; pp. 85–87. [Google Scholar]
- Valdivia, P.A.; Zenteno-Savin, T.; Gardner, S.C.; Aguirre, A.A. Basic Oxidative Stress Metabolites in Eastern Pacific Green Turtles (Chelonia mydas agassizii). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Browne, R.W.; Armstrong, D. Simulatneous Determination of Serum Retinol, Tocopherols, and Carotenoids by HPLC. In Methods in Molecular Biology: Free Radical and Antioxidant Protocols; Armstrong, D., Ed.; Humana Press, Inc.: Totowa, NJ, USA, 1998; Volume 108, pp. 269–275. ISBN 978-089-603-472-3. [Google Scholar]
- Armstrong, D.; Hiramitsu, T.; Ueda, T. In Vitro Screening for Antioxidant Activity. Methods Mol. Biol. 1998, 108, 315–323. [Google Scholar] [CrossRef]
- Miller, N. Nonvitamin Plasma Antioxidants. In Methods in Molecular Biology: Free Radical and Antioxidant Protocols; Armstrong, D., Ed.; Humana Press, Inc.: Totowa, NJ, USA, 1998; Volume 108, pp. 285–297. ISBN 978-089-603-472-3. [Google Scholar]
- Yagi, K. Simple Assay for the Level of Total Lipid Peroxides in Serum or Plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Aroni, K.; Aivaliotis, M.; Tsele, E.; Charalambopoulos, D.; Davaris, P. An Unusual Panniculitis Appearing in the Winter with Good Response to Tetracycline. J. Dermatol. 1998, 25, 677–681. [Google Scholar] [CrossRef]
- Milei, J.; Forcada, P.; Fraga, C.G.; Grana, D.R.; Iannelli, G.; Chiariello, M.; Tritto, I.; Ambrosio, G. Relationship Between Oxidative Stress, Lipid Peroxidation, and Ultrastructural Damage in Patients with Coronary Artery Disease Undergoing Cardioplegic Arrest/Reperfusion. Cardiovasc. Res. 2007, 73, 710–719. [Google Scholar] [CrossRef]
- Frutchey, K. Plasma Levels of Vitamin A and E in Marine Turtles (Chelonia mydas and Caretta caretta). Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2004. Available online: https://stars.library.ucf.edu/rtd/4580/ (accessed on 6 July 2020).
- Deem, S.L.; Dierenfeld, E.S.; Sounguet, G.P.; Alleman, A.R.; Cray, C.; Poppenga, R.H.; Norton, T.M.; Karesh, W.B. Blood Values in Free-Ranging Nesting Leatherback Sea Turtles (Dermochelys coriacea) on the Coast of the Republic of Gabon. J. Zoo Wildl. Med. 2006, 37, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Innis, C.; Merigo, C.; Dodge, K.; Tlusty, M.; Dodge, M.; Sharp, B.; Myers, A.; McIntosh, A.; Wunn, D.; Perkins, C.; et al. Health Evaluation of Leatherback Turtles (Dermochelys coriacea) in the Northwestern Atlantic During Direct Capture and Fisheries Gear Disentanglement. Chelonian Conserv. Biol. 2010, 9, 205–222. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, Oxidative Stress, and Inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef]
- Suantawee, T.; Tantavisut, S.; Adisakwattana, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Deepaisarnsakul, B.; Honsawek, S. Oxidative Stress, Vitamin E, and Antioxidant Capacity in Knee Osteoarthritis. J. Clin. Diagn. Res. 2013, 7, 1855–1859. [Google Scholar] [CrossRef]
- Drevon, C.A. Absorption, Transport and Metabolism of Vitamin E. Free Radic. Res. Commun. 1991, 14, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Ghelfi, M.; Atkinson, J.; Parker, R.; Qian, J.; Carlin, C.; Manor, D. Vitamin E and Phosphoinositides Regulate the Intracellular Localization of the Hepatic α-Tocopherol Transfer Protein. J. Biol. Chem. 2016, 291, 17028–17039. [Google Scholar] [CrossRef] [PubMed]
- Niza, M.; Vilela, C.; Ferreira, L. Feline Pansteatitis Revisited: Hazards of Unbalanced Home-Made Diets. J. Feline Med. Surg. 2003, 5, 271–277. [Google Scholar] [CrossRef]
- Fytianou, A.; Koutinas, A.F.; Saridomichelakis, M.N.; Koutinas, C.K. Blood Alpha-Tocopherol, Selenium, and Glutathione Peroxidase Changes and Adipose Tissue Fatty Acid Changes in Kittens with Experimental Steatitis (Yellow Fat Disease): A Comparative Study Between the Domestic Shorthaired and Siamese Breed. Biol. Trace Elem. Res. 2006, 112, 131–143. [Google Scholar] [CrossRef]
- Bricknell, I.R.; Bruno, D.W.; Bowden, T.J.; Smith, P. Fat Cell Necrosis Syndrome in Atlantic Halibut, Hippoglossus hippoglossus L. Aquaculture 1996, 144, 65–69. [Google Scholar] [CrossRef]
- Guarda, F.; Bertoja, G.; Zoccarato, I.; Tartari, E.; Biolatti, B. Spontaneous Steatitis of Epicardial Fat in Farmed White Sturgeon (Acipenser transmontanus). Aquaculture 1997, 158, 167–177. [Google Scholar] [CrossRef]
- Roberts, R.J.; Agius, C. Pan-Steatitis in Farmed Northern Bluefin Tuna, Thunnus thynnus (L.), in the Eastern Adriatic. J. Fish Dis. 2008, 31, 83–88. [Google Scholar] [CrossRef]
- Dierenfeld, E.S. Vitamin E Deficiency in Zoo Reptiles, Birds, and Ungulates. J. Zoo Wildl. Med. 1989, 20, 3–11. [Google Scholar]
- Pollock, C.G.; Sleeman, J.M.; Houle, C.D.; Ramsay, E.C. Vitamin E Deficiency and Pansteatitis in Juvenile Boat-Billed Herons (Cochlearius cochlearius). J. Zoo Wildl. Med. 1999, 30, 297–300. [Google Scholar] [PubMed]
- Wong, E.; Mikaelian, I.; Desnoyers, M.; Fitzgerald, G. Pansteatitis in a Free-Ranging Red-Tailed Hawk (Buteo jamaicensis). J. Zoo Wildl. Med. 1999, 30, 584–586. [Google Scholar]
- Myburgh, J.; Botha, A. Decline in Herons Along the Lower Olifants River—Could Pansteatitis Be a Contributing Factor? Vet News 2009, 3, 20–23. [Google Scholar]
- Jones, D.; Howard, A.N.; Gresham, G.A. Aetiology of “Yellow Fat” Disease (Pansteatitis) in the Wild Rabbit. J. Comp. Pathol. 1969, 79, 329–334. [Google Scholar] [CrossRef]
- Juan-Salles, C.; Prats, N.; Resendes, A.; Domingo, M.; Hilton, D.; Ruiz, J.M.; Garner, M.M.; Valls, X.; Marco, A.J. Anemia, Myopathy, and Pansteatitis in Vitamin E-Deficient Captive Marmosets (Callithrix spp.). Vet. Pathol. 2003, 40, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Bonar, C.J.; Wagner, R.A. A Third Report of “Golf Ball Disease” in an Amazon River Dolphin (Inia geoffrensis) Associated with Streptococcus iniae. J. Zoo Wildl. Med. 2003, 34, 296–301. [Google Scholar] [CrossRef]
- Hoopes, L.A.; Koutsos, E.A. Nutrition. In Sea Turtle Health and Rehabilitation; Manire, C.A., Norton, T.M., Stacy, B.A., Innis, C.J., Harms, C.A., Eds.; J. Ross Publishing, Inc.: Plantation, FL, USA, 2017; pp. 63–96. ISBN 978-160-427-099-0. [Google Scholar]
- Herring, Raw. Available online: https://ndb.nal.usda.gov//fdc-app.html#/food-details/782531/nutrients (accessed on 7 September 2020).
- Clams, Raw. Available online: https://ndb.nal.usda.gov//fdc-app.html#/food-details/782757/nutrients (accessed on 7 September 2020).
- Crustaceans, Crab, Blue, Raw. Available online: https://ndb.nal.usda.gov//fdc-app.html#/food-details/174204/nutrients (accessed on 7 September 2020).
- Innis, C.; Kennedy, A.; Wocial, J.; Burgess, E.; Papich, M.G. Comparison of Oxytetracycline Pharmacokinetics After Multiple Subcutaneous Injections in Three Sea Turtle Species. J. Herpetol. Med. Surg. 2020, 30, 142–147. [Google Scholar] [CrossRef]
- Mellanby, R.J.; Stell, A.; Baines, E.; Chantrey, J.C.; Herrtage, M.E. Panniculitis Associated with Pancreatitis in a Cocker Spaniel. J. Small Anim. Pract. 2003, 44, 24–28. [Google Scholar] [CrossRef]
- Mourad, F.H.; Hannoush, H.M.; Bahlawan, M.; Uthman, I.; Uthman, S. Panniculitis and Arthritis as the Presenting Manifestation of Chronic Pancreatitis. J. Clin. Gastroenterol. 2001, 32, 259–261. [Google Scholar] [CrossRef]
- O’Kell, A.L.; Inteeworn, N.; Diaz, S.F.; Saunders, G.K.; Panciera, D.L. Canine Sterile Nodular Panniculitis: A Retrospective Study of 14 Cases. J. Vet. Intern. Med. 2010, 24, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Comstock, G.W.; Alberg, A.J.; Helzlsouer, K.J. Reported Effects of Long-Term Freezer Storage on Concentrations of Retinol, Beta-Carotene, and Alpha-Tocopherol in Serum or Plasma Summarized. Clin. Chem. 1993, 39, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Brown Thomas, J.; Duewer, D.L.; Kline, M.C.; Sharpless, K.E. The Stability of Retinol, Α-Tocopherol, Trans-Lycopene, and Trans-Β-Carotene in Liquid-Frozen and Lyophilized Serum. Clin. Chim. Acta 1998, 276. [Google Scholar] [CrossRef]
- Young, I.S.; Trimble, E.R. Measurement of Malondialdehyde in Plasma by High Performance Liquid Chromatography with Fluorimetric Detection. Ann. Clin. Biochem. 1991, 28 Pt 5, 504–508. [Google Scholar] [CrossRef]

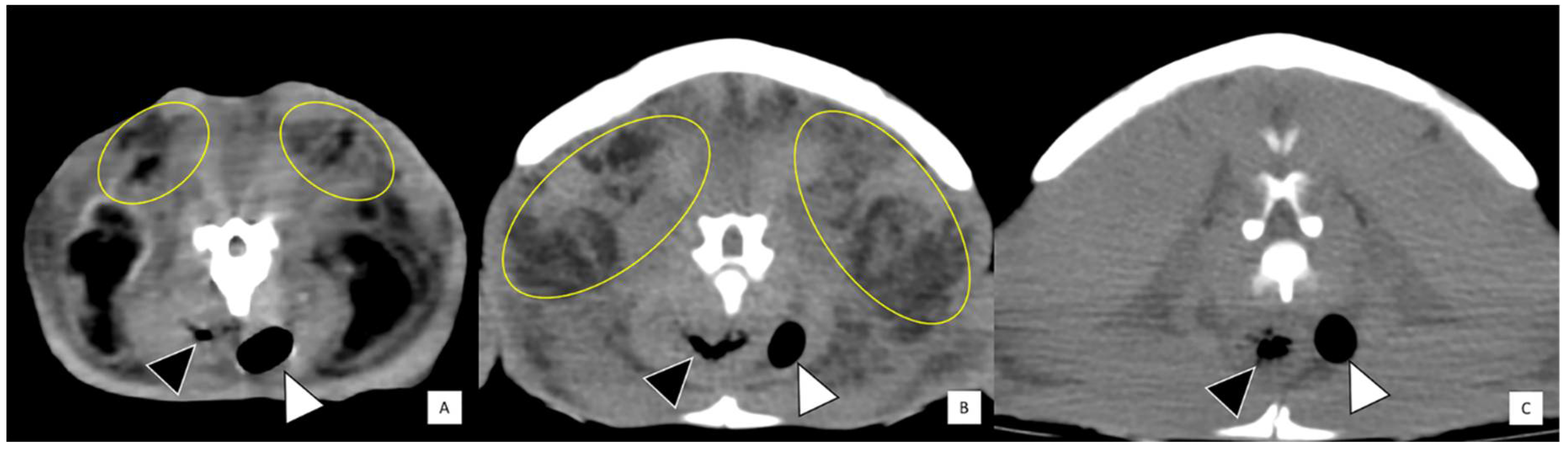
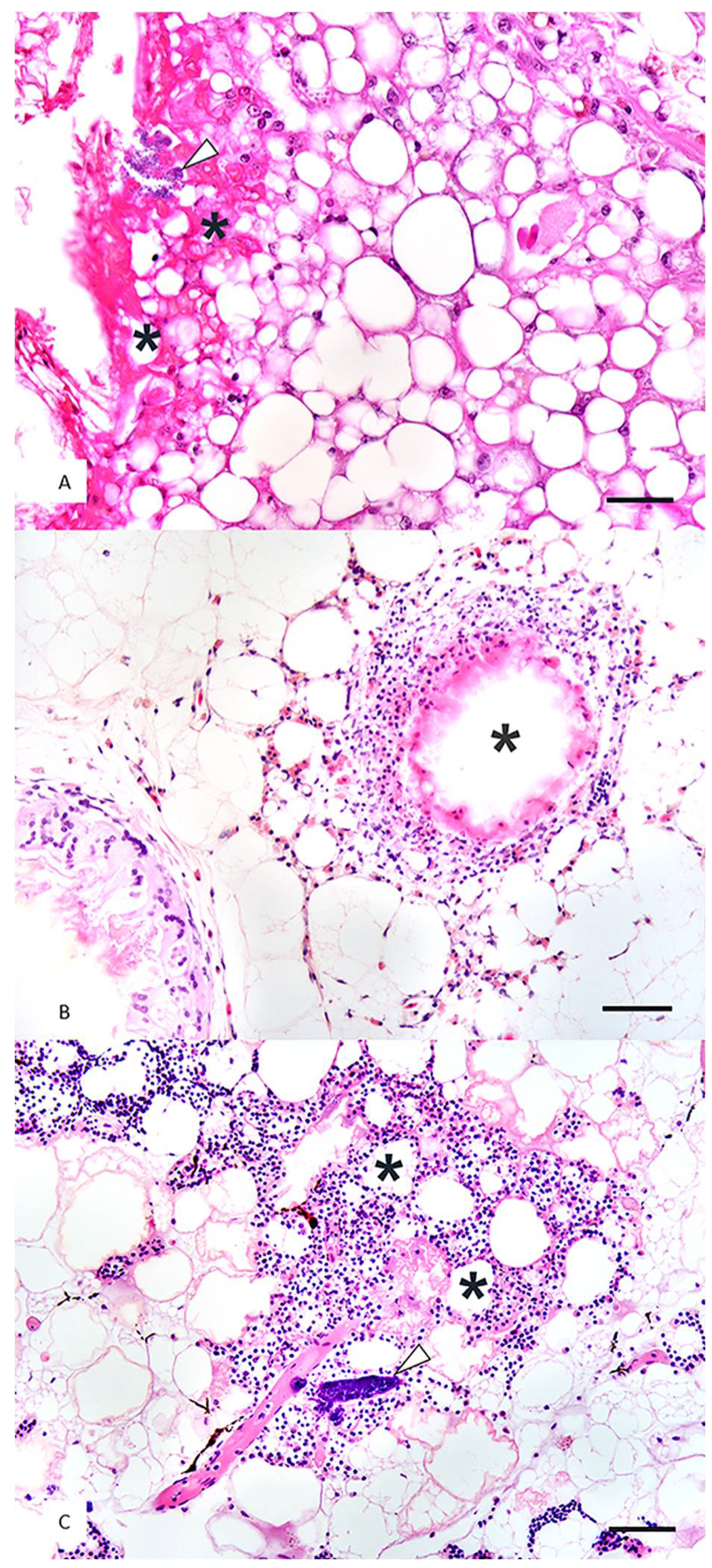
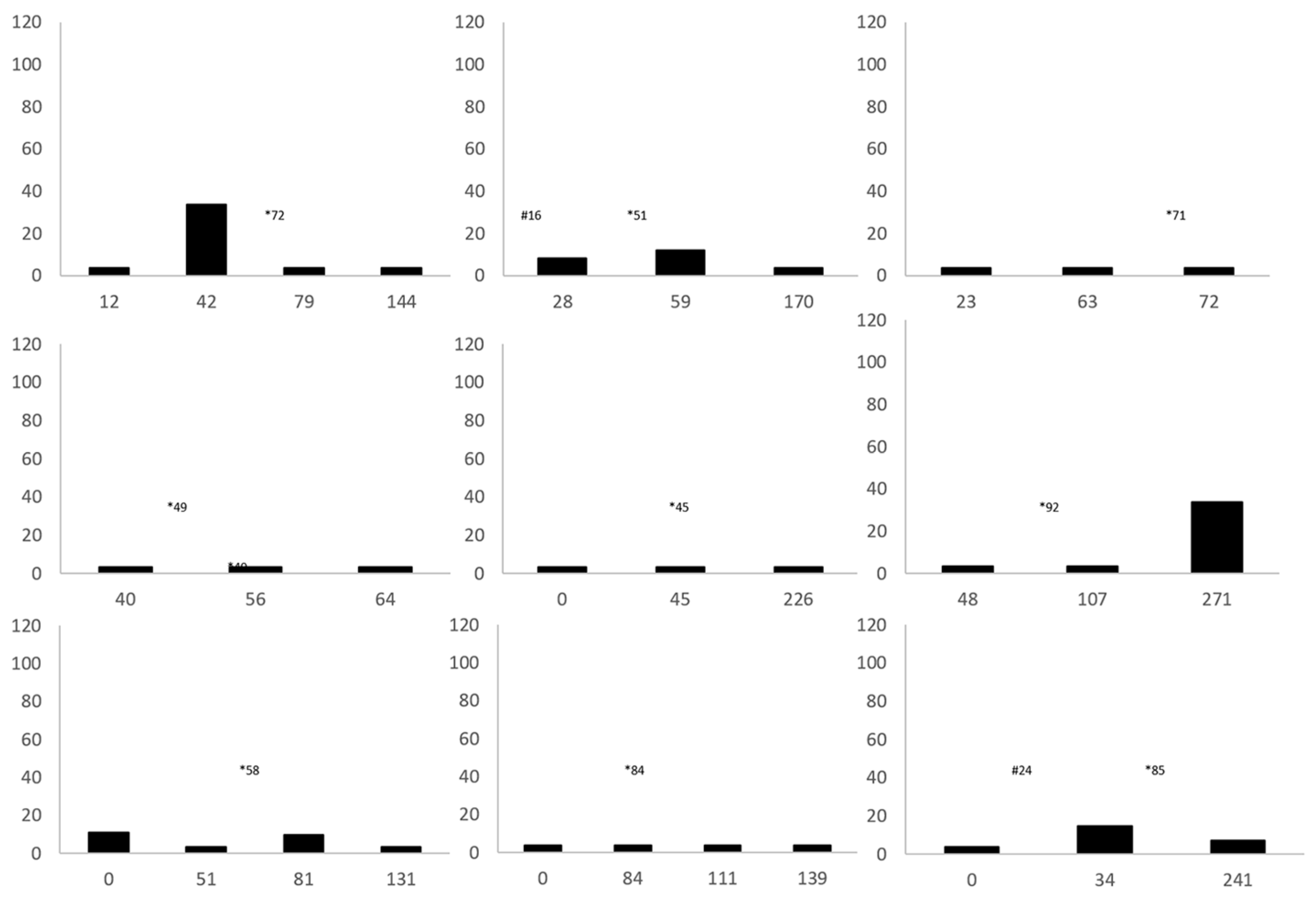

| ID | Steatitis Diagnosis by Palpation (Y/N) | Histopathology | Day of Steatitis Diagnosis after Admission | Blood Culture | Tissue Culture | Plasma Vitamin E (Y/N) | Plasma TBARS (Y/N) | Paired Vitamin E and TBARS Samples (Y/N) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Y | NP | 72 | NEG | NP | Y | N | N | Released |
| 2 | N | PM | 33 | NP | NP | Y | N | N | Died |
| 3 2 | Y | NP | 51 | NEG | NP | Y | N | N | Released |
| 4 | Y | NP | 50 | POS | POS | N | N | N | Released |
| 5 | N | PM | 469 | NP | NP | Y | Y | Y | Euthanized |
| 6 | N | PM | 84 | NP | NP | N | Y | N | Died |
| 7 1 | Y | NP | 71 | NEG | NP | Y | Y | Y | Released |
| 8 | Y | NP | 65 | NEG | NP | Y | Y | Y | Released |
| 9 | Y | NP | 70 | POS | NP | N | N | N | Released |
| 10 | Y | B (n = 2) | 55 | NEG | POS | Y | Y | Y | Released |
| 11 1 | Y | B | 49 | NEG | NP | Y | Y | N | Released |
| 12 | Y | NP | 85 | NEG | NP | Y | Y | Y | Released |
| 13 | Y | NP | 36 | NP | NEG | Y | N | N | Released |
| 14 | Y | NP | 45 | NP | NP | Y | N | N | Released |
| 15 | Y | NP | 92 | POS | NP | Y | Y | Y | Released |
| 16 | Y | NP | 84 | NEG | NP | Y | Y | Y | Released |
| 17 | Y | NP | 58 | NP | NP | Y | Y | Y | Released |
| 18 2 | Y | B (n = 2) | 115 | POS (n = 2) | NEG | Y | N | N | Released |
| 19 | N | PM | 437 | NP | NP | N | N | N | Died |
| 20 2 | Y | B | 75 | NEG | NP | Y | N | N | Released |
| 21 | Y | NP | 95 | POS (n = 3) | NP | Y | Y | Y | Released |
| 22 | Y | NP | 45 | NEG | NP | Y | Y | Y | Released |
| 23 | N | PM | 89 | POS (n = 2) | NP | N | N | N | Died |
| Histopathology ID # | Patient # | Necropsy or Biopsy | Heterophilic Inflammation | Necrosis of Adipose Tissue | Bacteria Present | Histiocytic Inflammation | Myo- Necrosis | Thrombi | Minerali-Zation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Biopsy | Y | Y | Y | Y | N | N | N |
| 2 | 1 | Biopsy | Y | Y | Y | Y | N | N | N |
| 3 | 2 | Biopsy | N | Y | N | N | Y | N | Y |
| 4 | 3 | Biopsy | Y | Y | N | N | N | N | N |
| 5 | 3 | Biopsy | N | Y | Y | N | N | N | N |
| 6 | 4 | Biopsy | Y | N | N | Y | N | N | N |
| 7 | 5 | Necropsy | Y | N | Y | N | Y | Y | N |
| 8 | 6 | Necropsy | Y | N | N | Y | N | N | N |
| 9 | 7 | Necropsy | Y | N | Y | Y | N | N | N |
| 10 | 8 | Necropsy | Y | Y | Y | N | N | N | N |
| 11 | 9 | Necropsy | Y | Y | N | N | N | N | N |
| Total | 9 | 7 | 6 | 5 | 2 | 1 | 1 |
| Variables | Cold-Stunned Turtles with Diagnosis of Steatitis | Free-Ranging Immature Control Turtles | Z | p |
|---|---|---|---|---|
| Mass (kg) | ||||
| N | 10 | 8 | ||
| Mean ± SD | 2.9 ± 0.9 | 17.9 ± 7.8 | ||
| Median (minimum, maximum) | 3.1 (1.6, 4.1) | 18.1 (3.4, 28.0) | 3.20 | <0.01 |
| Straight carapace length (cm) | ||||
| N | 10 | 8 | ||
| Mean ± SD | 27.5 ± 3.1 | 46.1 ± 8.7 | ||
| Median (minimum, maximum) | 28.5 (22.6, 31.4) | 47.7 (27.0, 55.3) | 2.98 | <0.01 |
| Vitamin E * nmol/g | ||||
| N | 10 | 9 | ||
| Mean ± SD | 3.6 ± 0.7 | 61.2 ± 23.3 | ||
| Median (minimum, maximum) | 3.5 (2.3, 5.5) | 62.3 (25.3, 90.9) | 3.67 | <0.01 |
| TBARS nmol/g | ||||
| N | 10 | 9 | ||
| Mean ± SD | 1.8 ± 0.6 | 2.5 ± 0.7 | ||
| Median (minimum, maximum) | 1.6 (1.2, 3.2) | 2.1 (1.8, 3.9) | 2.41 | 0.01 |
| TBARS to vitamin E ratio | ||||
| N | 10 | 9 | ||
| Mean ± SD | 0.52 ± 0.18 | 0.05 ± 0.03 | ||
| Median (minimum, maximum) | 0.50 (0.28, 0.92) | 0.03 (0.02, 0.10) | 3.63 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, R.C.; Innis, C.J.; Stacy, B.A.; Hernandez, J.A.; Hill, R.C.; Scott, K.C.; Frasca, S., Jr.; Garner, M.M.; Burns, R.E.; Arendt, M.D.; et al. Steatitis in Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii). Animals 2021, 11, 898. https://doi.org/10.3390/ani11030898
Turner RC, Innis CJ, Stacy BA, Hernandez JA, Hill RC, Scott KC, Frasca S Jr., Garner MM, Burns RE, Arendt MD, et al. Steatitis in Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii). Animals. 2021; 11(3):898. https://doi.org/10.3390/ani11030898
Chicago/Turabian StyleTurner, Rachel C., Charles J. Innis, Brian A. Stacy, Jorge A. Hernandez, Richard C. Hill, Karen C. Scott, Salvatore Frasca, Jr., Michael M. Garner, Rachel E. Burns, Michael D. Arendt, and et al. 2021. "Steatitis in Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii)" Animals 11, no. 3: 898. https://doi.org/10.3390/ani11030898
APA StyleTurner, R. C., Innis, C. J., Stacy, B. A., Hernandez, J. A., Hill, R. C., Scott, K. C., Frasca, S., Jr., Garner, M. M., Burns, R. E., Arendt, M. D., Brisson, J., Norton, T. M., Williams, S. R., Kennedy, A., Alexander, A. B., & Stacy, N. I. (2021). Steatitis in Cold-Stunned Kemp’s Ridley Sea Turtles (Lepidochelys kempii). Animals, 11(3), 898. https://doi.org/10.3390/ani11030898






