Posterior Mandibular Displacement—A Systematic Review Based on Animal Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection
2.4. Data Collection
2.5. Risk of Bias in Individual Studies
2.6. Summary Measures and Shaping of Results
3. Results
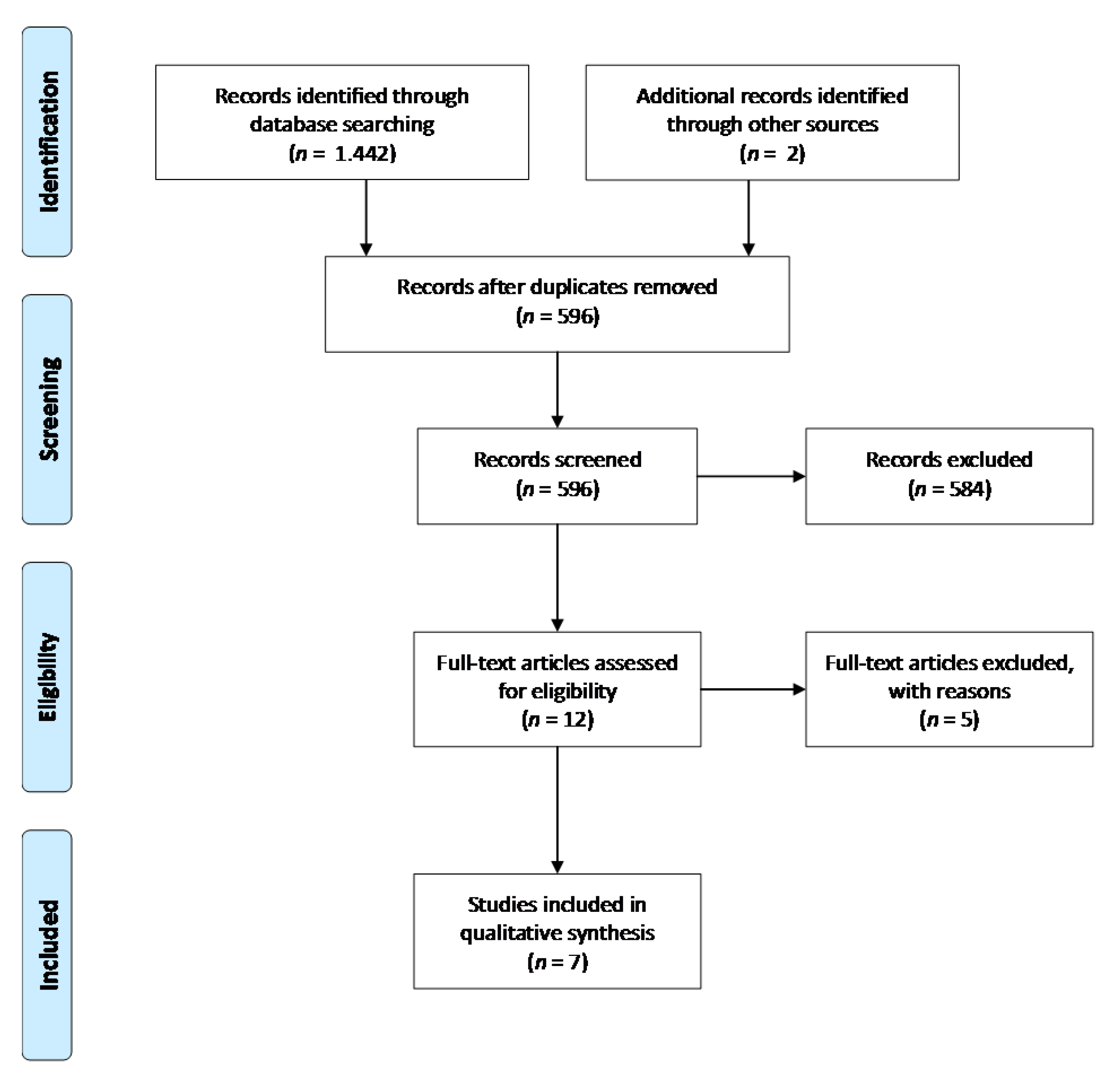
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Results of Individual Studies
4. Discussion
4.1. Summary of Evidence
4.2. Strengths and Limitations
4.3. Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 3–4. [Google Scholar]
- Lin, F.; Ren, M.; Yao, L.; He, Y.; Guo, J.; Ye, Q. Psychosocial impact of dental esthetics regulates motivation to seek orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 476–482. [Google Scholar] [CrossRef]
- Alabdulrazaq, R.S.; Al-Haj Ali, S.N. Parental Reported Bullying among Saudi Schoolchildren: Its Forms, Effect on Academic Abilities, and Associated Sociodemographic, Physical, and Dentofacial Features. Int. J. Pediatr. 2020. [Google Scholar] [CrossRef]
- Gavric, A.; Mirceta, D.; Jakobovic, M.; Pavlic, A.; Zrinski, M.T.; Spalj, S. Craniodentofacial characteristics, dental esthetics-related quality of life, and self-esteem. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 711–718. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Meira, A.C.L.; Custodio, W.; Vedovello Filho, M.; Borges, T.M.; Meneghim, M.D.C.; Santamaria, M., Jr.; Vedovello, S.A.S. How is orthodontic treatment need associated with perceived esthetic impact of malocclusion in adolescents? Am. J. Orthod. Dentofac. Orthop. 2020, 158, 668–673. [Google Scholar] [CrossRef]
- Hans, M.G.; Tsolakis, K.I.; Cain, D.A.; Elbarnashawya, S.G.; Valiathand, M. Animal studies in orthodontics—Are they useful for clinicians? Semin. Orthod. 2017, 23, 366–372. [Google Scholar] [CrossRef]
- Zere, E.; Chaudhari, P.K.; Sharan, J.; Dhingra, K.; Tiwari, N. Developing Class III malocclusions: Challenges and solutions. Clin. CosmetInvestig. Dent. 2018, 10, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.K.; Gaddikeri, S.; Singhal, A.; Hardin, S.; Tran, B.D.; Medina, J.A.; Curé, J.K. Imaging of the temporomandibular joint: An update. World J. Radiol. 2014, 6, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.A.; Becks, H.; Simpson, M.E.; Evans, H.M. Growth and Transformation of the Mandibular Joint in the Rat. I. Normal Female Rats. Am. J. Orthod. 1946, 32, 431–442. [Google Scholar] [CrossRef]
- Shaffer, S.M.; Brismée, J.-M.; Sizer, P.S.; Courtney, C.A. Temporomandibular disorders. Part 1: Anatomy and examination/diagnosis. J. Man. Ther. 2014, 22, 2–12. [Google Scholar] [CrossRef]
- Baume, L.J.; Derichsweiler, H. Is the condylar growth center responsive to orthodontic therapy? An Experimental study in macaca mulatta. Oral Surg. Oral Med. Oral Path. 1961, 14, 347–362. [Google Scholar] [CrossRef]
- Farias-Neto, A.; Varela Brown Martins, A.P.; Figueroba, S.R.; Groppo, F.C.; de Almeidad, S.M.; Rizzatti-Barbosa, C.M. Altered mandibular growth under functional posterior displacement in rats. Angle. Orthod. 2012, 82, 3–7. [Google Scholar] [CrossRef]
- Martina, S.; Martina, R.; Franchi, L.; D’Antò, V.; Valletta, R. A New Appliance for Class III Treatment in Growing Patients: Pushing Splints 3. Case Rep. Dent. 2019, 9597024. [Google Scholar] [CrossRef] [PubMed]
- Mousoulea, S.; Tsolakis, I.; Ferdianakis, E.; Tsolakis, A.I. The Effect of Chin-cup Therapy in Class III Malocclusion: A Systematic Review. Open Dent. J. 2016, 10, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Bryndahl, F.; Warfvinge, G.; Eriksson, L.; Isberg, A. Cartilage changes link retrognathic mandibular growth to TMJ disc displacement in a rabbit model. Int. J. Oral. Maxill. Surg. 2011, 40, 621–627. [Google Scholar] [CrossRef]
- Cholasueksa, P.; Warita, H.; Soma, K. Alterations of the Rat Temporomandibular Joint in Functional Posterior Displacement of the Mandible. Angle Orthod. 2004, 74, 677–683. [Google Scholar]
- Ingervall, B.; Fredén, H.; Heyden, G. Histochemical study of mandibular joint adaptation in experimental posterior mandibular displacement in the rat. Arch. Oral Biol. 1972, 17, 661–671. [Google Scholar] [CrossRef]
- Teramoto, M.; Kaneko, S.; Shibata, S.; Yanagishita, M.; Soma, K. Effect of compressive forces on extracellular matrix in rat mandibular condylar cartilage. J. Bone Miner. Metab. 2003, 21, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Von den Hoff, J.W.; Delatte, M. Interplay of mechanical loading and growth factorsin the mandibular condyle. Arch. Oral Biol. 2008, 53, 709–715. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Thilander, B.; Kjellberg, H.; Topouzelis, N.; Zafiriadis, A. Effect of low masticatory function on condylar growth: A morphometric study in the rat. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 121–125. [Google Scholar] [CrossRef]
- Kuroda, S.; Tanimoto, K.; Izawa, T.; Fujihara, S.; Koolstra, J.H.; Tanaka, E. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthr. Cartil. 2009, 17, 1408–1415. [Google Scholar] [CrossRef]
- Nickel, J.C.; Iwasaki, L.R.; Gonzalez, Y.M.; Gallo, L.M.; Yao, H. Mechanobehavior and Ontogenesis of the Temporomandibular Joint. J. Dent. Res. 2018, 97, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Utreja, A.; Dyment, N.A.; Yadav, S.; Villa, M.M.; Li, Y.; Jiang, X.; Nanda, R.; Rowe, D.W. Cell and matrix response of temporomandibular cartilage to mechanical loading. Osteoarthr. Cartil. 2016, 24, 335–344. [Google Scholar] [CrossRef]
- Carlson, D.S. Evolving concepts of heredity and genetics in orthodontics. Am. J. Orthod. Dent. Orthop. 2015, 148, 922–938. [Google Scholar] [CrossRef]
- Coombs, M.C.; She, X.; Brown, T.R.; Slate, E.H.; Lee, J.S.; Yao, H. Temporomandibular Joint Condyle-Disc Morphometric Sexual Dimorphisms Independent of Skull Scaling. J. Oral. Max. Surg. 2019, 77, 2245–2257. [Google Scholar] [CrossRef]
- He, S.; Hartsfield, J.K., Jr.; Guo, Y.; Cao, Y.; Wang, S.; Chen, S. Association between CYP19A1 genotype and pubertal sagittal jaw growth. Am. J. Orthod. Dent. Orthop. 2012, 142, 662–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vieira, A.R. Orthodontics and Genetics. Dent. Press J. Orthod. 2019, 24, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, T.; Reyes, B.C.; McNamara, J.A., Jr. Gender Differences in Class III Malocclusion. Angle Orthod. 2005, 75, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Baume, L.J. Growth and transformation of the mandibular joint in the rat thyroidectomized at birth: VII. The effect of growth hormone and thyroxin given separately or in combination. Am. J. Orthod. 1953, 39, 623–633. [Google Scholar] [CrossRef]
- Milam, S.B.; Aufdemorte, T.B.; Sheridan, P.J.; Triplet, R.G.; Van Sickels, J.E.; Halt, G.R. Sexual dimorphism in the distribution of estrogen receptors in the temporomandibular joint complex of the baboon. Oral. Surg. Oral. Med. Oral. Pathol. 1987, 64, 527–532. [Google Scholar] [CrossRef]
- Robinson, J.L.; Soria, P.; Xu, M.; Vrana, M.; Luchetti, J.; Lu, H.H.; Chen, J.; Wadhwa, S. Estrogen Promotes Mandibular Condylar Fibrocartilage Chondrogenesis and Inhibits Degeneration via Estrogen Receptor Alpha in Female Mice. Sci. Rep. 2018, 8, 8527. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tang, Q.; Xie, M.; Zhou, X.; Long, Y.; Xie, Y.; Guo, F.; Chen, L. Circadian BMAL1 regulates mandibular condyle development by hedgehog pathway. Cell Prolif. 2020, 53, e12727. [Google Scholar] [CrossRef]
- Du, J.; Jiang, Q.; Mei, L.; Yang, R.; Wen, J.; Lin, S.; Li, H. Effect of high fat diet and excessive compressive mechanical force on pathologic changes of temporomandibular joint. Sci. Rep. 2020, 10, 17457. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.; Ehrlich, P.; Feldman, M.; Sapolsky, R.; Wong, S. The Jaw Epidemic: Recognition, Origins, Cures, and Prevention. Bioscience 2020, 70, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Cedströmer, A.-L.; Andlin-Sobocki, A.; Abbu, N.; Hedenberg-Magnusson, B.; Dahlström, L.; Berntson, L. Condylar alterations and facial growth in children with juvenileidiopathic arthritis. J. Orofac. Orthop. 2020, 81, 163–171. [Google Scholar] [CrossRef]
- Chetty, M.; Roberts, T.S.; Stephen, L.; Beighton, P. Craniofacial manifestations in osteogenesis imperfecta type III in South Africa. BDJ Open 2017, 3, 17021. [Google Scholar] [CrossRef][Green Version]
- Sansare, K.; Gupta, A.; Khanna, V.; Karjodkar, F. Oral tuberculosis: Unusual radiographic findings. Dentomaxillofac. Radiol. 2011, 40, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, L.; Zhu, G.; Su, Y.; Chen, Y.; Sun, J.; Wang, Y.; Xian, J. Psychological stress induces alterations in temporomandibular joint ultrastructure in a rat model of temporomandibular disorder. Oral Surg. Oral Med. Oral Pathol. Oral RadiolEndod 2011, 112, e106–e112. [Google Scholar] [CrossRef]
- Wu, G.; Chen, L.; Su, Y.; Zhu, G.; Wang, P.; Wang, Y.; Chend, Y. The influence of psychological stress on the rat temporomandibular joint with the application of countermeasures. J. Surg. Res. 2012, 178, 728–736. [Google Scholar] [CrossRef]
- Mew, J.R.C. Factors Influencing Mandibular Growth. Angle Orthod. 1986, 56, 31–48. [Google Scholar] [CrossRef]
- Bouvier, M.; Hylander, W.L. The effect of dietary consistency on gross and histologic morphology in the craniofacial region of young rats. Am. J. Anat. 1984, 170, 117–126. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Xia, L.; Li, H.; Xu, Y.; Hua, X. Effects of Twin Inclined Plane Device on Adaptation and Ultrastructure Variations in Condyle of Growing Rats. Biomed. Res. Int. 2019, 3069347. [Google Scholar] [CrossRef]
- Figueroba, S.R.; Desjardins, M.P.; Ferreira, L.E.N.; Berto, L.A.; Valdrighi, H.C.; Groppo, F.C. The influence of altered occlusion on pro-inflammatory cytokine levels in the TMJ synovial tissues of rats. Archs. Dent. Biol. 2014, 59, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Johnson, D.L.; Howes, R.I.; Rohrer, M.D. Changes in the rabbit temporomandibular joint associated with posterior displacement of the mandible. Int. J. Prosthodont. 1996, 9, 46–57. [Google Scholar]
- Zurfluh, M.A.; Kloukos, D.; Patcas, R.; Eliades, T. Effect of chin-cup treatment on the temporomandibular joint: A systematic review. Eur. J. Orthod. 2015, 37, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Folke, L.E.A.; Stallard, R.E. Condylar adaptation to a change in intermaxillary relationship. J. Periodont. Res. 1966, 1, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Meikle, M.C. Remodeling the Dentofacial Skeleton: The Biological Basis of Orthodontics and Dentofacial Orthopedics. J. Dent. Res. 2007, 86, 12–24. [Google Scholar] [CrossRef]
- Breitner, C. Further investigations of bone changes resulting from experimental orthodontic treatment. Am. J. Orthod. Dent. Orthop. 1941, 27, 605–632. [Google Scholar] [CrossRef]
- Janzen, E.K.; Bluher, J.A. The cephalometric, anatomic, and histologic changes in Macaca mulatta after application of a continuous-acting retraction force on the mandible. Am. J. Orthod. 1965, 51, 803–878. [Google Scholar] [CrossRef]
- Almarza, A.J.; Brown, B.N.; Boaz Arzi, B.; Ângelo, D.F.; Chung, W.; Badylak, S.F.; Detamore, M. Preclinical Animal Models for Temporomandibular Joint Tissue Engineering. Tissue Eng. Part. B Rev. 2018, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.W. TMJ anatomy and animal models. J. Musculoskelet. Neuronal. Interact. 2003, 3, 391–394. [Google Scholar] [PubMed]
- Suzuki, A.; Iwata, J. Mouse genetic models for temporomandibular joint development and disorders. Oral. Dis. 2016, 22, 33–38. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, London. 2011. Available online: www.cochrane-handbook.org (accessed on 31 August 2018).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: Chichester, UK, 2009. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Asano, T. The effects of mandibular retractive force on the growing rat mandible. Am. J. Orthod. Dentofac. Orthop. 1986, 90, 464–474. [Google Scholar] [CrossRef]
- Hua, X.; Xiong, H.; Han, G.; Cheng, X. The effects of gradually induced backward movement of the mandible by a Twin Inclined Plane Device in rats. Angle. Orthod. 2012, 82, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, J.; Cistulli, P.A. Dental Treatment for Paediatric Obstructive Sleep Apnea. Paed. Resp. Rev. 2015, 16, 174–181. [Google Scholar] [CrossRef]
- Nguee, A.A.M.; Ongkosuwito, E.M.; Jaddoe, V.W.V.; Wolvius, E.B.; Kragt, L. Impact of orthodontic treatment need and deviant occlusal traits on oral health–related quality of life in children: A cross-sectional study in the Generation R cohort. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 764–772. [Google Scholar] [CrossRef]
- Sommerlad, B.C. Management of cleft lip and palate. Curr. Pediatr. 1994, 4, 189–195. [Google Scholar] [CrossRef]
- Ghafari, J.G. Centennial inventory: The changing face of orthodontics. Am. J. Orthod. Dentofac. Orthope. 2015, 148, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Aersens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Sim, H.Y.; Kim, H.S.; Jung, D.U.; Lee, H.; Han, Y.S.; Han, K.; Yun, K.I. Investigation of the association between orthodontic treatment and temporomandibular joint pain and dysfunction in the South Korean population. Korean J. Orthod. 2019, 49, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Farronato, M.; Bellincioni, F.; Cavagnetto, D.; Abate, A. Assessing mandibular body changes in growing subjects: A comparison of CBCT and reconstructed lateral cephalogram measurements. Sci. Rep. 2020, 10, 11722. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Cavagnetto, D.; Rusconi, F.M.E.; Cressoni, P.; Esposito, L. Safety and Effects of the Rapid Maxillary Expander on Temporomandibular Joint in Subjects Affected by Juvenile Idiopathic Arthritis: A Retrospective Study. Children 2021, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, G.B.; Crespi, R.; Capparè, P.; Gherlone, E. Transcrestal sinus floor elevation: A retrospective study of 46 patients up to 16 years. Clin. Implant. Dent. Relat. Res. 2012, 14, 759–767. [Google Scholar] [CrossRef]
- Hunter, W.S.; Baumrind, S.; Popovich, F.; Jorgensen, G. Forecasting the timing of peak mandibular growth in males by using skeletal age. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Mito, T.; Sato, K.; Mitani, H. Predicting mandibular growth potential with cervical vertebral bone age. Am. J. Orthod. Dentofac. Orthope. 2003, 124, 173–177. [Google Scholar] [CrossRef]
- Farronato, M.; Cavagnetto, D.; Abate, A.; Cressoni, P.; Fama, A.; Maspero, C. Assessment of condylar volume and ramus height in JIA patients with unilateral and bilateral TMJ involvement: Retrospective case-control study. Clin. Oral. Investig. 2020, 24, 2635–2643. [Google Scholar] [CrossRef]
- Abate, A.; Cavagnetto, D.; Fama, A.; Maspero, C.; Farronato, G. Relationship between Breastfeeding and Malocclusion: A Systematic Review of the Literature. Nutrients 2020, 12, 3688. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Y.; Xu, Y.; Xia, L.; Li, H.; Hua, X. Effect of twin inclined plane appliance on the mandible morphology in growing rats. Med. J. Wuhan Univ. 2019, 40, 323–327. [Google Scholar]
| Articles | Population | Intervention | Compared with | Outcome of Interest of Studies | Method of Assessment | Results |
|---|---|---|---|---|---|---|
| Asano, 1986 | 180 M, 4w-old Wistar rats | Orthopedic collar appliances for mandibular retractive force (8 h/d) | 20 rats in each group. | (1) 3D alterations on the growing mandible after retractive mandibular force | ||
| EG1:collar appliance with retractive force for 8w, | Radiographic data | Ø Volume and length of the mandibles: EG1 < CG1. | ||||
| EG2:10w, EG3:12w, EG4:16w | (2) mandibular growth after the orthopedic force was removed | Ø Height of anterior region and coronoid process, thickness of the retromolar corpus and condylar neck: EG1 > CG1. | ||||
| Ø Skull, condylar height and thickness of angular process: EG ≈ CG. | ||||||
| CG0:collar appliance without retractive force for 4w, | Ø Bone deposition lingually and buccally during force application: EG ≈ CG. | |||||
| CG1:8w, CG2:10w, CG3:12w, CG4:16w | Ø Bone deposition on the lingual surface EG > CG | |||||
| Ø Bone deposition on the buccal surface EG < CG. | ||||||
| Cholasueksa et al., 2004 | 39 M, 8w-old Wistar rats | Intermittent, functional posterior condylar displacement with modified guiding appliance attached to maxillary incisors | EG:24 rats, CG:15 rats, EG1:appliance for 4d, | Remodeling process of the TMJ | Lateral radiographs | |
| EG2:7d, | Ø Distal relationship of mandibular first molars compared to maxillary: EG > CG | |||||
| EG3:14d | Ø EG1,2,3: no incisal attrition of the mandibular incisors | |||||
| CG1:4d without appliance, CG2:7d, | ||||||
| CG3:14d | ||||||
| Desai et al., 1996 | 8, 9m old New Zealand white rabbits | Inclined planes on maxillary incisors. Functional continuous posterior mandibular displacement for 33 d | EG1:appliance for 2d, | TMJ morphological and spatial changes | Incisal relationships Radiographic data (lateral head X-rays) | Distalization of mandibular molars: |
| EG2:7d, | Ø EG1 > CG1 | |||||
| EG3:33d | Ø EG3 < EG1 | |||||
| CG1:2d, | ||||||
| CG2:7d, | ||||||
| CG3: 33d | ||||||
| Farias-Neto et al., 2012 | 20 F, 5w-old Wistar rats | Functional mandibular posterior displacement with occlusal guiding appliance attached to maxillary incisors | EG1:10 rats, appliance for 8w (Right side studied), EG2:the same 10 rats of EG1, appliance for 8w, (Left side studied) | Mandibular growth | Scan images with classic i-CAT and acrylic rapid prototyped templates of the mandibles | Ø Mandibular length: EG1,2 < CG, |
| CG: 10 rats without appliance for 8w, sham operation | EG1 ≈ EG2 | |||||
| Ø Ramus height and intercondylar distance between groups and sides: EG1,2 ≈ CG | ||||||
| Ø Altered mandibular bone morphology at grown age | ||||||
| Hua et al., 2012 | 8 M, 6w-old Wistar rats | Gradually induced backward movement of the mandible by a twin inclined plane device bonded to the posterior teeth | EG1:8 rats, device for 3d, EG2:8 rats, 14d, EG3:8 rats, 30d, EG4:8rats, 60d | Mandibular condyle remodeling | Radiographs and true-color video camera | Condylar remodeling |
| CG1:4 rats, 3d, no device, | Ø Length of condylar process, the dependent mandibular length and the condylar length: EG1,2 ≈ CG1,2; EG3 < CG3; EG4 < CG4 | |||||
| CG2:4 rats, 14d, CG3: 4 rats, 30d, CG4:4 rats, 60d | Ø Length of mandibular base: EG1,2,3,4 ≈ CG1,2,3,4 | |||||
| Ø Angle of the condylar process axis to the mandibular plane: EG1,2 ≈ CG1,2; EG3 > CG3; EG4 > CG4 | ||||||
| Ø Condylar width: EG1,2,3 ≈ CG1,2,3; EG4 < CG4 | ||||||
| Ø Flattening of the posterior condylar surface: EG3 > CG3; EG4 > CG4 | ||||||
| Ø Upwards shifting of the most posterior point of the condyle: EG4 > CG4 | ||||||
| Teramoto et al., 2003 | 24 M, 8w-old Wistar rats | Continuous compressive loading of the TMJ | EG1:7 rats appliance for 7d, EG2:5 rats for 1d, EG3:5 rats for 3d | Effects of compressive forces on extracellular matrix of mandibular condylar cartilage | Radiographic analysis (soft X-ray) | Ø EG1,2,3: the condyle remained under the articular eminence |
| CG: 7 rats, not treated | Ø CG: mandibular condyle moved anteriorly | |||||
| Wang et al., 2019 | 48 M 6w-old Wistar rats | Twin inclined plane device bonded to the posterior teeth to effect posterior mandibular movements | EG1:8 rats, appliance for 3d, EG2:8 rats, 14d, EG3:8 rats, 30d, EG4:8 rats, 60d | Posterior condylar area | Morphometric analysis by microcomputed tomography (micro-CT) | Flattening of the posterior region of the condyle |
| CG1: 4 rats, no appliance for 3d CG2: 4 rats, 14d, CG3:4 rats, 30d, CG4:4 rats, 60d | Ø CG1 ≈ CG2 ≈ CG3 ≈ CG4 ≈ EG1 ≈ EG2 | |||||
| Ø Lower part EG3 > EG1,2 | ||||||
| Ø Superior part: EG3 ≈ EG1,2 | ||||||
| Ø Entire posterior margin: EG4 > EG3 |
| Signaling Questions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Summary |
| Asano, 1986 | High | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Cholasueksaet al., 2004 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Desai et al., 1996 | High | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Farias-Neto et al., 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Hua et al., 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Teramotoet al., 2003 | High | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Wang et al.,2019 | High | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyros, I.; Makrygiannakis, M.A.; Lykogeorgos, T.; Ferdianakis, E.; Tsolakis, A.I. Posterior Mandibular Displacement—A Systematic Review Based on Animal Studies. Animals 2021, 11, 823. https://doi.org/10.3390/ani11030823
Lyros I, Makrygiannakis MA, Lykogeorgos T, Ferdianakis E, Tsolakis AI. Posterior Mandibular Displacement—A Systematic Review Based on Animal Studies. Animals. 2021; 11(3):823. https://doi.org/10.3390/ani11030823
Chicago/Turabian StyleLyros, Ioannis, Miltiadis A. Makrygiannakis, Theodoros Lykogeorgos, Efstratios Ferdianakis, and Apostolos I. Tsolakis. 2021. "Posterior Mandibular Displacement—A Systematic Review Based on Animal Studies" Animals 11, no. 3: 823. https://doi.org/10.3390/ani11030823
APA StyleLyros, I., Makrygiannakis, M. A., Lykogeorgos, T., Ferdianakis, E., & Tsolakis, A. I. (2021). Posterior Mandibular Displacement—A Systematic Review Based on Animal Studies. Animals, 11(3), 823. https://doi.org/10.3390/ani11030823








