Evaluation of Coated Biochar as an Intestinal Binding Agent for Skatole and Indole in Male Intact Finishing Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Diets and Feeding Concept
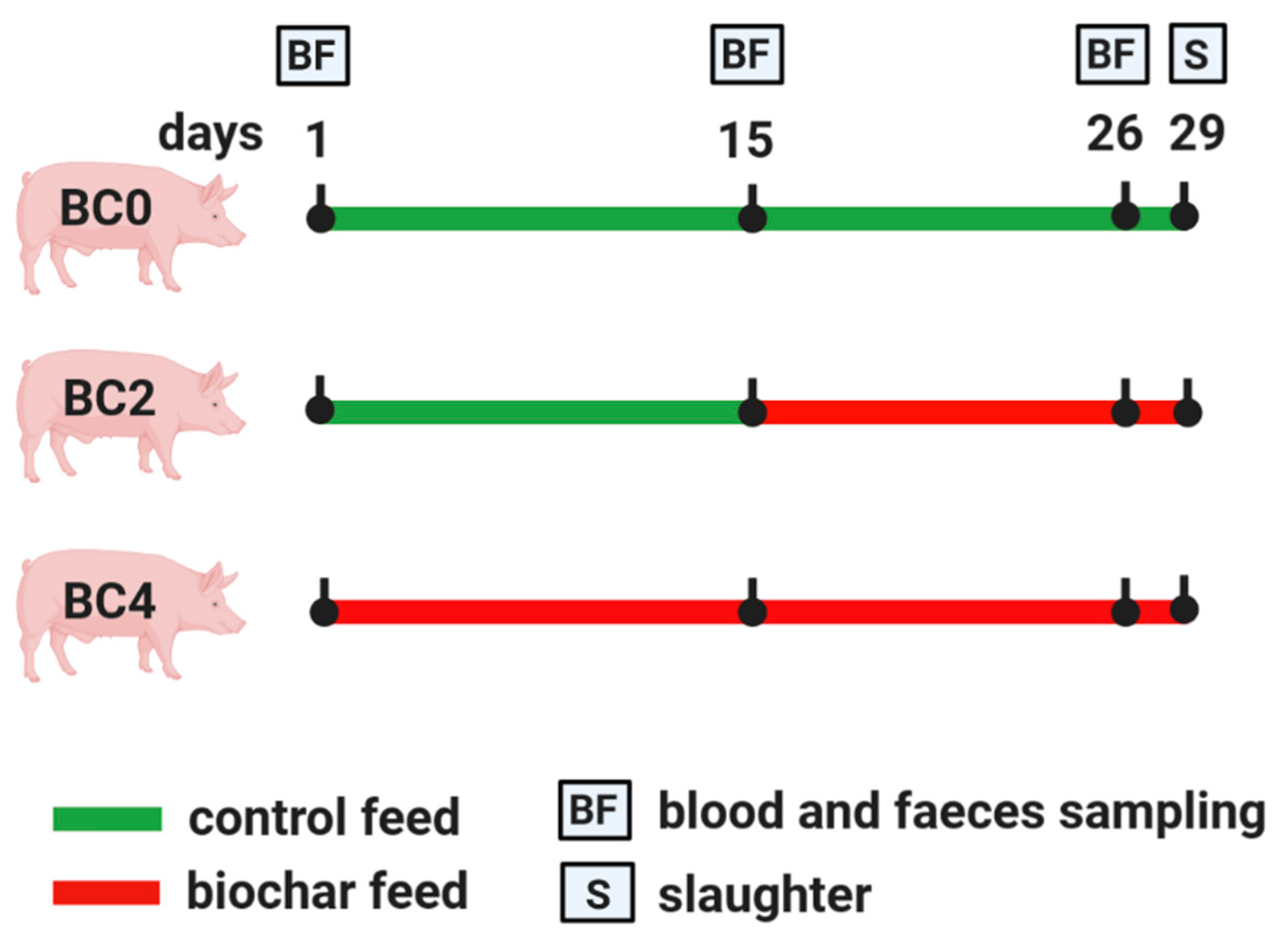
2.3. Experimental Design and Sampling
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
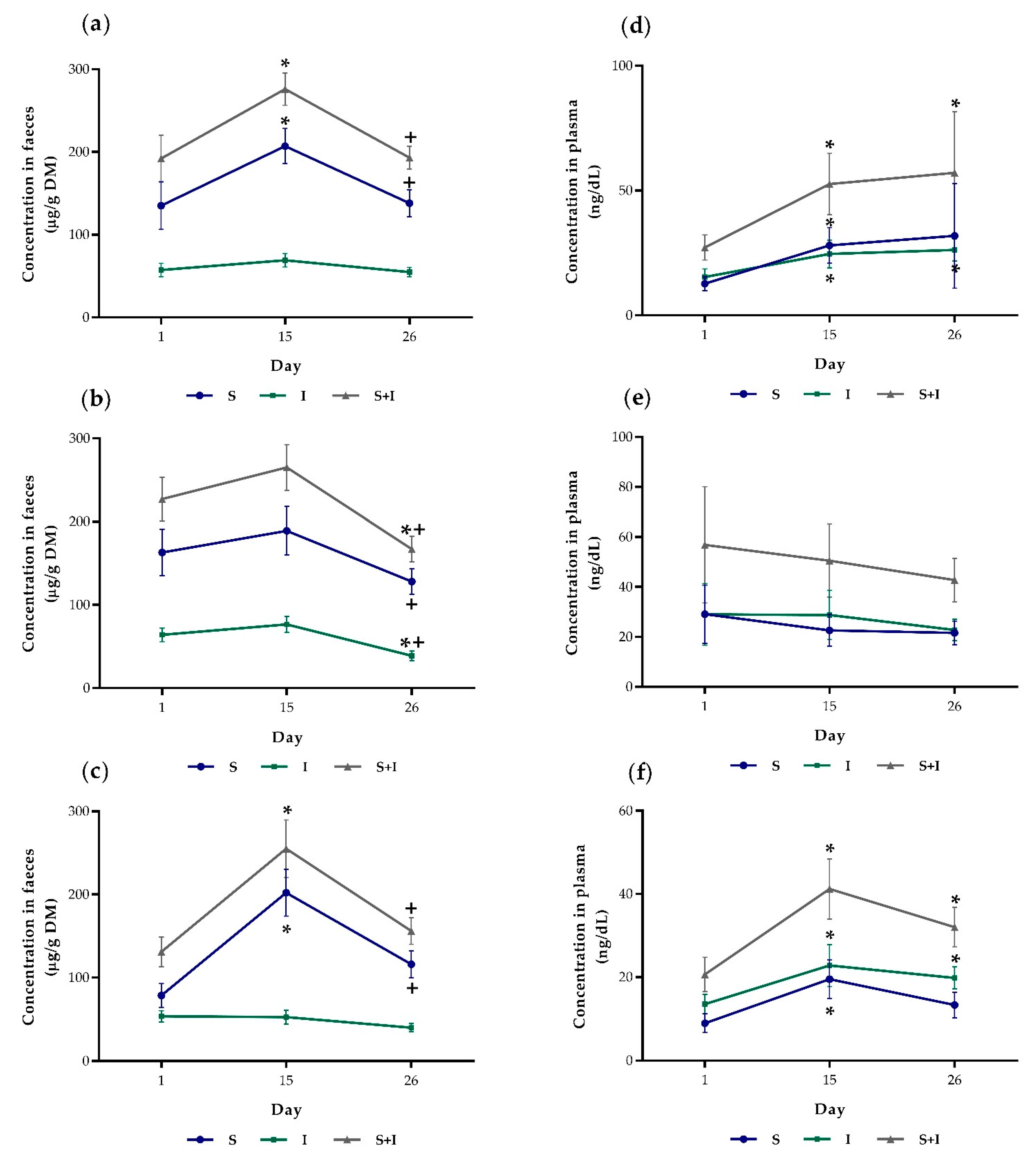
3.1. Skatole and Indole Concentrations in Faeces and Plasma
3.2. Dry Matter Content and pH-Values in Faeces
3.3. Performance Parameters
3.4. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viertes Gesetz zur Änderung des Tierschutzgesetzes Vom 17. December 2018. Available online: http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl118s2586.pdf (accessed on 23 September 2019).
- Lin-Schilstra, L.; Ingenbleek, P. Examining Alternatives to Painful Piglet Castration within the Contexts of Markets and Stakeholders: A Comparison of Four EU Countries. Animals 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Weiler, U.; Bonneau, M. Why it is so difficult to end surgical castration of boars in Europe: Pros and cons of alternatives to piglet castration. In Proceedings of the 60th International Meat Industry Conference MEATCON2019, Kopaonik, Serbia, 22–25 September 2019; p. 012001. [Google Scholar]
- Claus, R.; Weiler, U.; Herzog, A. Physiological aspects of androstenone and skatole formation in the boar—A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Vold, E. Fleischproduktionseigenschaften bei Ebern und Kastraten: III. Untersuchungen der Schlachtkörperzusammensetzung, sowie der Fleisch- und Speckqualität bei Ebern und Kastraten. Meld. Norges Landbr. Ogskole 1969, 49, 1–25. [Google Scholar]
- Patterson, R.L.S. 5α-androst-16-ene-3-one:—Compound responsible for taint in boar fat. J. Sci. Food Agric. 1968, 19, 31–38. [Google Scholar] [CrossRef]
- Bonneau, M.; Walstra, P.; Claudi-Magnussen, C.; Kempster, A.J.; Tornberg, E.; Fischer, K.; Diestre, A.; Siret, F.; Chevillon, P.; Claus, R.; et al. An international study on the importance of androstenone and skatole for boar taint: IV. Simulation studies on consumer dissatisfaction with entire male pork and the effect of sorting carcasses on the slaughter line, main conclusions and recommendations. Meat Sci. 2000, 54, 285–295. [Google Scholar] [CrossRef]
- Moss, B.W.; Haewe, S.M.; Walker, N. Sensory thresholds for skatole and indole. In Measurement and Prevention of Boar Taint in Entire Male Pigs; Bonneau, M., Ed.; INRA Edition: Paris, France, 1993; pp. 63–68. [Google Scholar]
- Garcia-Regueiro, J.; Diaz, I. Evaluation of the contribution of skatole, indole, androstenone and androstenols to boar-taint in back fat of pigs by HPLC and capillary gas chromatography (CGC). Meat Sci. 1989, 25, 307–316. [Google Scholar] [CrossRef]
- Weiler, U.; Fischer, K.; Kemmer, H.; Dobrowolski, A.; Claus, R. Influence of androstenone sensitivity on consumer reactions to boar meat. In Proceedings of the Boar Taint in Entire Male Pigs: Proceedings of a Meeting of the EAAP Working Group Production and Utilisation of Meat from Entire Male Pigs, Stockholm, Sweden, 1–3 October 1997. [Google Scholar]
- Weiler, U.; Furnols, M.F.; Fischer, K.; Kemmer, H.; Oliver, M.; Gispert, M.; Dobrowolski, A.; Claus, R. Influence of differences in sensitivity of Spanish and German consumers to perceive androstenone on the acceptance of boar meat differing in skatole and androstenone concentrations. Meat Sci. 2000, 54, 297–304. [Google Scholar] [CrossRef]
- Jensen, M.T.; Cox, R.P.; Jensen, B.B. 3-Methylindole (skatole) and indole production by mixed populations of pig fecal bacteria. Appl. Environ. Microbiol. 1995, 61, 3180–3184. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Carlson, J. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am. J. Clin. Nutr. 1979, 32, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Prusa, K.; Nederveld, H.; Runnels, P.; Li, R.; King, V.; Crane, J. Prevalence and relationships of sensory taint, 5α-androstenone and skatole in fat and lean tissue from the loin (Longissimus dorsi) of barrows, gilts, sows, and boars from selected abattoirs in the United States. Meat Sci. 2011, 88, 96–101. [Google Scholar] [CrossRef]
- Bonneau, M.; Weiler, U. Pros and cons of alternatives to piglet castration: Welfare, boar taint, and other meat quality traits. Animals 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, R.; Weiler, U. Nutritional Influences on Skatole Formation and Skatole Metabolism in the Pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Sander, S.J.; Osterhues, A.; Tabeling, R.; Kamphues, J. Geruchsabweichungen am Schlachtkörper bei der Ebermast—Einflüsse von Genetik, Fütterung und Haltung. Übers. Tierernährg. 2012, 40, 65–111. [Google Scholar]
- Squires, E.J.; Bone, C.; Cameron, J. Pork production with entire males: Directions for control of boar taint. Animals 2020, 10, 1665. [Google Scholar] [CrossRef]
- Claus, R.; Raab, S. Influences on skatole formation from tryptophan in the pig colon. In Tryptophan, Serotonin, and Melatonin; Huether, G., Kochen, W., Simat, T.J., Steinhart, H., Eds.; Springer: Boston, MA, USA, 1999; Volume 467, pp. 679–684. [Google Scholar]
- Raab, S.; Leiser, R.; Kemmer, H.; Claus, R. Effects of energy and purines in the diet on proliferation, differentiation, and apoptosis in the small intestine of the pig. Metabolism 1998, 47, 1105–1111. [Google Scholar] [CrossRef]
- Leong, J.; Morel, P.C.; Purchas, R.W.; Wilkinson, B.H. Effects of dietary components including garlic on concentrations of skatole and indole in subcutaneous fat of female pigs. Meat Sci. 2011, 88, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Losel, D.; Lacorn, M.; Mentschel, J.; Schenkel, H. Effects of butyrate on apoptosis in the pig colon and its consequences for skatole formation and tissue accumulation. J. Animal Sci. 2003, 81, 239–248. [Google Scholar] [CrossRef]
- Barragán, H.B. Physiologische und Nutritive Einflüsse auf die Bildung von Skatol (3-Methylindol) im Dickdarm von Schweinen; Universität Hohenheim: Stuttgart, Germany, 1992. [Google Scholar]
- Hansen, L.; Mejer, H.; Thamsborg, S.; Byrne, D.; Roepstorff, A.; Karlsson, A.; Hansen-Møller, J.; Jensen, M.; Tuomola, M. Influence of chicory roots (Cichorium intybus L) on boar taint in entire male and female pigs. Animal Sci. 2006, 82, 359–368. [Google Scholar] [CrossRef]
- Zammerini, D.; Wood, J.; Whittington, F.; Nute, G.; Hughes, S.; Hazzledine, M.; Matthews, K. Effect of dietary chicory on boar taint. Meat Sci. 2012, 91, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Aluwé, M.; Heyrman, E.; Theis, S.; Sieland, C.; Thurman, K.; Millet, S. Chicory fructans in pig diet reduce skatole in back fat of entire male pigs. Res. Vet. Sci. 2017, 115, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Whittington, F.; Nute, G.; Hughes, S.; McGivan, J.; Lean, I.; Wood, J.; Doran, E. Relationships between skatole and androstenone accumulation, and cytochrome P4502E1 expression in Meishan × Large White pigs. Meat Sci. 2004, 67, 569–576. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Zamaratskaia, G.; Ekstrand, B. In vivo effect of dried chicory root (Cichorium intybus L.) on xenobiotica metabolising cytochrome P450 enzymes in porcine liver. Toxicol. Lett. 2011, 200, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Baltic, M.; Raicevic, S.; Tadic, I.; Drljacic, A. Influence of zeolite on skatole content of swine fat tissue. In Boar Taint in Entire Male Pigs, Proceedings of a Meeting of the EAAP Working Group Production and Utilisation of Meat from Entire Male Pigs, Stockholm, Sweden, 1–3 October 1997; Wageningen Pers: Wageningen, The Netherlands, 1997. [Google Scholar]
- Aluwé, M.; Millet, S.; Nijs, G.; Tuyttens, F.; Verheyden, K.; de Brabander, H.; de Brabander, D.; van Oeckel, M. Absence of an effect of dietary fibre or clinoptilolite on boar taint in entire male pigs fed practical diets. Meat Sci. 2009, 82, 346–352. [Google Scholar] [CrossRef]
- Jen, K.; Squires, E. Efficacy of non-nutritive sorbent materials as intestinal-binding agents for the control of boar taint. Animal 2011, 5, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Jen, K.; Squires, E. In vitro assessment of the effectiveness of non-nutritive sorbent materials as binding agents for boar taint compounds. Animal 2011, 5, 1821. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 117–138. [Google Scholar]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Hagemann, N.; Draper, K.; Kammann, C. The use of biochar in animal feeding. PeerJ 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- BHZP. db.77(R) Ebermast-Fütterungsempfehlungen. Available online: https://www.bhzp.de/fileadmin/user_upload/Aktuelles/2016/folder-updates/5007_BHZP_2016%20BHZPlakativ-Ebermast%20Update2016-LowRes.pdf (accessed on 12 January 2020).
- Lanthier, F.; Lou, Y.; Terner, M.; Squires, E. Characterizing developmental changes in plasma and tissue skatole concentrations in the prepubescent intact male pig. J. Animal Sci. 2006, 84, 1699–1708. [Google Scholar] [CrossRef]
- Mörlein, D.; Grave, A.; Sharifi, A.R.; Bücking, M.; Wicke, M. Different scalding techniques do not affect boar taint. Meat Sci. 2012, 91, 435–440. [Google Scholar] [CrossRef]
- Naumann, C.; Bassler, R. Methoden der Landwirtschaftlichen Forschungs-und Untersuchungsanstalt, Biochemische Untersuchung von Futtermitteln. Methodenbuch III (Einschließlich der Achten Ergänzungen); VDLUFA: Darmstadt, Germany, 2012. [Google Scholar]
- Claus, R.; Dehnhard, M.; Herzog, A.; Bernal-Barragan, H.; Giménez, T. Parallel measurements of indole and skatole (3-methylindole) in feces and blood plasma of pigs by HPLC. Livest. Prod. Sci. 1993, 34, 115–126. [Google Scholar] [CrossRef]
- Dehnhard, M.; Bernal-Barragan, H.; Claus, R. Rapid and accurate high-performance liquid chromatographic method for the determination of 3-methylindole (skatole) in faeces of various species. J. Chromatogr. B Biomed. Sci. Appl. 1991, 566, 101–107. [Google Scholar] [CrossRef]
- Gibis, M.; Dehnhard, M.; Fischer, A. Bestimmung von Skatol und Indol in Rückenspeck und Muskelfleisch von Schweinen durch Hochleistungs-Flüssigchromatographie (HPLC) mit fluorimetrischer Detektion. Z. Lebensm. Unters. Forsch. 1991, 193, 220–223. [Google Scholar] [CrossRef]
- American Veterinary Medicine Association, (A.V.M.A). Literature Review on the Welfare Implications of Swine Castration. 2018. Available online: https://www.avma.org/resources-tools/literature-reviews/welfareimplications-swine-castration (accessed on 8 June 2020).
- Rasmussen, M.K.; Brunius, C.; Zamaratskaia, G.; Ekstrand, B. Feeding dried chicory root to pigs decrease androstenone accumulation in fat by increasing hepatic 3β hydroxysteroid dehydrogenase expression. J. Steroid Biochem. Mol. Biol. 2012, 130, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.L.; Stolzenbach, S.; Jensen, J.A.; Henckel, P.; Hansen-Møller, J.; Syriopoulos, K.; Byrne, D.V. Effect of feeding fermentable fibre-rich feedstuffs on meat quality with emphasis on chemical and sensory boar taint in entire male and female pigs. Meat Sci. 2008, 80, 1165–1173. [Google Scholar] [CrossRef]
- Babol, J.; Zamaratskaia, G.; Juneja, R.; Lundström, K. The effect of age on distribution of skatole and indole levels in entire male pigs in four breeds: Yorkshire, Landrace, Hampshire and Duroc. Meat Sci. 2004, 67, 351–358. [Google Scholar] [CrossRef]
- Kress, K.; Weiler, U.; Schmucker, S.; Čandek-Potokar, M.; Vrecl, M.; Fazarinc, G.; Škrlep, M.; Batorek-Lukač, N.; Stefanski, V. Influence of Housing Conditions on Reliability of Immunocastration and Consequences for Growth Performance of Male Pigs. Animals 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Meiirkhanuly, Z.; Koziel, J.A.; Bialowiec, A.; Banik, C.; Brown, R.C. The proof-of-the concept of biochar floating cover influence on swine manure pH: Implications for mitigation of gaseous emissions from area sources. Front. Chem. 2020, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Knarreborg, A.; Beck, J.; Jensen, M.; Laue, A.; Agergaard, N.; Jensen, B.B. Effect of non-starch polysaccharides on production and absorption of indolic compounds in entire male pigs. Animal Sci. 2002, 74, 445–453. [Google Scholar] [CrossRef]
- Škrlep, M.; Batorek, N.; Bonneau, M.; Fazarinc, G.; Šegula, B.; Čandek-Potokar, M. Elevated fat skatole levels in immunocastrated, surgically castrated and entire male pigs with acute dysentery. Vet. J. 2012, 194, 417–419. [Google Scholar] [CrossRef]
- Schubert, D.C.; Chuppava, B.; Witte, F.; Terjung, N.; Visscher, C. Effect of two different biochars as a component of compound feed on nutrient digestibility and performance parameters in growing pigs. Front. Animal Sci. 2021, 2, 2. [Google Scholar] [CrossRef]
| Feedstuffs | Variant 1 | Variant 2 |
|---|---|---|
| Barley | 21.0 | 21.0 |
| Wheat | 15.0 | 20.0 |
| Triticale | 14.0 | 16.0 |
| Rye | 13.0 | 15.0 |
| Wheat bran | 13.0 | 8.0 |
| Soybean meal * | 5.0 | 6.0 |
| Maize | 5.0 | 5.0 |
| Oat hulling bran | 5.0 | – |
| Wheat semolina bran | 2.0 | 2.0 |
| Maize gluten feed | 2.0 | 2.0 |
| Rapeseed meal | 2.0 | 2.0 |
| Premix ** | 3.0 | 3.0 |
| Item | CON 1 | BCF 2 | |
|---|---|---|---|
| Metabolizable energy (ME) 3 | MJ per kg diet | 13.0 | 13.1 |
| Organic matter | g/kg DM 4 | 951 | 952 |
| Crude protein | g/kg DM | 157 | 160 |
| Ether extract | g/kg DM | 56.9 | 52.1 |
| Crude fibre | g/kg DM | 56.6 | 52.0 |
| Nitrogen-free extract (NfE) 5 | g/kg DM | 681 | 688 |
| Calcium | g/kg DM | 5.95 | 6.48 |
| Phosphorus | g/kg DM | 4.87 | 4.70 |
| Magnesium | g/kg DM | 2.00 | 1.94 |
| Sodium | g/kg DM | 2.36 | 2.21 |
| Zinc | mg/kg DM | 130 | 133 |
| Iron | mg/kg DM | 315 | 344 |
| Selenium | mg/kg DM | 0.676 | 0.835 |
| Copper | mg/kg DM | 27.9 | 29.8 |
| Faecal Properties | Feed Affiliation | p | |||
|---|---|---|---|---|---|
| CON 2 | n | BCF 3 | n | ||
| DMf day 1 (g/kg) | 274 ± 24.9 | 52 | -- | 0 | -- |
| DMf day 8 (g/kg) | 279 ± 24.1 | 35 | 287 ± 29.9 | 18 | 0.295 |
| DMf day 15 (g/kg) | 283 b ± 23.4 | 36 | 301 a ± 29.2 | 18 | 0.015 |
| DMf day 22 (g/kg) | 293 ± 23.6 | 18 | 296 ± 24.7 | 35 | 0.711 |
| DMf day 26 (g/kg) | 286 b ± 27.4 | 18 | 305 a ± 31.7 | 36 | 0.034 |
| pHf day 1 | 6.77 ± 0.361 | 53 | -- | 0 | -- |
| pHf day 8 | 6.77 ± 0.271 | 36 | 6.80 ± 0.395 | 18 | 0.719 |
| pHf day 15 | 6.83 ± 0.304 | 35 | 6.76 ± 0.268 | 18 | 0.463 |
| pHf day 22 | 6.78 ± 0.257 | 18 | 6.82 ± 0.359 | 36 | 0.680 |
| pHf day 26 | 6.98 ± 0.255 | 18 | 7.03 ± 0.338 | 35 | 0.614 |
| Performance Parameter | Treatment | p | Trial | p | ||||
|---|---|---|---|---|---|---|---|---|
| BC0 | BC2 | BC4 | T1 | T2 | T3 | |||
| BW day 1 (kg) | 97.3 ± 5.56 | 96.7 ± 7.47 | 97.7 ± 7.76 | 0.927 | 97.5 ± 8.58 | 98.6 ± 6.57 | 95.6 ± 5.08 | 0.471 |
| BW day 28 (kg) | 119 ± 5.10 | 119 ± 7.01 | 120 ± 6.69 | 0.767 | 122 a ± 7.37 | 120 ab ± 6.05 | 117 b ± 4.10 | 0.063 |
| ADWG (g/d) | 806 ± 159 | 837 ± 126 | 865 ± 102 2 | 0.262 | 925 a ± 79.0 2 | 798 b ± 128 | 788 b ± 136 | 0.001 |
| G:F (kg/kg) | 0.336 ± 0.067 | 0.360 ± 0.047 | 0.362 ± 0.041 2 | 0.134 | 0.387 a ± 0.034 2 | 0.332 b ± 0.049 | 0.334 b ± 0.058 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, D.C.; Chuppava, B.; Witte, F.; Terjung, N.; Visscher, C. Evaluation of Coated Biochar as an Intestinal Binding Agent for Skatole and Indole in Male Intact Finishing Pigs. Animals 2021, 11, 760. https://doi.org/10.3390/ani11030760
Schubert DC, Chuppava B, Witte F, Terjung N, Visscher C. Evaluation of Coated Biochar as an Intestinal Binding Agent for Skatole and Indole in Male Intact Finishing Pigs. Animals. 2021; 11(3):760. https://doi.org/10.3390/ani11030760
Chicago/Turabian StyleSchubert, Dana Carina, Bussarakam Chuppava, Franziska Witte, Nino Terjung, and Christian Visscher. 2021. "Evaluation of Coated Biochar as an Intestinal Binding Agent for Skatole and Indole in Male Intact Finishing Pigs" Animals 11, no. 3: 760. https://doi.org/10.3390/ani11030760
APA StyleSchubert, D. C., Chuppava, B., Witte, F., Terjung, N., & Visscher, C. (2021). Evaluation of Coated Biochar as an Intestinal Binding Agent for Skatole and Indole in Male Intact Finishing Pigs. Animals, 11(3), 760. https://doi.org/10.3390/ani11030760






