Changes in Rumen Microbiota Affect Metabolites, Immune Responses and Antioxidant Enzyme Activities of Sheep under Cold Stimulation
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Experimental Animals and Design
2.3. Feeding and Management
2.4. Sampling
2.5. Determination of Inflammatory Factors and Antioxidant Enzymes
2.6. Total DNA Extraction from Rumen Fluid, 16S rRNA Gene Sequencing, Data Processing and Functional Predictions
2.7. Determination of Volatile Fatty Acids (VFA)
2.8. Statistical Analysis
3. Results
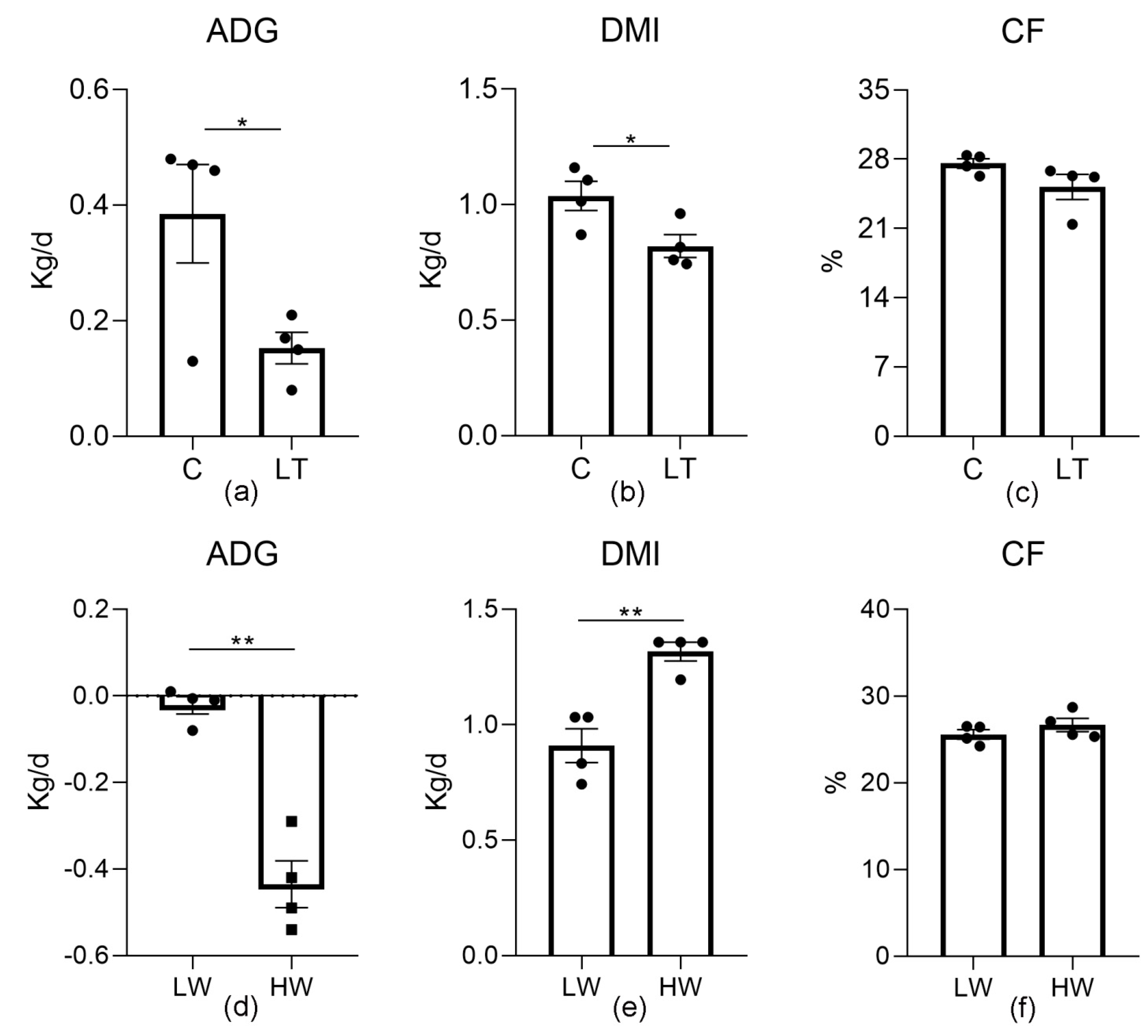
3.1. Growth Performance
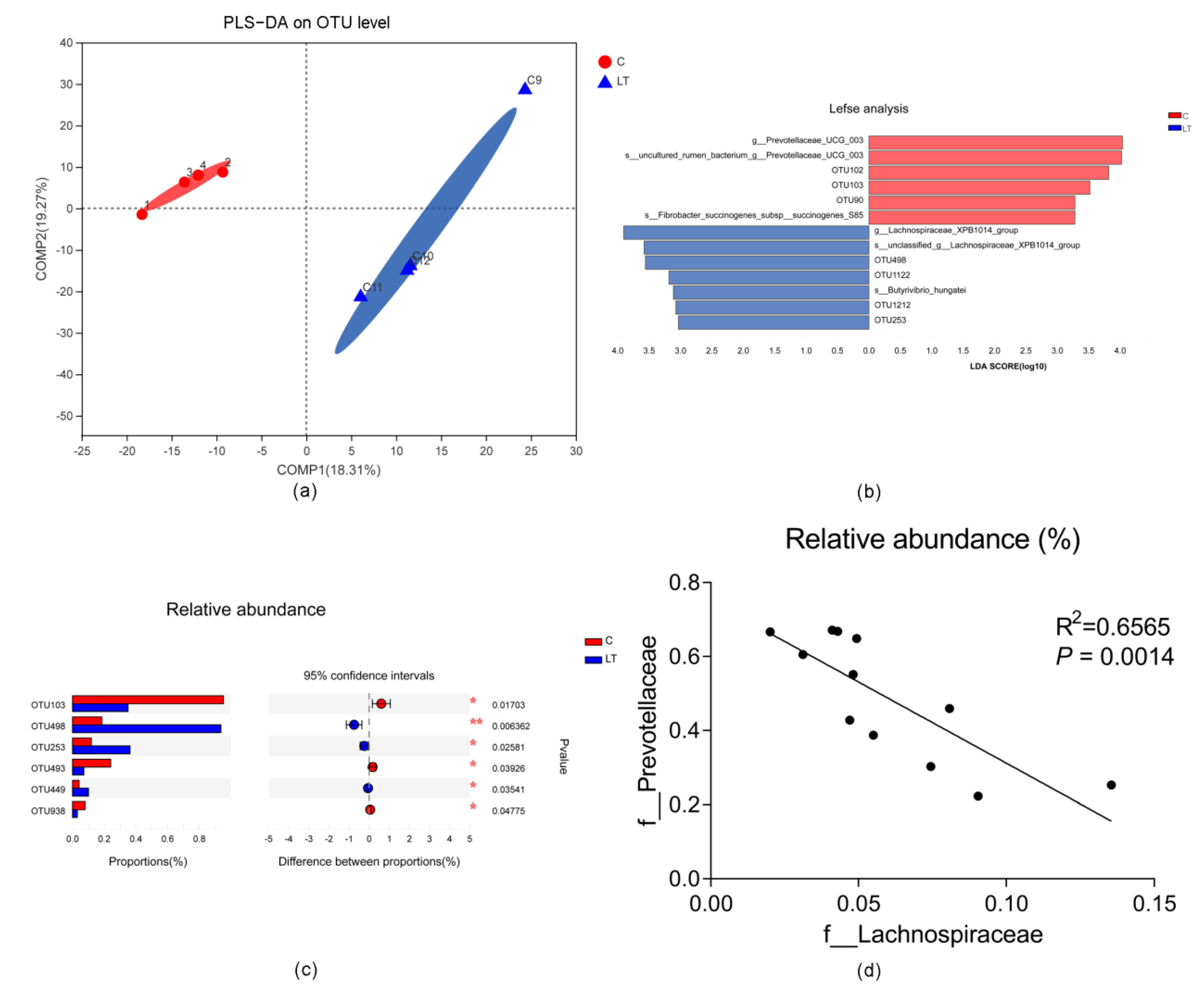
3.2. Rumen Microbiota Changes under Cold Temperatures
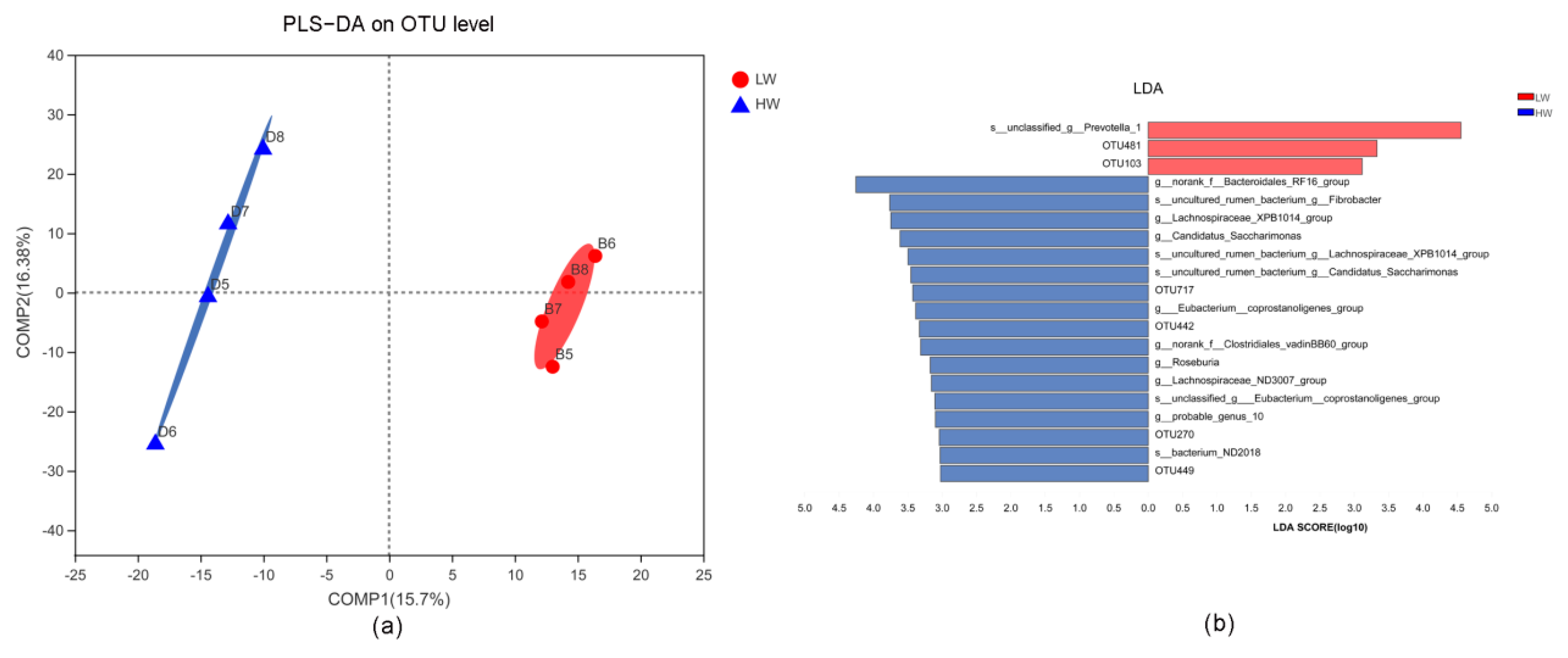
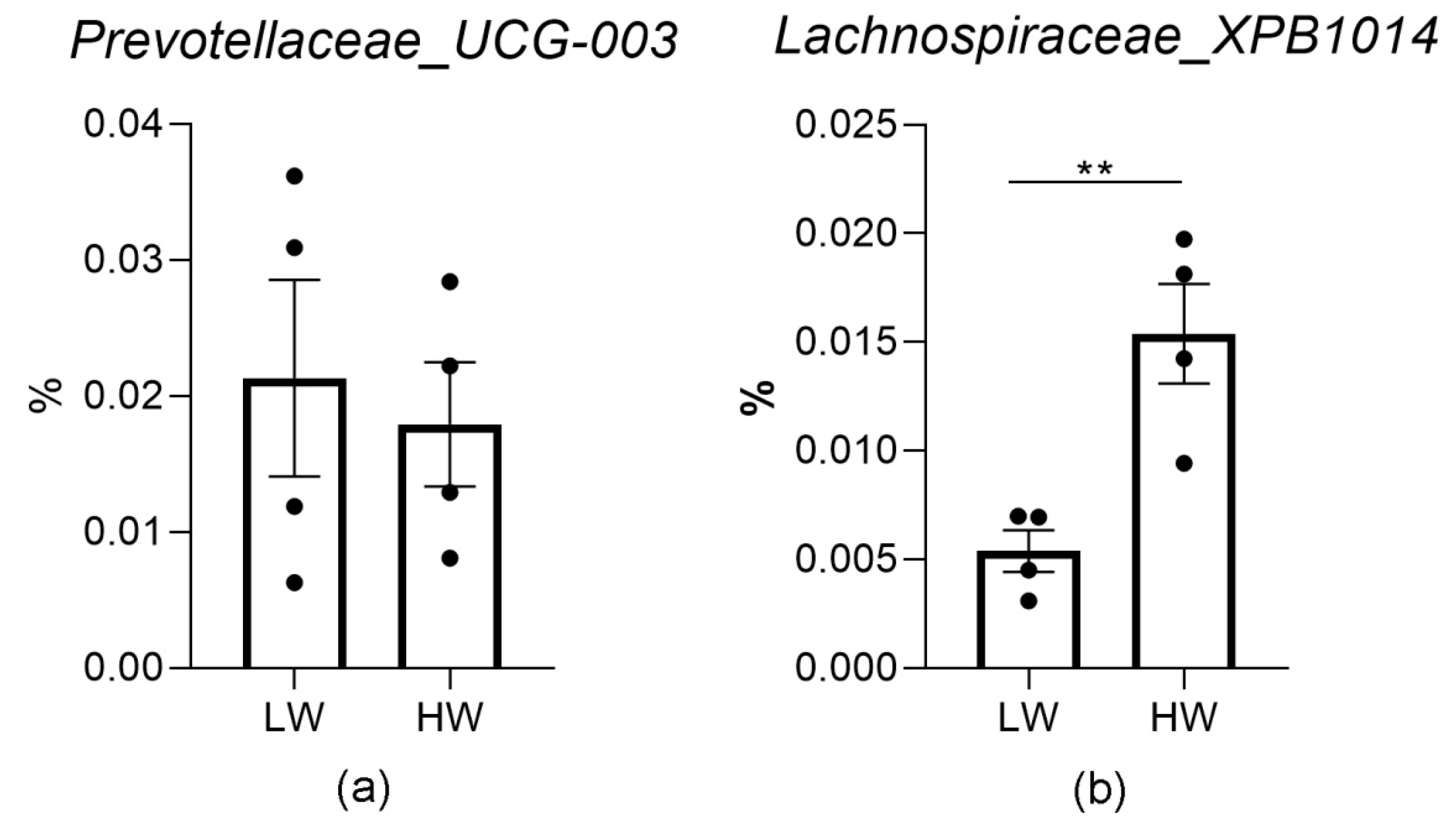
3.3. Rumen Microbiota Altered with the Increase in Wind Treatment under Cold Temperatures
3.4. Functional Differences in the Bacteria in a Cold Environment
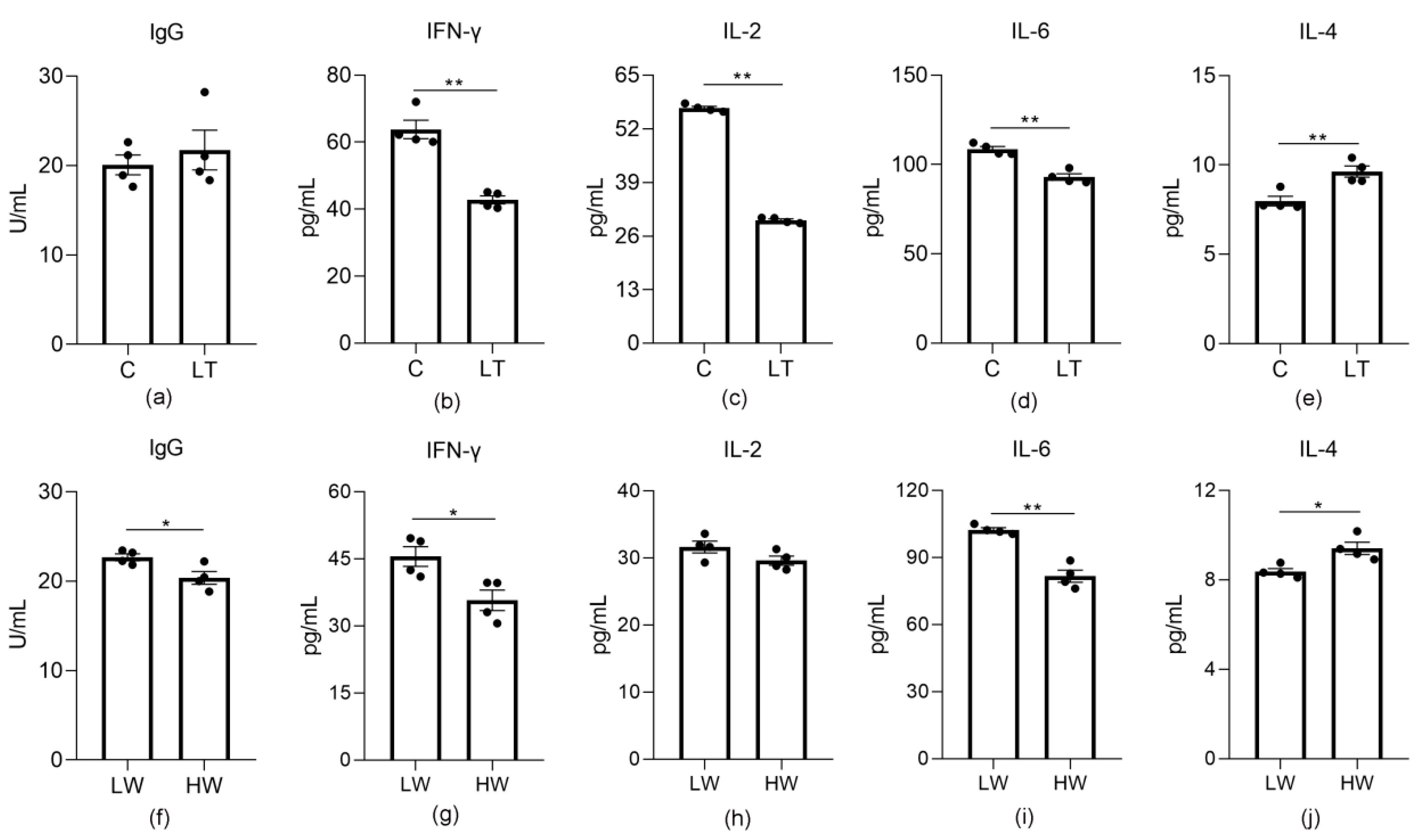
3.5. Changes in Inflammatory Factors and Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crater, A.R.; Barboza, P.S. The rumen in winter: Cold shocks in naturally feeding muskoxen (Ovibos moschatus). J. Mammal. 2007, 88, 625–631. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, P.; Araujo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Fan, P.X.; Bian, B.L.; Teng, L.; Nelson, C.D.; Driver, J.; Elzo, M.A.; Jeong, K.C. Host genetic effects upon the early gut microbiota in a bovine model with graduated spectrum of genetic variation. ISME J. 2020, 14, 302–317. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.L.; Yuan, J.H.; Li, J.; Jiang, D.D.; Zhang, J.J.; Li, H.; Wang, R.Y.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Audebert, M.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. High-protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clin. Nutr. 2019, 38, 1012–1022. [Google Scholar] [CrossRef]
- Han, X.; Li, B.; Wang, X.; Chen, Y.; Yang, Y. Effect of dietary concentrate to forage ratios on ruminal bacterial and anaerobic fungal populations of cashmere goats. Anaerobe 2019, 59, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Dadi, T.H.; Pieper, L.; Vahjen, W.; Franke, A.; Reinert, K.; Zentek, J. Concentration and chemical form of dietary zinc shape the porcine colon microbiome, its functional capacity and antibiotic resistance gene repertoire. ISME J. 2020, 14, 2783–2793. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, L.; Hu, F.; Zhu, W.; Mao, S. Disruption of ruminal homeostasis by malnutrition involved in systemic ruminal microbiota-host interactions in a pregnant sheep model. Microbiome 2020, 8, 138–152. [Google Scholar] [CrossRef]
- Zhong, S.; Ding, Y.; Wang, Y.Y.; Zhou, G.C.; Guo, H.R.; Chen, Y.L.; Yang, Y.X. Temperature and humidity index (THI)-induced rumen bacterial community changes in goats. Appl. Microbiol. Biot. 2019, 103, 3193–3203. [Google Scholar] [CrossRef]
- Bo, T.B.; Zhang, X.Y.; Wen, J.; Deng, K.; Qin, X.W.; Wang, D.H. The microbiota-gut-brain interaction in regulating host metabolic adaptation to cold in male Brandt’s voles (Lasiopodomys brandtii). ISME J. 2019, 13, 3037–3053. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simao, A.N.C.; de Souza, A.H.B.; Alfieri, D.F.; Segura, L.G.; Costa, G.N.; Dichi, I. Effect of Bifidobacterium lactis HN019 on inflammatory markers and oxidative stress in subjects with and without the metabolic syndrome. Brit. J. Nutr. 2018, 120, 645–652. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal. Immunol. 2018, 11, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; McLaren, J.S.; Himanka, M.J.; Speakman, J.R. Effect of long-term cold exposure on antioxidant enzyme activities in a small mammal. Free Radical. Bio. Med. 2000, 28, 1279–1285. [Google Scholar] [CrossRef]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Singh, M.; Hossain, S.A. Improved milk production through PG-PL system by provision of in-house shelter management in lactating Murrah buffaloes during winter season. J. Anim. Physiol. Anim. Nutr. 2018, 102, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Yu, J.; Zhang, Y.; Hu, S.; Ouyang, R.; Liu, W. Temporal variation of wind speed in China for 1961–2007. Theor. Appl. Climatol. 2010, 104, 313–324. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, G.C.; Tian, G.; Liu, Y.L.; Dong, N.; Zhang, S.; Chai, H.; Chen, Y.L.; Yang, Y.X. Effects of low temperature and wind treatment on physiological indexes, rumen microbiota, immune responses and hormones in sheep. Preprint 2020. Available online: https://europepmc.org/article/ppr/ppr179910 (accessed on 4 March 2021). [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Liang, Z.C.; Li, W.X.; He, Z.G.; Lin, X.Z.; Ren, X.Y.; Lin, X.J. Changes in Cold and Hot Syndrome and Gastrointestinal Bacterial Community Structure in Mice by Intervention with Food of Different Nature. Chin. J. Integr. Med. 2020, 26, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Sukhchuluun, G.; Bo, T.B.; Chi, Q.S.; Yang, J.J.; Chen, B.; Zhang, L.; Wang, D.H. Huddling remodels gut microbiota to reduce energy requirements in a small mammal species during cold exposure. Microbiome 2018, 6, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Froehlich, K.; Casper, D.P.; St-Pierre, B. Feeding Essential Oils to Neonatal Holstein Dairy Calves Results in Increased Ruminal Prevotellaceae Abundance and Propionate Concentrations. Microorganisms 2019, 7, 120. [Google Scholar] [CrossRef]
- Liang, X.; Jin, J.; Bi, X.; Kamruzzaman, M.; Kudo, T.; Sano, H. Effects of Chinese herbal medicine and cold exposure on plasma glucose, leucine and energy metabolism in sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, e534–e541. [Google Scholar] [CrossRef]
- Song, S.; Wu, J.; Zhao, S.; Casper, D.P.; Zhang, L.; He, B.; Lang, X.; Wang, C.; Gong, X.; Wang, F.; et al. The effect of periodic energy restriction on growth performance, serum biochemical indices, and meat quality in sheep. J. Anim. Sci. 2018, 96, 4251–4263. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Poeker, S.A.; Geirnaert, A.; Berchtold, L.; Greppi, A.; Krych, L.; Steinert, R.E.; de Wouters, T.; Lacroix, C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS). Sci. Rep. UK 2018, 8, 4318–4330. [Google Scholar] [CrossRef]
- Herrmann, E.; Young, W.; Rosendale, D.; Conrad, R.; Riedel, C.U.; Egert, M. Determination of Resistant Starch Assimilating Bacteria in Fecal Samples of Mice by In vitro RNA-Based Stable Isotope Probing. Front. Microbiol. 2017, 8, 1331–1344. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal. Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Lavoy, E.C.P.; McFarlin, B.K.; Simpson, R.J. Immune Responses to Exercising in a Cold Environment. Wild. Environ. Med. 2011, 22, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.A.; Rifkin, I.R.; Hohlbaum, A.M.; Beaudette, B.C.; Shlomchik, M.J.; Marshak-Rothstein, A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002, 416, 603–607. [Google Scholar] [CrossRef]
- Cripps, A.W.; Lascelles, A.K. The biological ’half-lives’ of IgG1 and IgG2 in young milk-fed lambs and in non-pregnant colostrum-forming sheep. Aust. J. Exp. Biol. Med. Sci. 1974, 52, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.P.; Papasian, C.J.; Ritter, R.C. The effects of cold stress on neonatal calves. II. Absorption of colostral immunoglobulins. Can. J. Comp. Med. 1980, 44, 19–23. [Google Scholar] [PubMed]
- Belay, T. Cold-induced stress impairs the production of Th1 protective cytokines during co-culturing of dendritic cells and naive T helper cells of Chlamydia muridarum infected mice. J. Immunol. 2018, 200, 117–135. [Google Scholar]
- Makarova, O.V.; Trunova, G.V.; Diatroptov, M.E.; Serebryakov, S.N.; Kondashevskaya, M.V.; Malaitsev, V.V. Comparative characterization of cytokine production by concanavalin A-activated splenocytes from BALB/c and C57BL/6 mice after cold exposure. Bull. Exp. Biol. Med. 2005, 139, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Redox signaling mediated by the gut microbiota. Free Radical. Res. 2013, 47, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Huang, F.F.; Li, X.; Su, Y.Y.; Li, H.T.; Bao, J. Effects of intermittent cold stimulation on antioxidant capacity and mRNA expression in broilers. Livest. Sci. 2017, 204, 110–114. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, K.J.; Suzuki, K.; Lee, I.S. Effect of cold acclimation on antioxidant status in cold acclimated skaters. J. Physiol. Anthropol. 2008, 27, 255–262. [Google Scholar] [CrossRef]
| VFAs | C | LT | SEM | p Value | LW | HW | SEM | p Value |
|---|---|---|---|---|---|---|---|---|
| Acetate (%) | 61.90 | 65.08 | 1.130 | 0.176 | 64.50 | 63.49 | 0.610 | 0.454 |
| Propionate (%) | 24.85 | 23.82 | 1.026 | 0.655 | 24.29 | 20.46 | 0.842 | 0.006 |
| Butyrate (%) | 10.47 | 9.026 | 0.600 | 0.259 | 8.504 | 8.745 | 0.382 | 0.778 |
| Acetate (mM) | 52.40 | 55.52 | 4.734 | 0.769 | 48.44 | 25.50 | 5.440 | 0.018 |
| Propionate (mM) | 21.41 | 21.09 | 2.623 | 0.956 | 18.28 | 8.123 | 2.254 | 0.007 |
| Butyrate (mM) | 8.797 | 8.209 | 1.106 | 0.813 | 6.506 | 3.474 | 0.843 | 0.064 |
| Pathway Level1 | Pathway Level2 | Pathway Level3 | Description | C | LT | SEM | p Value | LW | HW | SEM | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental Information Processing | 12.44 | 12.56 | 0.358 | 0.885 | 11.95 | 13.05 | 0.280 | 0.034 | |||
| Environmental Information Processing | Signal transduction | 8.444 | 8.462 | 0.264 | 0.976 | 11.89 | 13.11 | 0.270 | 0.007 | ||
| Environmental Information Processing | Signal transduction | ko02020 | Two-component system | 12.33 | 12.67 | 0.430 | 0.718 | 11.77 | 13.23 | 0.325 | 0.009 |
| Environmental Information Processing | Signal transduction | Ko04066 | HIF-1 signaling pathway | 12.82 | 12.18 | 0.478 | 0.547 | 11.87 | 13.13 | 0.281 | 0.009 |
| Environmental Information Processing | Signal transduction | Ko04152 | AMPK signaling pathway | 12.32 | 12.68 | 0.563 | 0.774 | 11.52 | 13.48 | 0.408 | 0.002 |
| Environmental Information Processing | Signal transduction | Ko04016 | MAPK signaling pathway | 13.74 | 11.26 | 1.318 | 0.389 | 11.09 | 13.91 | 0.692 | 0.026 |
| Cellular Processes | Cell motility | 8.043 | 8.673 | 0.746 | 0.706 | 10.81 | 14.19 | 0.868 | 0.038 | ||
| Cellular Processes | Cell motility | Ko02040 | Flagellar assembly | 12.09 | 12.91 | 1.294 | 0.779 | 10.74 | 14.26 | 0.975 | 0.061 |
| Cellular Processes | Cell motility | Ko02030 | Bacterial chemotaxis | 11.96 | 13.04 | 0.924 | 0.597 | 10.91 | 14.09 | 0.763 | 0.020 |
| C | LT | SEM | p Value | LW | HW | SEM | p Value | |
|---|---|---|---|---|---|---|---|---|
| MDA (nmol/mL) | 2.189 | 1.478 | 0.141 | 0.000 | 1.676 | 1.376 | 0.112 | 0.200 |
| T-AOC (U/mL) | 8.043 | 10.74 | 0.540 | 0.000 | 8.395 | 9.641 | 0.343 | 0.060 |
| SOD (U/mL) | 65.38 | 82.52 | 3.321 | 0.000 | 82.73 | 88.96 | 1.952 | 0.114 |
| GSH-PX (U/mL) | 258.2 | 378.4 | 24.55 | 0.001 | 319.3 | 349.1 | 14.93 | 0.356 |
| CAT (U/mL) | 17.24 | 25.56 | 1.607 | 0.000 | 20.87 | 21.22 | 0.837 | 0.854 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Zhou, G.; Tian, G.; Liu, Y.; Dong, N.; Li, L.; Zhang, S.; Chai, H.; Chen, Y.; Yang, Y. Changes in Rumen Microbiota Affect Metabolites, Immune Responses and Antioxidant Enzyme Activities of Sheep under Cold Stimulation. Animals 2021, 11, 712. https://doi.org/10.3390/ani11030712
Guo H, Zhou G, Tian G, Liu Y, Dong N, Li L, Zhang S, Chai H, Chen Y, Yang Y. Changes in Rumen Microbiota Affect Metabolites, Immune Responses and Antioxidant Enzyme Activities of Sheep under Cold Stimulation. Animals. 2021; 11(3):712. https://doi.org/10.3390/ani11030712
Chicago/Turabian StyleGuo, Hongran, Guangchen Zhou, Guangjie Tian, Yuyang Liu, Ning Dong, Linfang Li, Shijun Zhang, Haochen Chai, Yulin Chen, and Yuxin Yang. 2021. "Changes in Rumen Microbiota Affect Metabolites, Immune Responses and Antioxidant Enzyme Activities of Sheep under Cold Stimulation" Animals 11, no. 3: 712. https://doi.org/10.3390/ani11030712
APA StyleGuo, H., Zhou, G., Tian, G., Liu, Y., Dong, N., Li, L., Zhang, S., Chai, H., Chen, Y., & Yang, Y. (2021). Changes in Rumen Microbiota Affect Metabolites, Immune Responses and Antioxidant Enzyme Activities of Sheep under Cold Stimulation. Animals, 11(3), 712. https://doi.org/10.3390/ani11030712





